Case Report
A 51-year-old woman presented with progressive isolated cerebellar symptoms. She had no history of neurological disease, but had undergone splenectomy at the age of 24 years for refractory lymphopenia of unknown etiology. She had a 1-month history of progressive gait instability and decreased right-sided coordination with truncal ataxia and right hand dysmetria. Brain computerized tomography and MRI failed to show any abnormality.
Four months after symptoms onset, she was unable to walk alone, her speech became slurred and she developed severe postural ataxia, bilateral gaze-evoked horizontal nystagmus with saccadic pursuit, appendicular dysmetria, dysarthria and brisk and pendular deep tendon reflexes bilaterally. Cognitive, motor and sensory functions were spared. MRI showed new-onset non-enhancing cerebellar vermis atrophy without any white matter signal anomaly. CSF analysis revealed 1 white blood cell/μl, a protein count of 0.29 g/L and a normal glucose level. CSF cultures, syphilis VDRL, HSV 1 and 2, and cytomegalovirus PCR were all negative. Work-up for metabolic, auto-immune and paraneoplastic conditions including cerebellar-specific auto-antibodies Anti-Hu, Anti-Ri and Anti-Yo was unrevealing.
Human immunodeficiency virus-1 (HIV-1) was diagnosed 5 months after symptoms onset. Baseline plasma viral load was 19,530 copies/ml, CD4+ lymphocyte count was 111 cells/μl and virus was pan-sensitive to antiretroviral therapy (ART). Considering newly recognized immunosuppression, CSF analysis was repeated and an extended infectious work-up was negative, except for JC virus (JCV) quantitative PCR assay of 60,000 copies/ml and HIV CSF viral load of 3521 copies/ml. JC virus DNA sequencing of the VP1 sub-unit capsid protein gene revealed a co-infection with the previously described JCVGCN1 variant and the novel variant JCVGCN8 (Figure 1). In the absence of clinical or radiological signs compatible with PML, the patient was diagnosed with isolated JCV granule cell neuronopathy (JCV GCN).

Figure 1 JC virus (JCV) DNA sequence variations among variants responsible for granule cell neuronopathy (GCN). JCV DNA sequencing revealing co-existence of JCVGCN1 and JCVGCN8.Reference Agnihotri, Dang and Carter 9
Central nervous system (CNS)-penetrating ART including abacavir/lamivudine and lopinavir/ritonavir was initiated and eventually switched to darunavir/ritonavir. Despite HIV-1 viral load becoming undetectable after 8 weeks and CD4+ lymphocyte count rising to 222 cells/μl, she experienced relentless neurological worsening and cerebellar atrophy (Figure 2). Six months after ART initiation, CSF HIV viral load was undetectable, but JCV remained elevated at 30,000 copies/ml. The absence of gadolinium enhancement or mass effect on MRI and weak JC-virus-specific CD4+ and CD8+ T-cell responses argued against PML immune reconstitution syndrome (PML-IRIS). To investigate this possibility, proton magnetic resonance spectroscopy was proposed, but the patient declined further investigation.
