Introduction
Foraminifera are single-celled microorganisms that are one of the most important and widely distributed groups of organisms in marine environments (Gooday, Reference Gooday and Southward2003; Sen Gupta, Reference Sen Gupta2003). They occupy all marine habitats, including marginal (i.e. lagoons, estuaries, mangroves and salt marshes), coastal and deep-sea environments (Murray, Reference Murray2006). Benthic foraminifera are usually classified based on the structure of their test (shell). The calcareous type secretes calcium carbonate to form its tests, which can be divided into two groups: hyaline tests (perforated wall) and porcelaneous tests (imperforate wall) (Armstrong & Brasier, Reference Armstrong and Brasier2005). Meanwhile, agglutinated test species build their test by cementing foreign particles; usually sand grains or other small, fragmented shells (Tuckwell et al., Reference Tuckwell, Allen, Roberts and Murray1999). The preservation of the test depends on their surroundings, whether conductive to carbonate preservation or dissolution (Scott et al., Reference Scott, Medioli and Schafer2004). Distinct species assemblages preserved in large numbers/unit volume makes foraminifera a potential bioindicator of pollution, and several studies have shown the suitability of this benthic organism as a bioindicator (Hallock et al., Reference Hallock, Lidz, Cockey-Burkhard and Donnelly2003; Le Cadre & Debenay, Reference Le Cadre and Debenay2006; Frontalini & Coccioni, Reference Frontalini and Coccioni2011; Debenay, Reference Debenay, Marchand, Molnar, Aschenbroich and Meziane2015; Alve et al., Reference Alve, Korsun, Schönfeld, Dijkstra, Golikova, Hess, Husum and Panieri2016). Some studies have also addressed the relationship of species assemblages to floral zone at salt marshes and used to reconstruct sea level changes (Callard et al., Reference Callard, Gehrels, Morrison and Grenfell2011; Wright et al., Reference Wright, Edwards and van de Plassche2011; Horton et al., Reference Horton, Vann, Engelhart, Grand Pre, Vane, Nikitina and Anisfeld2012; Kemp et al., Reference Kemp, Telford, Horton, Anisfeld and Sommerfield2013; Strachan et al., Reference Strachan, Finch, Hill and Barnett2015; Barnett et al., Reference Barnett, Garneau and Bernatchez2016; Shaw et al., Reference Shaw, Kirby, Holgate, Tutman and Plater2016).
Benthic foraminifera represent a significant part of the total biomass of the meiofaunal community and play an essential role in the consumption of organic carbon in surface sediments (Mojtahid et al., Reference Mojtahid, Zubkov, Hartmann and Gooday2011). Some species of benthic foraminifera convert organic matter by means of kleptoplastidy where the species retain chloroplast from food sources and integrate them into their own pathway (e.g. genus Haynesina), while other species such as Ammonia rapidly ingest organic matter into cellular biomass (Pillet et al., Reference Pillet, Voltski, Korsun and Pawlowski2011; Jauffrais et al., Reference Jauffrais, Jesus, Metzger, Mouget, Jorissen and Geslin2016; Wukovits et al., Reference Wukovits, Enge, Wanek, Watzka and Heinz2017; Lintner et al., Reference Lintner, Biedrawa, Wukovits, Wanek and Heinz2020; Jesus et al., Reference Jesus, Jauffrais, Trampe, Goessling, Lekieffre, Meibom, Kühl and Geslin2022). The process of food uptake in foraminifera was induced by salinity in the surrounding environment. A recent laboratory experiment carried out has found that Ammonia tepida consumed green algae (Dunaliella tertiolecta) with average C of 0.8 and 1 μg mg−1 and N of about 0.3 1 μg mg−1 at salinity between 24 and 37 PSU, while Haynesina germanica recorded a lower average C of 0.3 μg mg−1 and N of about 0.05–1 μg mg−1(Lintner et al., Reference Lintner, Biedrawa, Wukovits, Wanek and Heinz2020).
