Neonatal early-onset sepsis (EOS), defined as a positive blood culture within the first 3 days after birth, increases the risk of morbidity and mortality in newborn infants. Reference Ting, Roberts and Sherlock1 The overall incidence of EOS in the United States is 1 per 1,000 live births, but EOS occurs at a 10-fold higher rate in premature infants. Reference Flannery, Edwards, Puopolo and Horbar2 Premature infants are highly susceptible to bacterial infections due to their immature immune systems, Reference Melville and Moss3 and inflammation due to infection often triggers preterm labor. Mortality rates of premature infants diagnosed with EOS reaches up to 33%. Reference Flannery, Edwards, Puopolo and Horbar2,Reference Mukhopadhyay and Puopolo4,Reference Stoll, Puopolo and Hansen5 The diagnosis of EOS is challenging due to the nonspecific clinical presentation, with signs and symptoms that overlap with manifestations of prematurity, respiratory distress syndrome, and the transition from fetal to neonatal physiology. Reference Flannery, Edwards, Puopolo and Horbar2
Due to the potentially lethal consequences of untreated neonatal sepsis, the nonspecific signs, and the common presence of risk factors, most premature infants receive empiric antibiotics for early-onset bacterial infection. Reference Flannery, Ross, Mukhopadhyay, Tribble, Puopolo and Gerber6 Preterm labor, prolonged rupture of membranes, and chorioamnionitis in the mother’s perinatal history indicate increased risk to the infant and prompt an evaluation with blood culture and empiric antibiotics to rule out sepsis. Reference Romero, Pacora and Kusanovic7,Reference Mukhopadhyay and Puopolo8 A propensity toward caution in these high-risk infants often results in a longer course of antibiotics (≥5 days) despite negative blood cultures. Neonatologists often define this condition, in which the infant is sick but the blood culture is negative, as “clinical sepsis.” They treat clinical sepsis with empiric antibiotics, presuming that the blood culture was falsely negative. Consequently, most infants in NICUs have prolonged early antibiotic exposure. Reference Ting, Roberts and Sherlock1,Reference Flannery, Ross, Mukhopadhyay, Tribble, Puopolo and Gerber6,Reference Songer, Calip, Srinivasan, Barbosa and Pham9
In the short term, prolonged antibiotic exposure increases the likelihood of antibiotic resistance and the risk of neonatal morbidities and mortality in premature infants. Reference Ting, Roberts and Sherlock1,Reference Cantey, Pyle, Wozniak, Hynan and Sánchez10,Reference Fajardo, Alshaikh and Harabor11 The long-term effects of neonatal antibiotic exposure are also important because empiric treatment of EOS disrupts the intestinal microbiome during a critical time in its development. Reference Gasparrini, Crofts, Gibson, Tarr, Warner and Dantas12–Reference Thanabalasuriar and Neonates14 Early antibiotic exposure reduces the abundance and diversity of intestinal microbiota, which may lead to stunted childhood growth Reference Uzan-Yulzari, Turta and Belogolovski15 or obesity, Reference Bailey, Forrest, Zhang, Richards, Livshits and DeRusso16 and poor neurodevelopmental outcomes. Reference Lu and Claud17
Many NICUs use 48 hours of empiric antibiotics in EOS evaluations, yet the growth of most EOS pathogens in culture in most cases are identified within 24 hours. Reference Mukhopadhyay and Puopolo8,Reference Goldberg, Sokolover and Bromiker18–Reference Kuzniewicz, Mukhopadhyay, Li, Walsh and Puopolo20 The duration of antimicrobial activity extends hours to days beyond the last dose for some antibiotics in preterm infants, including ampicillin and gentamicin. Reference Le, Greenberg and Benjamin21,Reference Le, Greenberg and Yoo22 To reduce the length of early antibiotic exposure for neonates, we implemented a change in the unit guideline to shorten the duration of empiric EOS antibiotic coverage from 48 (last dose at 36 hours) to 24 hours (last dose at 12 hours) while ensuring no adverse effects.