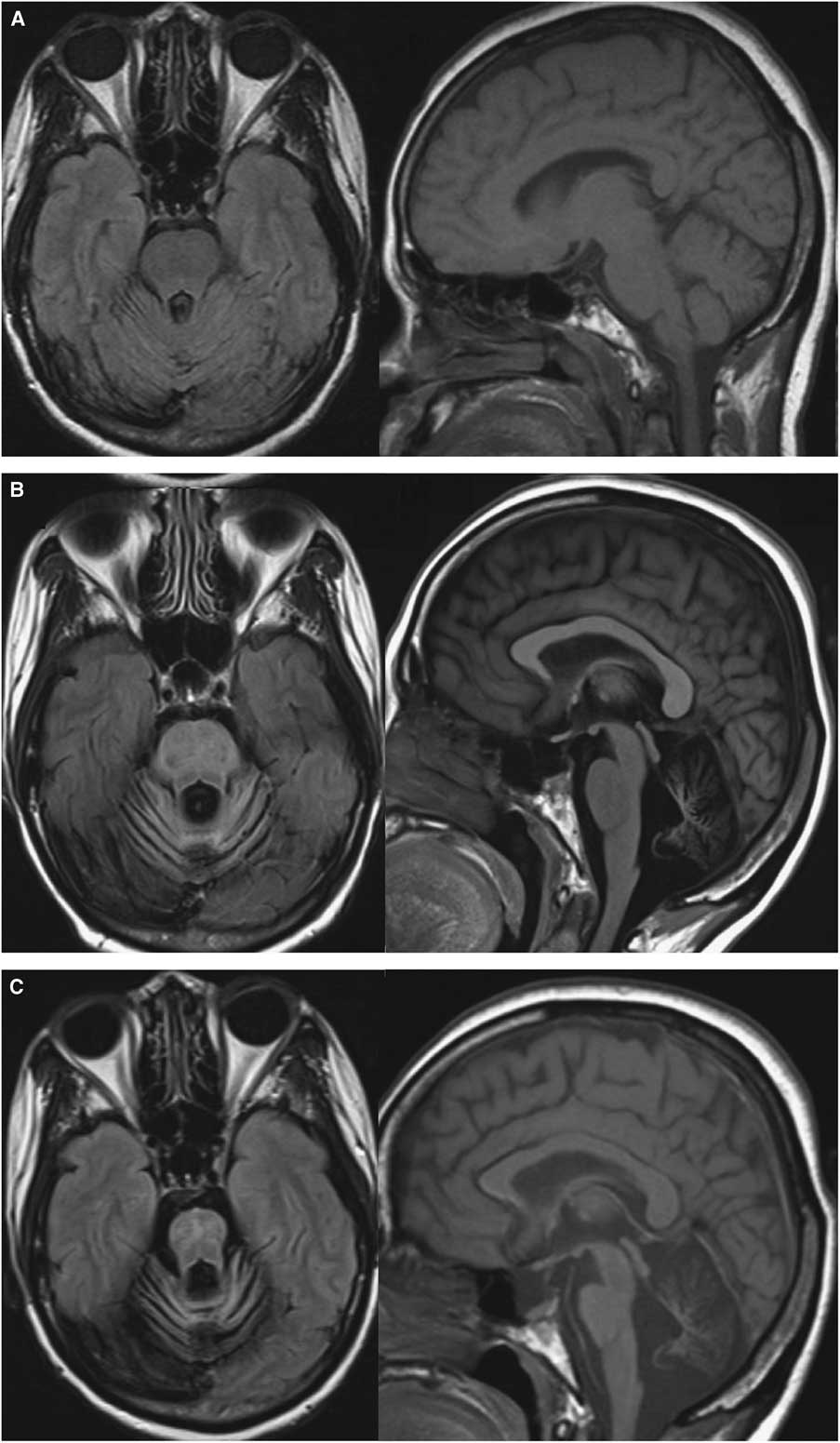
Figure 2 Evolution of MRI abnormalities at (A) 1 month, (B) 10 months and (C) 22 months after symptoms onset. Axial FLAIR sequences showed marked posterior fossa atrophy without signal abnormality (left) and Sagittal T1 sequences showed progressive pontocerebellar and pons atrophy (right).
Three years after initial presentation, cerebellar symptoms finally stabilized, but the patient showed no sign of neurological recovery. This is the first reported case of isolated JCV GCN, without clinical or radiological evidence of PML, manifesting as an AIDS-presenting illness in a previously undiagnosed HIV-infected individual.