Under natural conditions in nearshore marine environments, common factors contributing to the distribution and abundance of benthic foraminifera include salinity, temperature, dissolved oxygen, pH, substrate type, tidal regime and biotic influences such as competition, food supply and local disturbance (Scott et al., Reference Scott, Medioli and Schafer2004; Woodroffe et al., Reference Woodroffe, Horton, Larcombe and Whittaker2005; Murray, Reference Murray2006; Horton & Culver, Reference Horton and Culver2008; Kemp et al., Reference Kemp, Buzas, Horton and Culver2011). Species of benthic foraminifera in marshes/mangroves environments have been identified by some authors worldwide (e.g. Scott et al., Reference Scott, Medioli and Schafer2004; Woodroffe et al., Reference Woodroffe, Horton, Larcombe and Whittaker2005; Murray, Reference Murray2006; Berkeley et al., Reference Berkeley, Perry and Smithers2009a, Reference Berkeley, Perry, Smithers, Horton and Cundy2009b; Culver et al., Reference Culver, Mallinson, Corbett, Leorri, Rouf, Shazili, Yaacob, Whittaker, Buzas and Parham2012, Reference Culver, Leorri, Corbett, Mallinson, Shazili, Mohammad, Parham and Yaacob2013; Debenay, Reference Debenay2012; Gómez & Bernal, Reference Gómez and Bernal2013; Satyanarayana et al., Reference Satyanarayana, Husain, Ibrahim, Ibrahim and Dahdouh-Guebas2014; Camacho et al., Reference Camacho, Maria, Moura, Connor, Scott and Boski2015; Langer et al., Reference Langer, Olugbenga and Mannl2016). Common benthic foraminifera species that were found in mangrove ecosystems mostly consist of agglutinated taxa such as Ammotium morenoi, Arenoparrella mexicana, Haplophragmoides wilberti, Miliammina fusca, Entzia macrescens and Trochammina inflata, with some calcareous hyaline taxa (e.g. Ammonia tepida, Bolivina striatula, Elphidium fijiense, Elphidium hispidulum, Elphidium advenum) (Scott et al., Reference Scott, Medioli and Schafer2004; Woodroffe et al., Reference Woodroffe, Horton, Larcombe and Whittaker2005; Murray, Reference Murray2006; Berkeley et al., Reference Berkeley, Perry and Smithers2009a, Reference Berkeley, Perry, Smithers, Horton and Cundy2009b; Debenay, Reference Debenay2012; Culver et al., Reference Culver, Leorri, Corbett, Mallinson, Shazili, Mohammad, Parham and Yaacob2013; Camacho et al., Reference Camacho, Maria, Moura, Connor, Scott and Boski2015; Langer et al., Reference Langer, Olugbenga and Mannl2016; Abd Malek et al., Reference Abd Malek, Frontalini, Yahya, Talib and Zakaria2021).
Ecological baseline studies are essential to monitoring environmental changes, especially in fragile ecosystems such as mangroves. Mangrove refers to assemblages of tropical trees and shrubs that grow in the intertidal zone with adaptation to a wet, saline habitat. Mangrove is a highly productive habitat on earth and provides numerous ecosystems services for human livelihoods (Carugati et al., Reference Carugati, Gatto, Rastelli, Lo Martire, Coral, Greco and Danovaro2018). Despite that, mangroves have been constantly cleared for aquaculture shrimp farms, industrial developments and enlargement of housing settlements (Chee et al., Reference Chee, Othman, Sim, Mat Adam and Firth2017). The mangrove destruction has a profound effect on biodiversity and causes serious threats on human sustainability (Rawat & Agarwal, Reference Rawat and Agarwal2015; Carugati et al., Reference Carugati, Gatto, Rastelli, Lo Martire, Coral, Greco and Danovaro2018). Despite that, little attention has been paid to this fragile ecosystem. Thus, recording biodiversity and monitoring ecological changes in this habitat are essential.
Environmental settings
Malaysia is divided into two parts by the South China Sea, which shares maritime borders with Singapore, Thailand, Indonesia, Brunei, Vietnam and the Philippines (Figure 1A). Located in the equatorial region, the country experiences a tropical climate with three monsoon seasons. These monsoon seasons are divided into South-west Monsoon (SWM) from early May to early October and North-east Monsoon (NEM) from early November to March. In between changing of these monsoons, the Monsoon Transitional Period (MTP) occurs from April to early May and early October to early November. Usually, a long dry period and low precipitation occurs during SWM, while NEM generates higher rainfall and tidal events (Malaysian Meteorological Department, 2017). During the sampling period, the lowest monthly precipitation occurred in June (63.8 mm) while the highest volume was recorded in September (487.8 mm).