Methods
Setting and context
This study was a single-center quality improvement project following SQUIRE 2.0 guidelines for the study design and reporting. 23 We conducted the study in a 51-bed, level IV NICU at the University of Virginia’s Children’s Hospital in Charlottesville, Virginia. The study was categorized as a quality improvement effort by our local institutional review board and consent was not required. The NICU serves as a major regional referral center for the state, and nearly two-thirds of admissions to the unit each year are inborn. The study population included all inborn infants <35 weeks’ gestation (ie, preterm infants) admitted to the NICU and evaluated for EOS with a blood culture obtained within 72 hours after birth. We focused on this subpopulation for analysis and data collection, but the implemented guideline change applied to all inborn NICU admissions.
We collected retrospective data on antibiotic exposures and EOS blood culture results from January through November 2019 to assess the baseline condition and to plan guideline changes and prospective data collection. A multidisciplinary team composed of neonatologists, pediatric infectious disease experts, pharmacists, neonatal advanced practice providers, and nurses were consulted regarding the proposed guideline change. Blood cultures were obtained from the newborn using sterile technique from a peripheral arterial or venous blood draw, from an umbilical line during placement, or from the placenta immediately after delivery. Our unit guidelines recommend a blood culture at the time of NICU admission for any infant born premature with risk factors, including delivery due to preterm labor, prolonged rupture of membranes, clinical concern for chorioamnionitis, and any other indication as determined by the clinical team. Blood cultures obtained after admission but before 3 days of age are ordered based on clinical suspicion of sepsis.
Intervention
In December 2019, we changed the unit guideline to reduce the standard duration of empiric treatment for negative EOS blood cultures from 48 hours to 24 hours. Prior to implementation, we educated all staff and providers on the rationale for the change and presented the baseline data. In January 2020, we changed the default antibiotic doses in the electronic medical record order set to end after 2 doses of ampicillin (100 mg/kg given every 12 hours) and 1 dose of gentamicin (4 mg/kg). Project champions gave frequent periodic reminders and brief educational sessions during group practice meetings, daily unit huddles, and rounds. A detailed infographic outlining data on time to blood-culture positivity and deleterious effects of early antibiotic exposure on neonates with culture-negative sepsis was published in the NICU newsletter to educate providers. The study team met every 3 months to conduct a new Plan–Do–Study–Act (PDSA) cycle, in which we evaluated process measures, identified areas for improvement, and re-educated NICU clinicians.
Data collection and measures
We prospectively collected data on antibiotic exposures, EOS blood-culture results, late infections, and patient characteristics. To ensure that the interventions had no unintended negative impact, we tracked the following data as balancing measures: time to positivity for EOS blood cultures positive for any organism, the percentage of infants with a repeat blood culture drawn within 7 days of a negative EOS blood culture, and rates of late-onset sepsis, clinical sepsis, necrotizing enterocolitis (NEC), and urinary tract infection (UTI). We defined clinical sepsis as a negative EOS blood culture treated with antibiotics for ≥5 days. We analyzed the following process measures monthly: (1) guideline adherence, defined as the proportion of negative EOS blood cultures treated with ≤24 hours of antibiotics, (2) mean and standard deviation of treatment duration for a negative EOS blood culture, defined as the number of consecutive days of antibiotics following the EOS blood culture, and (3) antibiotic days per 30 patient days, analyzed in the first month after birth. We defined an antibiotic day as a calendar day in which an infant received 1 or more doses of any intravenous antibiotic. We also recorded patient days as the number of calendar days from birth until 30 days of age, NICU discharge, death, or transfer to another unit, whichever came first.
Analysis
We used control charts to assess process measures for significant changes using standard rules Reference Gupta and Kaplan24 and QI Macros for Excel (KnowWare International, Denver, CO, and Microsoft, Redmond, WA). To assess guideline adherence, we used a P chart to analyze the percentage of neonates with negative EOS blood cultures who received antibiotics for ≤24 hours. Mean and standard deviation of antibiotic days for a negative EOS blood culture by month and were charted using X-bar and S charts. Patient characteristics, demographics, and clinical outcomes were compared across the preintervention and postintervention groups using the Fisher exact test for categorical data and the Kruskal-Wallis test for continuous data using GraphPad Prism version 8.0.0 software for Windows (GraphPad Software, San Diego, CA). A P value ≤.05 was considered statistically significant.