PML was first described in 1958 and JCV was identified as its causative agent in 1971. JC virus is recognized as the causative agent of multiple neurological syndromes.Reference Miskin and Koralnik 1 Two cases of JCV encephalitis, one case of JCV meningitis and an increasing number of JCV granule cell neuropathy and PML-IRIS have now been reported.Reference Agnihotri, Wuthrich and Dang 2 , Reference Bialasiewicz, Hart and Oliver 3 Initially mainly reported in the context of hematological malignancies, the HIV epidemic and use of chemotherapy and immunomodulatory drugs including natalizumab and rituximab have significantly modified the epidemiology of JCV-associated CNS diseases.Reference Bloomgren, Richman and Hotermans 4 , Reference Casado, Corral and Garcia 5
JC virus is believed to initially infect children through the upper respiratory tract or the urine-oral route before establishing latency in kidney, bone marrow and spleen. In immunocompromised hosts, JCV can reactivate, spread to the CNS and enter permissive host cells, a process facilitated by serotonin receptors. Lytic infection of oligodendrocytes and astrocytes target cells results in multifocal white matter and white-gray matter junction demyelination, a disease known as classical PML. VP1 capsid protein is thought to play a crucial role in pathophysiology, immune response mediation and cell entry. Although capsid proteins coding regions express high genetic stability, mutations and subsequent changes in capsid structure may alter target cell specificity and clinical presentation.Reference Gorelik, Reid and Testa 6 Multiple JCV variants with mutations in the VP1 coding region were described and linked to lytic infection of cerebellar granule cell neurons and cerebellar atrophy. Infection with one of these mutants results in JCV GCN, a rare neurologic condition that can present with or without classical PML. JC virus GCN typically occurs in the same immunocompromised populations as classical PML. Specific clinical features are those of a subacute progressive cerebellar syndrome associated with cerebellar atrophy on brain imaging.Reference Koralnik, Wuthrich and Dang 7 Combined with a positive CSF JCV PCR, these clinical features yield a virological diagnosis of JCV GCN.Reference Dang, Vidal and Oliveira 8 , Reference Agnihotri, Dang and Carter 9 A histological diagnosis requires hypochromatic, enlarged granule cell neurons expressing JCV proteins on cerebellum biopsy. As it is the case in HIV-negative patients, JCV GCN may occur with or without classical PML. Probable JCV GCN was previously described in a newly diagnosed HIV patient with cerebellar deficits, but JCV PCR testing on CSF remained negative and no biopsy was performed.Reference Henry, Jouan and De Broucker 10
PML 1-year survival increased from a few months 20 years ago to 50%-60% in HIV-positive patients with the advent of ART. As no specific treatment showed efficacy against JCV, no further progress was made since. Serotonin receptors targeting drugs chlorpromazine and mirtazapine were inconsistently reported as effective. DNA replication inhibitors cytarabine and cidofovir showed no benefit in HIV patients. Despite in vitro activity against JCV, a trial with mefloquine was terminated after interim analysis suggested that data were unlikely to show a significant difference between groups. In HIV-negative cases, discontinuation of immunosuppressive agents is essential. In HIV-positive cases, factors associated with favorable outcome are CD4 count greater than 100 cells/μl, CSF inflammatory response and early diagnosis and ART initiation.Reference Casado, Corral and Garcia 5
This is the first reported confirmed JCV GCN case presenting as inaugural HIV/AIDS-related illness. Isolated cerebellar disease caused by JCV GCN should be considered as an HIV/AIDS opportunistic infection. Isolated cerebellar disease should evoke the possibility of undiagnosed HIV infection and trigger subsequent testing since early diagnosis, and rapid reversal of immunosuppression with ART only can prevent progression to irreversible disease. Furthermore, CSF JCV testing should always be performed in immunocompromised patients presenting with isolated cerebellar disease or atrophy regardless of their HIV status.
Acknowledgment
This study was funded in part by NIH grant R01 NS047029 and R01 NS074995 to IJK. The authors have no conflicts of interest to declare.
Disclosures
SGL, XD, DG, SL and LL have nothing to disclose. IJK has the following disclosure: NIH Grant R01 NS047029 and R01 NS074995.
Statement of Authorship
SGL carried out infectious disease clinical care, chart review, data gathering and manuscript writing, and is the corresponding author; DG performed diagnostic radiology examination interpretation and follow-up; SL performed neurology clinical care; XD and IJK carried out CSF molecular analysis, viral genome sequencing and lymphocyte activity assessment; LL carried out infectious disease clinical care, long-term patient follow-up and manuscript writing.