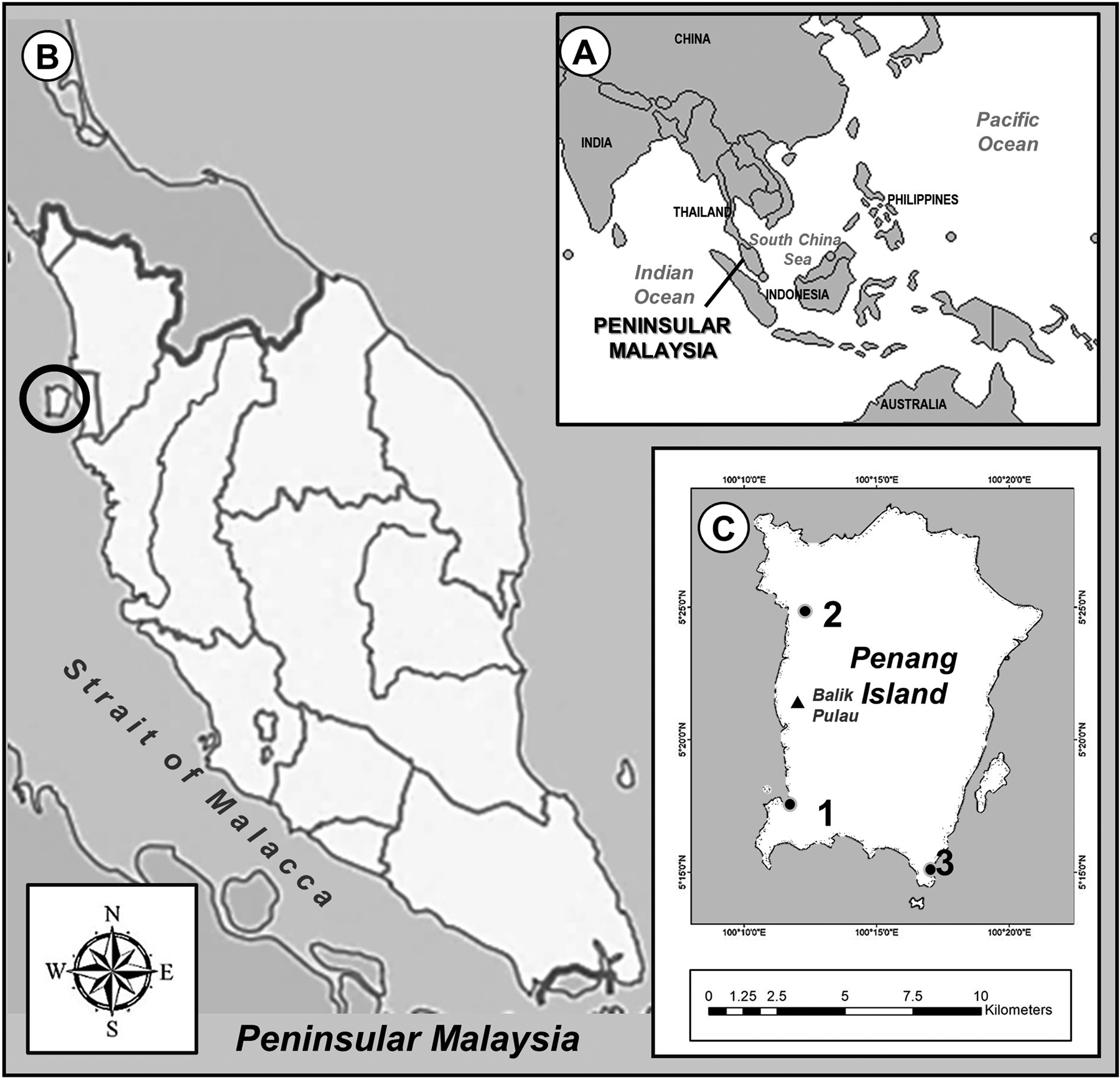
Fig. 1. (A) Location of Malaysia and bordering countries; (B) Location of Penang Island in Peninsular Malaysia; (C) Location of three mangrove forest; (1) Pulau Betong; (2) Kuala Sungai Pinang; (3) Teluk Tempoyak.