Results
From January 2019 to December 2021, the UVA NICU admitted 347 inborn infants <35 weeks gestational age at birth (ie, preterm infants): 206 infants before and 141 infants after the change in guideline or intervention. Table 1 compares the characteristics of infants with an EOS blood culture before and after the intervention. Figure 1 shows a diagram summarizing the numbers and proportions of infants categorized by blood culture results and treatment durations. More infants had an EOS blood culture in the postintervention phase (70% before the intervention vs 94% after the intervention), and the incidence of EOS was the same (3%) in each phase. The indications for delivery among those evaluated for EOS were similar overall. Preterm labor was the primary indication for delivery in 67% of infants, while 15% (preintervention) and 11% (postintervention) were delivered for maternal indications (Table 1).
Table 1. Patient Characteristics Before (Preintervention) and After (Postintervention) the Implementation of the 24-Hour Recommended Duration of Empiric Antibiotics for a Negative Early-Onset Sepsis Blood Culture
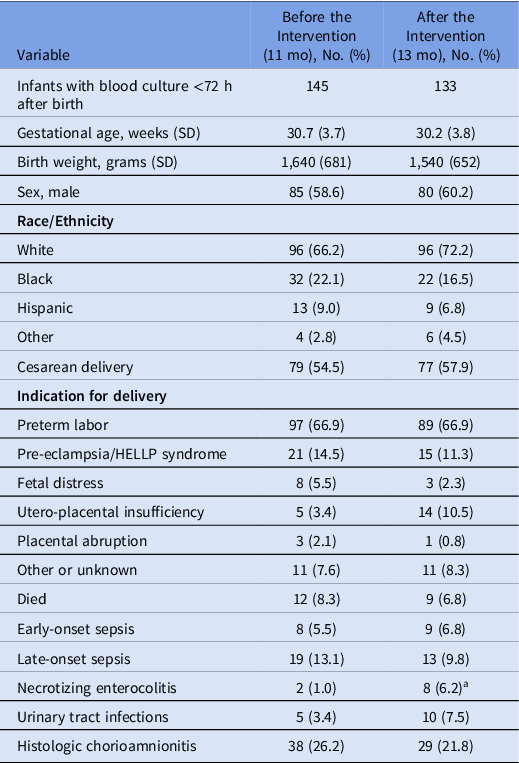
Note. SD, standard deviation. Continuous variables are shown as mean (standard deviation) and categorical variables as number (%).
a P < .05, Fisher exact test.

Fig. 1. Diagram of results. The numbers and proportions of infants with an EOS blood culture (ie, obtained ≤3 days after birth) during the study period, categorized by culture results and treatment durations are displayed in the boxes. Organisms isolated in cases of EOS are listed under each phase. Note. EOS, early-onset sepsis; CONS, coagulase-negative Staphylococcus spp; GBS, group B Streptococcus spp; GA, gestational age.
Excluding contaminants, the median time to positivity was 13.2 hours (range, 8–23). Organisms isolated from EOS blood cultures are listed in Figure 1. Moreover, 5 blood cultures grew bacteria that the clinical team considered to be contaminants and all resulted after 24 hours (range, 25–103), including 4 blood cultures with coryneform gram-positive rods and 1 that grew Olligella urethralis. Notably, all of these blood cultures were obtained from the placenta.
Rates of late-onset sepsis and UTI did not change after implementation. The incidence of NEC was higher in the postintervention phase (1% vs 6.2%) but was comparable to the historical NEC rate in the unit of 5%–6% per year. Of 8 NEC cases in the postintervention phase, 4 infants were diagnosed beyond 30 days old and therefore were not included in the analysis of antibiotic days per patient days.
Baseline data evaluation revealed that 15 infants (10%) with negative EOS blood cultures were treated for <48 hours. The rare practice of prescribing <48 hours of antibiotics for infants with suspected EOS informed our plan for education and guideline change implementation. After the implementation of the new guideline, 71 infants (53%) with negative EOS blood cultures were treated according to the new guideline and received no more than 24 hours of empiric antibiotic therapy. These 71 infants comprised 77% of infants with negative EOS blood cultures after excluding those treated for clinical sepsis (ie, received ≥ 5 days of empiric antibiotics with a negative blood culture). Figure 2 shows a P chart of the percentage of infants with negative EOS blood cultures treated for ≤24 hours with empiric antibiotics. Also, 12 data points in the postintervention period fell above the UCL of the preintervention mean, indicating a significant change in this process measure. Reference Gupta and Kaplan24

Fig. 2. P chart of the monthly percent of infants with a negative early-onset sepsis blood culture who received 24 hours or less of empiric antibiotics. The upper control limit (UCL) and lower control limit (LCL) lines are drawn at 3 standard deviations from the mean. The LCL before the intervention and the UCL after the intervention are not shown because they were <0% or >100%, respectively. The yellow solid vertical line represents the start of the intervention phase.
