Introduction
The distribution of species is highly influenced by both abiotic (ocean warming, Burrows et al., Reference Burrows, Schoeman, Buckley, Moore, Poloczanska, Brander and Richardson2011; Chen et al., Reference Chen, Hill, Ohlemuller, Roy and Thomas2011; Poloczanska et al., Reference Poloczanska, Brown, Sydeman, Kiessling, Schoeman, Moore and Richardson2013; Büscher et al., Reference Büscher, Form and Riebesell2017) and biotic factors (i.e. facilitation as a positive interaction among organisms, Michalet & Pugnaire, Reference Michalet and Pugnaire2016).
Marshes are environments exposed to terrestrial and aquatic conditions (both marine and freshwater) that present marked gradients of physical stress (e.g. anoxia, salinity) that have important effects on the structuring and, in particular, on the community's zonation patterns (Traut, Reference Traut2005; Lortie & Callaway, Reference Lortie and Callaway2006). Additionally, these environments suffer the effects of globalization, such as maritime transport and aquaculture, favouring the transport of marine species outside their native geographic ranges (Carlton & Geller, Reference Carlton and Geller1993; Cohen & Carlton, Reference Cohen and Carlton1998; Mack et al., Reference Mack, Simberloff, Mark Lonsdale, Evans, Clout and Bazzaz2000).
Gobiids are mostly from tropical and subtropical areas, being predominantly found in marine and brackish environments, but some species are catadromous; they prefer shallow coastal waters and live around coral reefs in all the world's seas (Acha, Reference Acha1994). Most are cryptic bottom-dwelling carnivores of small benthic invertebrates; others are planktivores (Nelson, Reference Nelson2006). Some species have symbiotic relationships with invertebrates (e.g. shrimps) and others are known to remove ecto-parasites from other fishes (Vázquez & Bas, Reference Vázquez and Bas2019). Gobiidae, one of the largest family of marine fishes, according to Nelson et al. (Reference Nelson, Grande and Wilson2016) is among the most species-rich of marine fish families, and is often the most abundant fish family in freshwater habitats on oceanic islands. The family shows some particular morphology, i.e. pelvic fins are usually fused into an adhesive disc, and most species are below 10 cm in total length (Figueroa, Reference Figueroa2019). They are common inhabitants on the coast of Uruguay and Buenos Aires, but are difficult to observe due to their cryptic habits, with the southernmost distribution limit of Gobiosoma hemigymnum being Mar del Plata (38°00′S 57°33′W), Argentina (Van Tassell et al., Reference Van Tassell, Joyeux, Macieira and Tornabene2015; Figueroa, Reference Figueroa2019). Another gobiid species (Ophiogobius jenynsi) has been reported from Argentina, and it was found at the Beagle Channel, Ushuaia (Menni et al., Reference Menni, Ringuelet and Aramburu1984).
Usually, salt marshes do not present favourable conditions for cryptic species such as gobiid fishes to inhabit these areas, mainly due to dryness and high temperatures at low tides (Piccolo & Perillo, Reference Piccolo and Perillo1990), and scarce places to hide. However, the presence of an invasive species of Pacific oyster, Magallana gigas could have a positive effect over fish species. Invasive species could usually be considered as ecosystem engineers (Fei et al., Reference Fei, Phillips and Shouse2014). Howard et al. (Reference Howard, Francis, Côté and Therriault2019) described: ‘Ecosystem engineering may be autogenic, where the invasive species itself creates habitat, or allogenic, where the habitat is transformed by the invasive organism through its activities’. Magallana gigas was first reported by Dos Santos & Fiori (Reference Dos Santos and Fiori2010) when they found a few oysters on port docks at the Bahía Blanca estuary. Twelve years later the Bahía Blanca population of M. gigas is very abundant with apparently large impacts on ecosystem structure and functionality (Molina, pers. obs.).
The aim of this paper is to report the occurrence of Gobiosoma hemigymnum more southerly than the so far (Van Tassell et al., Reference Van Tassell, Joyeux, Macieira and Tornabene2015; Figueroa, Reference Figueroa2019) documented distribution range of the species, associated to an uncommon habitat, salt marshes. We propose the hypothesis that the finding of Gobiosoma hemigymnum might be a consequence of a positive effect of autogenic ecosystem engineer and invasive species Magallana gigas.