Penang Island is situated in the Strait of Malacca, off the north-western coast of Peninsular Malaysia located between 5°15´N–5°30´N and 100°10´–100°21´E with altitude ranging from 0–817 m above sea level, and slope degree of 0–61.598° (Khodadad & Dong-Ho, Reference Khodadad and Dong-Ho2015) (Figure 1B). The island is a highly developed and populated area in Malaysia with a population of 752,800 and a density of 1663/km2 on the total area of 299 km2 (Chee et al., Reference Chee, Othman, Sim, Mat Adam and Firth2017). The mangrove forests on the island cover ~6.8 km2 and are mostly found along the west coast of Balik Pulau (Chee et al., Reference Chee, Othman, Sim, Mat Adam and Firth2017) (Figure 1C). The island experiences a tropical climate with relative humidity varying from 60.9–96.8% and the annual rainfall ranges from 2670–3250 mm (Gao et al., Reference Gao, San and Zhu2021). Over the past three decades, the island experienced an average sea level rise rate of 3.2 mm year−1 and this is expected to rise from 320–7320 mm above 2000 levels by 2100 (Gao et al., Reference Gao, San and Zhu2021).
Previous foraminiferal studies in Penang Island were conducted in coastal waters and most species identified were common species found worldwide (Minhat et al., Reference Minhat, Yahya, Talib and Ahmad2014; Yahya et al., Reference Yahya, Shuib, Minhat, Ahmad and Talib2014). No studies of foraminifera in the mangrove area in Penang Island have been done. The present study was conducted to determine the community structure of benthic foraminifera by analysing their assemblages and the ecological factors that contribute to their distribution at the mangrove forest. The results of this study will provide a database for monitoring ecological changes in the Penang Island mangrove forest.
Materials and methods
Sampling
For this study, three locations of mangrove forests were selected (Figure 2): Pulau Betong (PB, Figure 2.1), Kuala Sungai Pinang (KSP, Figure 2.2) and Teluk Tempoyak (TT, Figure 2.3). At each location, the mangrove areas were divided into three intertidal zones, lower: 0–0.5 m, middle: 0.5–1.0 m and upper: 1.0–1.5 m, according to the watermark label classification from Hogarth (Reference Hogarth2015). In each zone, six sampling points were laid out, with ~3–5 m between points. Sampling was performed monthly for a one-year period (from March 2017 until February 2018) during the lowest spring tide. The details of the sampling points are provided in Supplementary Table S1. Mangrove flora were identified to genus level based on Lee et al. (Reference Lee, Muhamad and Tong2015).

Fig. 2. Sampling points and zonation depicted at each mangrove forests: (1) Pulau Betong; (2) Kuala Sungai Pinang; (3) Teluk Tempoyak.
At each station, ~50 cm3 volume (50 cm2 surface sample by 1 cm thick) of surface sediment was collected at each point using a scoop and stored in labelled plastic bags. The samples were separated into two parts, one part was used for foraminiferal analysis and another part for organic matter (OM) and particle size analysis. In situ parameters were measured at each point (Table 1). A refractometer (Milwaukee model MA887) was used to measure pore-water salinity, and a pH meter (Thermo Scientific Eutech Expert) was employed to measure pore-water temperature and pH.