For all infants with negative EOS blood cultures, the mean duration of treatment decreased by half a day (3.6 vs 3.1 days). As a process measure, this did not change over time but was not a stable process at baseline, indicated by multiple points above and below the control limits in the s-chart of monthly standard deviation (Fig. 3). Subsequent sepsis evaluations within 7 days of a negative EOS blood culture did not increase: 30 (21%) before implementation versus 26 (19%) after implementation. However, the percentage of infants with negative EOS blood cultures treated as clinical sepsis increased from 17% before the intervention to 26% after the intervention. Infants treated for 5 or more days in either phase had lower mean body weight (1,270 ± 750 grams vs 1,700 ± 605 grams) and gestational age (28 ± 4 weeks vs 31 ± 3 weeks) compared to those whose antibiotic treatment was discontinued earlier. The average antibiotic days in the first month (per 30 patient days) increased by half a day from 7.2 ± 7.3 days to 7.5 ± 8.3 days.

Fig. 3. X-bar and S charts of antibiotic days given for a negative early-onset sepsis blood culture by month. The upper control limit (UCL) and lower control limit (LCL) lines are drawn at 3 standard deviations from the mean. The top panel shows an x-bar chart of the mean monthly antibiotic days and the bottom panel shows an s-chart of the standard deviation (SD) by month. Red points indicate months in which the SD was above or below the control limit.
A subanalysis of extremely preterm infants (ie, gestational age <28 weeks at birth) revealed that a smaller proportion of infants were treated according to the new guideline after the intervention compared to the overall cohort, indicating that most of the change occurred in the bigger or more mature infants (Fig. 1). The new guideline was followed in 30% of extremely preterm infants who had a negative EOS blood culture after the intervention, compared with 66% of extremely preterm infants. The reduction in mean duration of treatment of an infant with a negative EOS blood culture was slightly higher in this subgroup than the overall study population, with a decrease in mean duration from 6.9 ± 8.3 days to 6.0 ± 7.2 days.
Discussion
In this single-center, quality improvement initiative, we demonstrated that the duration of empiric antibiotic coverage for well-appearing preterm infants with a negative EOS blood culture can be safely reduced from 48 to 24 hours. Much of the work on reducing early antibiotic exposure has focused on asymptomatic term infants with risk factors, but growing evidence suggests that we need to focus on premature infants as well. Reference Ting, Roberts and Sherlock1,Reference Flannery, Ross, Mukhopadhyay, Tribble, Puopolo and Gerber6
Studies on time to positivity have shown that most gram-negative organisms and many, but not all, gram-positive pathogens grow within 24 hours of culture. Reference Kumar, Qunibi, Neal and Yoxall19,Reference Kuzniewicz, Mukhopadhyay, Li, Walsh and Puopolo20,Reference Garcia-Prats, Cooper, Schneider, Stager and Hansen25–Reference Mukhopadhyay, Briker and Flannery28 In a recent, large study of EOS blood cultures growing pathogens, 68% were positive by 24 hours, 94% were positive by 36 hours, and 97% were positive by 48 hours. Reference Kuzniewicz, Mukhopadhyay, Li, Walsh and Puopolo20 In our cohort, all organisms determined to be clinical pathogens grew within 24 hours and those isolated beyond 24 hours were potential contaminants based on the treatment duration and organism. Although these results suggest that the 24-hour duration is safe, the results of the much larger study conducted at 19 centers over a longer duration indicate that this practice may not be generalizable. This discrepancy warrants ongoing monitoring not only time to positivity in our unit but also differences in laboratory processes or result reporting procedures. We also noted a high rate of contaminant organisms, possibly because most of our EOS blood cultures are obtained from the placenta. Reference Newberry29
Several studies support the safety of the 24-hour rule in well-appearing term infants, but these studies used clinical or laboratory criteria to guide the early discontinuation of antibiotics for low-risk infants. Reference Goldberg, Sokolover and Bromiker18,Reference Murphy and Weiner30,Reference Escobar, Zukin and Usatin31 Time to positivity >36 hours is common with coagulase-negative staphylococci (CONS) isolation, Reference Mukhopadhyay, Briker and Flannery28 so empiric treatment for >24 hours is appropriate for late-onset neonatal sepsis, many of which are caused by CONS. Reference Flannery, Puopolo and Hansen32 In our center, urine culture results also routinely require 24–36 hours for a positive result.