Materials and methods
Study area
The Bahía Blanca Estuary is a wide coastal wetland complex in temperate South America, comprising a total surface of 2300 km2 which includes about 410 km2 of marshes and more than 1150 km2 of mudflats (Perillo et al., Reference Perillo, Piccolo, Parodi and Freije2001; Isacch et al., Reference Isacch, Costa, Rodríguez-Gallego, Conde, Escapa, Gagliardini and Iribarne2006). Mean tidal range varies from 2 m at the mouth to 3.8 m at the middle and upper reaches, and salinity typically increases from the mouth to the head, where the restricted circulation and the high evaporation may produce concentrations higher than 38 (Piccolo & Perillo, Reference Piccolo and Perillo1990). Located in the northern limit of the Patagonian desert, vegetation in the intertidal zone is scarce and, unlike the better known counterparts of Eastern North America, Spartina alterniflora marshes only occur in discontinuous patches at the mouth of the estuary. Under the seasonally hypersaline conditions in the inner estuary, vegetation is virtually absent in the intertidal zone except for the circular mounds of Sarcocornia perennis, colonizing the upper marshes (Perillo & Iribarne, Reference Perillo and Iribarne2003).
Within the study area, major transformations relate to the presence of the largest deep-water harbour system in Argentina, that comprises Ingeniero White Port and a series of subsidiary harbours related to a petrochemical industrial park, and a naval base (Perillo & Sequeira, Reference Perillo and Sequeira1989). The specific site where Gobiosoma hemigymnum was found corresponds to a S. alterniflora marsh of about 30 ha located 15 km south-east of Ingeniero White Port, and just 4 km north-west of the Puerto Belgrano Naval Base (Figure 1). Substrate is composed of a mixture of sand and mud, with extensive bare flats occupying most of the intertidal fringe, and high densities of Magallana gigas could be found covering the sediment surface, forming reefs (Molina, pers. obs.). Vegetation is restricted to the upper intertidal zone, with plant densities varying from 100 to more than 300 ramets m2, according to a strong seasonal pattern, and as happens also with tidal flats, lots of oyster are growing between plants, with a high bio-geomorphological impact (Molina, pers. obs.).
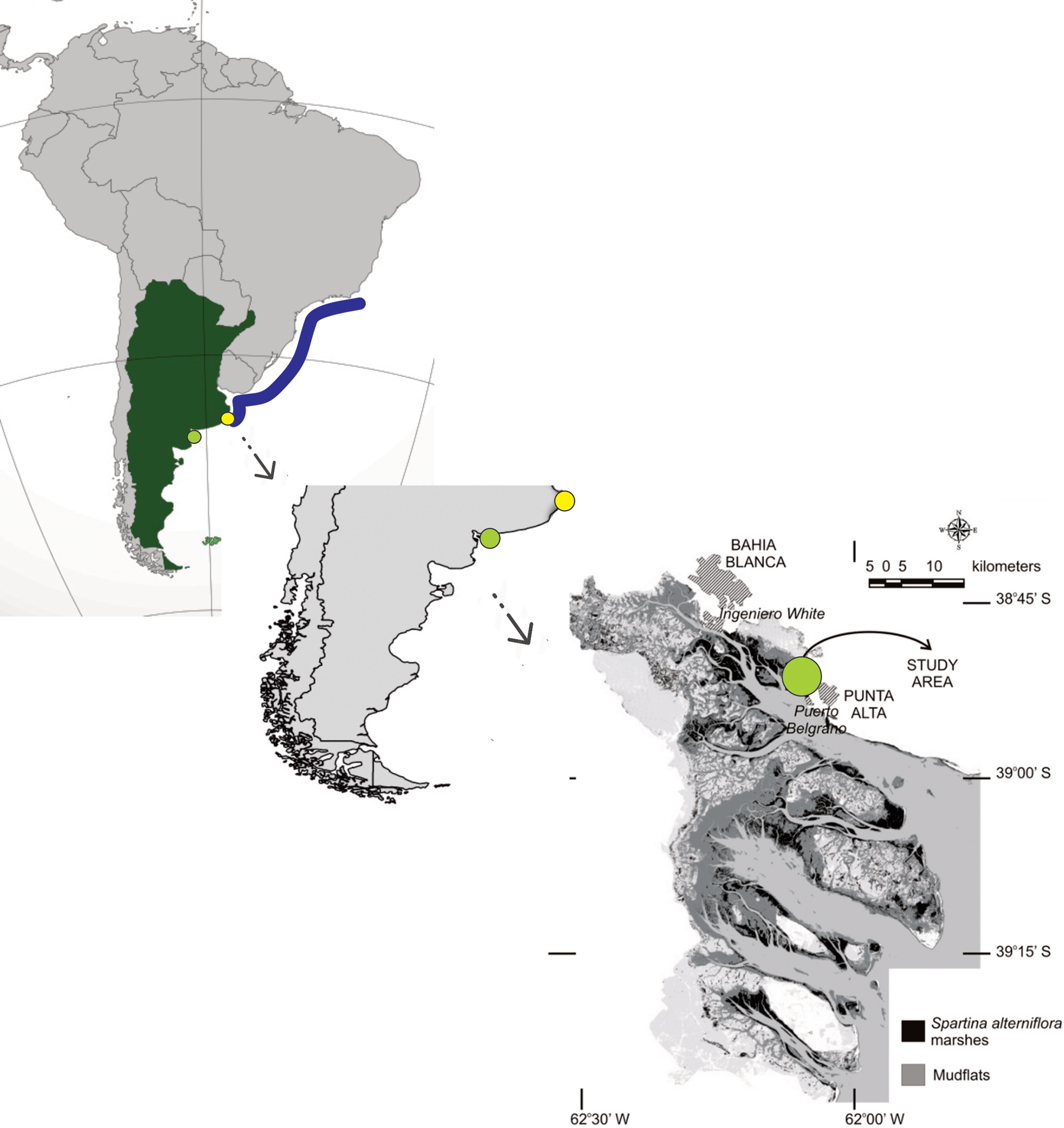
Figure 1. The map shows the geographic distribution range of Gobiosoma hemigymnum (blue line), with the location of the southern limit of distribution before this study (yellow dot), and the new southernmost documented record (light green dot). The Bahía Blanca estuary is shown in detail, with the main harbours, as well as the area covered by Spartina alterniflora marshes and mudflats.