Table 1. Summary of in situ parameters recorded at each sampling locations

Samples taken for foraminiferal analysis were preserved in 80% ethanol and stained with Rose Bengal (2 g l−1), following methods described by Schönfeld et al. (Reference Schönfeld, Alve, Geslin, Jorissen, Korsun and Spezzaferri2012). About 10 cm3 of the stained samples was washed through a 500 μm sieve and then a 63 μm sieve (Buzas-Stephens et al., Reference Buzas-Stephens, Buzas, Price and Courtney2018). Only the 63 μm sieve fractions were used for the collection of benthic foraminifera (Edwards et al., Reference Edwards, Wright and Van De Plassche2004; Hayward et al., Reference Hayward, Grenfell, Sabaa, Kay and Clark2011; Schönfeld et al., Reference Schönfeld, Alve, Geslin, Jorissen, Korsun and Spezzaferri2012; Camacho et al., Reference Camacho, Maria, Moura, Connor, Scott and Boski2015; Debenay et al., Reference Debenay, Marchand, Molnar, Aschenbroich and Meziane2015). Samples smaller than this size fraction are known to contain juvenile species which are harder to identify and may cause species misidentification (Murray & Alve, Reference Murray and Alve2000; Bouchet et al., Reference Bouchet, Alve, Rygg and Telford and2012). When possible, at least 300 specimens of benthic foraminifera were counted from each sample under a dissecting microscope (Olympus SZ51, Japan). Specimen that stained bright red colour was considered as living during the time of sampling (Schönfeld et al., Reference Schönfeld, Alve, Geslin, Jorissen, Korsun and Spezzaferri2012). In ecological studies of foraminifera, it is presumed that staining with Rose Bengal is the most practical method for distinguishing living specimens because the solution will stain the organisms' protoplasm, indicating that the specimens were alive during the sample collection (Murray & Alve, Reference Murray and Alve2000). The benthic foraminifera were wet picked to ensure that the stained tests would be recognizable (Edwards & Horton, Reference Edwards and Horton2006; Berkeley et al., Reference Berkeley, Perry and Smithers2009a; Semensatto-Jr et al., Reference Semensatto-Jr, Funo, Dias-Brito and Coelho-Jr2009; Shennan et al., Reference Shennan, Long and Horton2015; Strachan et al., Reference Strachan, Finch, Hill and Barnett2015; Alve et al., Reference Alve, Hess, Bouchet, Dolven and Rygg2019). All foraminiferal specimens were stored on micropalaeontological slides and labelled accordingly. Since ecological studies are dealing with living organisms at a particular time and space, only live count data were used for statistical analysis as they provide more ecological insights (Murray, Reference Murray2006). Census data for live and dead assemblages at each location were expressed as individuals/10 cm3 and are provided in Supplementary file Tables S2 and S3.
Selected specimens of benthic foraminifera were observed using a scanning electron microscope (Carl Zeiss Leo Supra 50 VP Field Emission) at the School of Biological Sciences, Universiti Sains Malaysia (USM), Penang. A complete taxonomy list of species from this study and other mangrove locations around Malaysia was recently published (Abd Malek et al., Reference Abd Malek, Frontalini, Yahya, Talib and Zakaria2021).
The OM content in the sediments was analysed using the loss on ignition method based on the procedures described by Dean (Reference Dean1974), Heiri et al. (Reference Heiri, Lotter and Lemcke2001) and Minhat et al. (Reference Minhat, Ghandhi, Ahzan, Haq, Manaf, Sabohi, Lee, Akhir and Abdullah2021). Approximately 100 g of sediments was weighed and dried overnight in an oven at 105°C. Later, 5 g of dry subsample was placed in a labelled crucible and heated in a furnace at 550°C for 4 h. The percentage of OM content was determined based on the differences between the initial and final weight. Another part of the sediment samples was analysed for particle size (sand, silt and clay) using the initial (dry) sieving method, followed by pipette analysis (Krumbein & Pettijohn, Reference Krumbein and Pettijohn1938).
Data analysis
At each mangrove location, the species abundance of benthic foraminifera was expressed as a percentage of relative abundance. Additionally, two-way PERMANOVA test was conducted on temporal and spatial benthic foraminifera data to test for homogeneity between the assemblages. This non-parametric test was calculated using Euclidean distance measure that was performed using Paleontological Statistics data analysis package (PAST) (Hammer et al., Reference Hammer, Harper and Ryan2001).