We took the approach of implementing a universal guideline of 24-hour empiric treatment for preterm infants with a negative EOS blood culture and without clinical sepsis. Some have questioned whether intrapartum antibiotics impact the positivity of neonatal blood cultures, but this theory has since been disproved. Reference Kuzniewicz, Mukhopadhyay, Li, Walsh and Puopolo20 Our guideline left room for clinical judgment regarding which infants could be treated for clinical sepsis despite a negative blood culture. Clinicians and units vary widely on diagnostic criteria for culture-negative clinical sepsis. We noticed an increase in the proportion of infants treated for presumed clinical sepsis after the implementation of the new guideline, which was greater among extremely preterm infants. We did not collect data on reasons for not discontinuing antibiotics during this study period, but we plan to do so to inform future PDSA cycles and interventions. A revised guideline that uses specific criteria to identify low-risk preterm infants might improve adherence and reduce prolonged treatment of a negative EOS blood culture.
The mean duration of treatment for a negative EOS blood culture in extremely preterm infants decreased by 1 day. Our guideline change only reduced the recommended antibiotic duration by 1 day, so this magnitude of change in treatment duration for infants with negative EOS blood cultures in the smaller subpopulation was encouraging despite incomplete adherence to the guideline and the large proportion of infants with negative blood cultures treated empirically for clinical sepsis. However, a much smaller proportion of infants <28 weeks gestation age at birth (ie, extremely preterm infants) with negative EOS blood cultures were treated for 24 hours compared to those 28–34 weeks of gestational age at birth (30% vs 66%). Further work is needed in our unit and likely others to safely reduce the duration of treatment for infants with negative blood cultures. Reference Cantey and Baird33,Reference Cantey, Wozniak, Pruszynski and Sánchez34 In addition, a substantial proportion of infants evaluated for EOS were delivered due to maternal indications, so they presumably had a low risk of EOS. The risk of undertreating a negative blood culture in an acutely ill infant must be balanced by data showing that every additional day of antibiotics administered to premature infants increases the risk of adverse outcomes including late-onset sepsis, NEC, and death. Reference Kuppala, Meinzen-Derr, Morrow and Schibler35 Hence, a reduction in empiric antibiotic exposure in premature infants has the potential to make a clinically significant impact.
We did not detect an increase in late-onset sepsis during the postintervention period, but the number of NEC cases increased. Based on historical NEC rates in our unit, we suspect that this increase was due to the unusually low NEC rate in 2019, followed by a typical year in 2020. Ongoing data collection and analysis will be used to investigate this change.
The impact of this quality improvement study is limited by the wide variation among NICUs in antibiotic prescribing practices. Reference Flannery, Ross, Mukhopadhyay, Tribble, Puopolo and Gerber6 Our intervention may have had a greater impact on antibiotic exposure in a different unit with more low-risk or moderately premature infants.
Next steps and future directions
The results of this study identified areas for further improvements in antibiotic stewardship using quality improvement methodology and analyses. In the next PDSA cycle, we aim to revise guidelines for initiating and stopping antibiotics using objective criteria based on risk factors. After unit education and implementation, we will track an updated set of process measures to evaluate adherence to these guidelines as well as their safety and efficacy with balancing measures. Although limited to a single center, we anticipate that the findings of this study and future results will inform quality improvement efforts at similar NICUs.
In conclusion, implementation of a guideline to change the empiric antibiotic duration for suspected EOS from 48 to 24 hours safely reduced antibiotic exposure in 77% of preterm infants (ie, <35 weeks gestational age at birth) in whom EOS was ruled out. The intervention reduced the mean antibiotic days for infants with a negative EOS blood culture by half a day. All clinically significant pathogens grew within 24 hours. Although the data suggest that this practice is safe, the change did not reduce overall antibiotic days, likely due to incomplete adherence to the guideline and an increase in the proportion of infants with negative EOS blood cultures treated empirically for clinical sepsis.
Acknowledgments
Financial support
This study was supported by the National Institutes of Health (grant no. K23 HD 097254 to B. Sullivan).
Conflicts of interest
The authors have no conflicts to disclose.