Field sampling and data analysis
Sampling was made from 29 October to 5 November 2022, during low tide at afternoon. Two specimens of an unidentified fish were found in caves formed between oysters and Spartina stems (Figure 2A), which retained water, picking them up by hand, in isolated events. The specimens were kept in sampling bags with seawater for at least 2 h in order to allow fishes to be in a relaxed condition before being fixed in 10% formalin. Water temperature and salinity were also measured.

Figure 2. Plate showing record site and morphological characteristic of Gobiosoma hemigymnum. (A) Detail of the place where one of the specimens was collected, showing individuals of Magallana gigas and stems of Spartina alterniflora; (B) drawing showing standard length is ~5 times body height (from Figueroa, Reference Figueroa2019). (C) fixed specimen; (D) showing first dorsal fin with VI spines and second dorsal fin with XII rays; (E) detail of the mature female gonads occupying almost the entire fish cavity; (F) pelvic fins fused into an adhesive disc; (G) scaled specimen.
The specimens were photographed in the lab, with a Canon 7D Dsrl camera, with a 32 mm objective, considering several taxonomic features (following Van Tassell et al., Reference Van Tassell, Joyeux, Macieira and Tornabene2015 and Figueroa, Reference Figueroa2019). The morphological characteristics were described and specimens measured to the nearest 0.05 mm with a digital calliper. Meristic data were taken, gonads and livers were extracted and weighed on a SF-400C Venezia digital scale (± 0.01 mg), and the stomachs as well. Subsequently, the gonadosomatic IGS and hepatosomatic IHS indices were calculated according to Ferré et al. (Reference Ferré, Medesani, García, Grodzielski and Rodríguez2012): IGS (%) = (PG/PSP) × 100 and IHS = (PH/PSP) × 100, where PG is the wet weight of the gonad, PH the wet weight of the liver and PSP the wet weight of the specimen. Gonads were macroscopically staged according to the gonad development classification for gobies based on a five-point scale of maturity (Miller, Reference Miller1961). The specimens were stored in the fish collection of the Universidad Nacional de Río Negro under the catalogue number 44.
Results and discussion
Measurements and counts are given in Table 1. Standard length is ~5 times the body height (Figure 2B, C), dorsal fin with 6–8 spines and 11–12 rays (Figure 2C, D). These features, following Van Tassell et al. (Reference Van Tassell, Joyeux, Macieira and Tornabene2015) and Figueroa (Reference Figueroa2019), allowed to identify the two specimens as Gobiosoma hemigymnum. Anatomical dissection determined that the specimens were two females. The gonads occupied most of the whole abdominal cavity in one of the observed specimens, while in the other individual the gonads were smaller in size (Figure 2E). The two female specimens were sexually mature, according to the gonad development classification for gobiids based on a five-stage scale of maturity (Miller, Reference Miller1961). The high values of IGS and the low values of IHS indices for each specimen indicated that the two females were in breeding season (following Fouda et al., Reference Fouda, Hanna and Fouda1993).