The environmental parameters measured were analysed using principal component analysis (PCA) to reduce the dimensions of the data and to determine the parameters that best explained the environmental gradient (Hotelling, Reference Hotelling1933; Paliy & Shankar, Reference Paliy and Shankar2016). PCA was performed using PRIMER software version 7 (Clark et al., Reference Clark, Gorley, Somerfield and Warwick2014). The results of the PCA were then used to investigate the relationship with the most abundant benthic foraminiferal species.
For this purpose, canonical correspondence analysis (CCA) was applied to the species-environmental parameters data, and was conducted in PAST software (Hammer et al., Reference Hammer, Harper and Ryan2001). The data were initially screened to reduce background ‘noise’ that could complicate the visualization of the data pattern. Thus, for this analysis, only samples containing more than 50 specimens of benthic foraminifera and species with a relative abundance greater than 5% in at least one sample were used. The species abundance was logarithmically transformed into log (1 + x) to reduce the influence of highly dominant species and allow a pattern of subsidiary species to appear (Frontalini et al., Reference Frontalini, Buosi, Da Pelo, Coccioni, Cherchi and Bucci2009; Strachan et al., Reference Strachan, Finch, Hill and Barnett2015). For the environmental parameters, the data with different scale units were transformed and standardized so that those combined parameters could be analysed together (Hammer et al., Reference Hammer, Harper and Ryan2001). Monte Carlo permutation tests were applied to the data to test the statistical significance of the measured environmental variables on assemblage variations based on Legendre & Birks (Reference Legendre, Birks, Birks, Lotter, Juggins and Smol2012).
Results
Benthic foraminiferal abundance and environmental parameters
Based on two-way PERMANOVA results, there was a significant difference between temporal and spatial foraminifera distribution from the mangrove (P < 0.05) throughout the sampling period (Table 2).
Table 2. Results of PERMANOVA between foraminifera counts data for monthly and zones between sampling sites

df, degrees of freedom; SS, sum of squares; MS, mean squares; F, Fisher's statistic.
Significant factors (P < 0.05) are shown in bold.
Foraminiferal abundances and measured environmental parameters are shown in Figure 3 (Supplementary file Table S4). In comparison between three mangrove forests, PB mangrove recorded the highest test abundance (3814 individuals/10 cm3) followed by TT (3027 individuals/10 cm3 and KSP mangroves (780 individuals/10 cm3). Highest assemblages at each mangrove were recorded during February (PB: 872 individuals/10 cm3; TT: 494 individuals/10 cm3; KSP: 209 individuals/10 cm3).

Fig. 3. Monthly foraminiferal abundance and mean environmental parameters.
The measured environmental parameters revealed variation throughout the year. The pore-water pH recorded was in a range of pH 6–8 at each mangrove location. The maximum pH value was recorded at TT in May (pH 7.7 ± 0.3), at PB in March (pH 7.6 ± 0.3) and at KSP in May (pH 7.8 ± 0.2). In TT mangrove, pH value >6.8 occurred during May, June, July and September with a low total number of foraminifera (<200 individuals/10 cm3). In PB mangrove, low foraminiferal abundance occurred during May–July while KSP mangrove occurred throughout sampling period except in February. The pore-water temperature generally ranged from 27–32°C. The highest temperature was at TT in August and September (30°C ± 1.1), at PB in April (31°C ± 1.9) and at KSP in March (32°C ± 1.0). Pore-water salinity also fluctuated during certain months, with a range of 24–34 PSU. The highest PSU was recorded in June at every location: TT (31 PSU ± 3.5), PB (34 PSU ± 2.6) and KSP (31 PSU ± 1.7). Organic matter content in the mangrove sediments varied between 5–15%. The highest OM was recorded at different months at the three sites: TT: September = 17% ± 4.3; PB: February = 13% ± 8.9; KSP: June = 14% ± 12).
Sediment particle size
The sediments in the mangrove areas in all three locations were predominantly sand. The sediment particle size ranges were similar between zones in each mangrove area. The mean percentage ranged from 56–63% for sand, 11–16% for silt and 5–6% for clay (Table 3).