Table 1. Meristic and environmental data, and the results of morphological indices

Within its normal distribution range this family is a numerically dominant component of larval, juvenile and adult fish assemblages in tropical and subtropical estuaries (Barletta-Bergan et al., Reference Barletta-Bergan, Barletta and Saint-Paul2002; Sanvicente-Añorve et al., Reference Sanvicente-Añorve, Salgado-Ugarte, Castillo-Rivera, Browman and Skiftesvik2003; Joyeux et al., Reference Joyeux, Campanha Filho and Jesus2004; Bonecker et al., Reference Bonecker, Nagae, Bletller, Velho and Lansac-To^ha2007; Coser et al., Reference Coser, Pereira and Joyeux2007; Shervette et al., Reference Shervette, Aguirre, Blacio, Cevallos, Gonzalez, Pozo and Gelwick2007; Ooi & Chong, Reference Ooi and Chong2011). The salt marshes fish assemblages of Bahía Blanca estuary were extensively studied by Lopez Cazorla (Reference Lopez Cazorla1987), Valiñas (Reference Valiñas2010), Valiñas et al. (Reference Valiñas, Molina, Addino, Montemayor, Acha and Iribarne2012) and Molina et al. (Reference Molina, Valiñas, Pratolongo, Elias and Perillo2017), as were the macrobenthic communities of vegetated and non-vegetated sediments (Molina et al., Reference Molina, Valiñas, Pratolongo, Elias and Perillo2009, Reference Molina, Valiñas, Pratolongo, Elias and Perillo2017; Molina, Reference Molina2013). The occurrence of G. hemigymnum has not been recorded in Bahía Blanca estuary before and all these observations and studies were made before the oyster M. gigas became an invader, with the consequential physical and biological alteration of the shore (Molina, pers. obs). Gobiosoma hemigymnum has been documented to occur, based on recent collections, in the south-western Atlantic from Rio de Janeiro, Brazil (23 S), to Mar del Plata, Argentina (38 S) (Van Tassell et al., Reference Van Tassell, Joyeux, Macieira and Tornabene2015; Figueroa, Reference Figueroa2019). The half-naked goby is associated to the Ficopomatus enigmaticus reefs, an invasive species and ecosystem engineer (Schwindt et al., Reference Schwindt, De Francesco and Iribarne2004), in Mar Chiquita lagoon (Cervigon & Bastida, Reference Cervigon and Bastida1971) and inhabits the encrusting community in both the harbour and the mesolittoral zone of Cabo Corrientes, near Mar del Plata (Vázquez & Bas, Reference Vázquez and Bas2019), both sites in the Buenos Aires province, Argentina. Accordingly, we hypothesize that the modifications introduced by Magallana gigas, in term of spatial complexity, created new habitats, suitable to G. hemigymnum.
There are several reasons to think that G. hemigymnum has become a frequent inhabitant of this environment, in relation to the presence of M. gigas. A general pattern of increased diversity and/or abundance with increased habitat complexity (Hosack et al., Reference Hosack, Dumbauld, Ruesink and Armstrong2006), with some exceptions were found (Castel et al., Reference Castel, Labourg, Escaravage, Auby and Garcia1989). Some authors support the idea that fishes are more abundant in vegetated adjacent habitats to oyster reefs (Holsman et al., Reference Holsman, McDonald and Armstrong2006; Kelly & Volpe, Reference Kelly and Volpe2007; Kelly et al., Reference Kelly, Proctor and Volpe2008) despite benthic organisms’ diversity being higher in M. gigas reefs (Hosack et al., Reference Hosack, Dumbauld, Ruesink and Armstrong2006), generating contradictory evidence. Leonard & Croft (Reference Leonard and Croft2006) propose that the presence of S. alterniflora reduces wave-related disturbance, and it could explain the occurrence of G. hemigymnum between S. alterniflora stems.
Conclusions
The introduction of Magallana gigas, that competes with existing forms of habitat structure, such as Spartina, may affect the availability of important habitat refugia and foraging resources for estuarine fishes (Hosack et al., Reference Hosack, Dumbauld, Ruesink and Armstrong2006) increasing complexity (Castel et al., Reference Castel, Labourg, Escaravage, Auby and Garcia1989), and in this case of a positive way, generating a new habitat type for the gobid G. hemigymnum, with potential prey items and refuge (Grabowski et al., Reference Grabowski, Hughes, Kimbro and Dolan2005). Further studies are needed to understand the impacts and modifications that communities and their habitats are undergoing, thus being able to predict new dynamics and improve management tools, especially taking into account that many species that inhabit these habitats can be negatively affected.
Acknowledgements
We thank the authorities of the Puerto Belgrano Club for allowing us to use the facilities for surveys.
Author contributions
LMM collected the specimens. The specimens were photographed by LMM and dissected by MCGD. ALC made the taxonomic identification. LMM wrote the manuscript with support from MCGD and ALC. All authors discussed the results and contributed to the final manuscript.
Financial support
LMM is supported by UNRN and CONICET, but this research was carried out with LMM personal financing.
Conflict of interest
The authors declare no conflict of interest.