Table 3. Mean and standard deviation (SD) of particle size percentage in the mangrove sediments

Benthic foraminifera species composition and zonation
Overall, 28 species of live benthic foraminifera were successfully identified from the three mangrove forests, belonging to five orders, 15 families and 22 genera (Plate A and B). The test type was predominantly agglutinated (17 species), followed by 10 hyaline and one porcelaneous. The highest number of species was found in PB mangrove (27 species), followed by TT mangrove (22 species), and the lowest was recorded in KSP mangrove (12 species).

Plate A. 1. Ammonia aoteana spiral view, 100×, 100 μm, 2. A. aoteana umbilical view, 150×, 100 μm, 3. Aubignyna perlucida spiral view, 230×, 30 μm, 4. A. perlucida umbilical view, 150×, 30 μm, 5. Elphidium fijiense spiral view, 200×, 100 μm, 6. Elphidium neosimplex spiral view, 250×, 30 μm, 7. Elphidium hispidulum spiral view, 150×, 20 μm, 8. Elphidium sandiegoense spiral view, 350 × , 30 μm, 9. Haynesina depressula spiral view, 150×, 30 μm, 10. Rosalina globularis spiral view, 280× 30 μm, 11. R. globularis umbilical view, 280×, 30 μm, 12. Bolivina striatula lateral view, 350×, 20 μm, 13. Asterorotalia pulchella spiral view, 448 200×, 30 μm, 14. Quinqueloculina seminula lateral view, 350×, 30 μm. Scale bar = 100 μm.

Plate B. 1. Acupeina triperforata dorsal view, 200×, 20 μm, 2. Ammoastuta salsa lateral view, 200×, 100 μm, 3. Haplophragmoides wilberti lateral view, 150×, 100 μm, 4. Ammobaculites exiguus lateral view, 300×, 300 μm, 5. Ammotium directum lateral view, 200×, 20 μm, 6. Ammotium fragile lateral view, 180×, 100 μm, 7. Ammotium pseudocassis lateral view, 100×, 100 μm, 8. Caronia exilis lateral view, 201×, 30 μm, 9. Monotalea salsa lateral view, 180×, 100 μm, 10. Glomospira fijiensis lateral view, 300×, 30 μm, 11. Entzia macrescens umbilical view, 200×, 20 μm, 12. Arenoparrella mexicana umbilical view, 150×, 20 μm, 13. Tiphotrocha comprimata umbilical view, 150×, 20 μm, 14. Trochammina inflata spiral view, 180×, 100 μm, 15. Siphotrochammina lobata spiral view, 180×, 100 μm, 16. Miliammina fusca lateral view, 250×, 30 μm, 17. Miliammina obliqua lateral view, 360×, 20 μm. Scale bar = 100 μm.
Pulau Betong assemblages
In the lower zone, hyaline tests dominated the assemblages with species such as A. aoteana (max = 276 individuals/10 cm3), E. hispidulum (max = 122 individuals/10 cm3), A. perlucida (max = 71 individuals/10 cm3) and E. neosimplex (max = 54 individuals/10 cm3). In the middle zone, agglutinated tests recorded higher abundance with T. inflata (max = 60 individuals/10 cm3) and M. obliqua (max = 22 individuals/10 cm3). Towards the upper zone, T. inflata remained abundant in the assemblages (max = 86 individuals/10 cm3) together with other agglutinated tests such as M. fusca (max = 57 individuals/10 cm3) and S. lobata (max = 50 individuals/10 cm3) (Figure 4A).

Fig. 4. Abundance of six main foraminiferal species: (A) PB mangrove; (B) KSP mangrove; and (C) TT mangrove. Water level, approximate tidal heights and sampling points are indicated.
Kuala Sungai Pinang assemblages
Species abundance in KSP was very low and mostly found between lower to middle zones. The lower zone recorded low species numbers which comprised mainly A. aoteana (max = 15 individuals/10 cm3). Middle zone recorded higher abundance of agglutinated tests such as A. mexicana (max = 96 individuals/10 cm3), T. inflata (max = 69 individuals/10 cm3) and S. lobata (max = 27 individuals/10 cm3) (Figure 4B).
Teluk Tempoyak assemblages
Foraminiferal assemblages in TT were mostly similar to those at PB and KSP mangroves. The lower zone contained a high number of A. aoteana (max = 229 individuals/10 cm3), E. hispidulum (max = 85 individuals/10 cm3) and A. perlucida (max = 74 individuals/10 cm3). In the middle zone, abundance of A. aoteana remained high (max = 82 individuals/10 cm3). Other species found in the middle zone were T. inflata (max = 26 individuals/10 cm3) and A. mexicana (max = 20 individuals/10 cm3). In the upper zone, only T. inflata was recorded with high abundance (max = 67 individuals/10 cm3) (Figure 4C).
Relationship between environmental parameters and species abundance
Environmental parameters analysed with PCA revealed that the sum of PC1 (eigenvalue = 2.3; variance = 33%) and PC2 (eigenvalue = 1.3; variance = 18%) explained half of the total environmental variation. In PC1, a higher correlation coefficient (>0.5) was contributed by OM and particle size, and in PC2, by pore-water temperature and pore-water salinity (Table 4). Based on the PCA loading scores, higher correlations were contributed by OM percentage and particle size sediments. These parameters were further analysed using CCA.
Table 4. Values of correlation coefficient from PCA

The results of CCA showing the relationship between the environmental parameters and the dominant species assemblages are presented in Figure 5. The first two axes explained 89% (axis 1 = 71.3%; axis 2 = 17.3%) of the total variation within species and the environmental parameters. The hyaline tests were significantly correlated with sand particles. Meanwhile, agglutinated tests were associated with OM content, together with silt and clay.

Fig. 5. CCA of the dominant species (>5% relative abundance) and the gradient of primary environmental parameters. (A) Test type as categorical factor; (B) tidal zone as categorical factor.
Discussion
Species numbers in mangrove areas
In general, foraminiferal diversity in mangrove forests is usually lower (<60 species) than that in normal marine lagoons and the deep-sea environment (>200 species) (Murray, Reference Murray2006; Ortiz et al., Reference Ortiz, Alegret, Payros, Orue-Etxebarria, Apellaniz and Molina2011; Contreras-Rosales et al., Reference Contreras-Rosales, Koho, Duijnstee, de Stigter, García, Koning and Epping2012; Debenay, Reference Debenay2012; Milker & Schmiedl, Reference Milker and Schmiedl2012). The mangrove environment is often regarded as an extreme condition for benthic foraminifera owing to its high fluctuations in salinity, temperature and OM availability (Murray, Reference Murray2006; Debenay, Reference Debenay2012). Conversely, stable environmental conditions from nearshore to deep sea have reported higher ranges of species diversity (after Murray, Reference Murray2006; Debenay, Reference Debenay2012; Milker & Schmiedl, Reference Milker and Schmiedl2012).
Species distribution pattern according to intertidal zonation
The zonation of the benthic foraminiferal test types showed a distinct microhabitat between lower and upper zones (Figure 5). The assemblages that contained only agglutinated tests were found mostly in PB and TT middle to upper zones, while hyaline tests dominated the lower zone. Agglutinated test species are commonly recorded in marshes, mangroves and brackish environments because this test type is able to endure low salinity and high pH conditions (Debenay & Guillou, Reference Debenay and Guillou2002; Hayward et al., Reference Hayward, Grenfell, Sabaa, Kay and Clark2011; Shennan et al., Reference Shennan, Long and Horton2015). At all studied locations, the upper zone was the driest area as it is periodically inundated during spring high tides. These severe conditions may not favour the establishment of benthic foraminiferal assemblages, especially the hyaline tests.
The mangrove forests in the studied locations were mostly in small patches with low floral species diversity. In this study, there was no clear relationship between benthic foraminiferal assemblages and mangrove floral zones because the mangrove forests in Penang Island are overwash type mangrove. Due to the strong impact of tidal activity, overwash mangroves often cover small areas with fewer mangrove species and no plant zonation (Sukardjo, Reference Sukardjo2006; Rodriguez et al., Reference Rodriguez, Urish, Feller and Wright2009). Similarly, Hadiyanto et al. (Reference Hadiyanto, Widyastuti, Arbi, Vimono, Ulumuddin, Dharmawan and Prayudha2018) found that in an overwash mangrove macrobenthos study, sediment type more significantly influenced the total abundance and species composition than mangrove vegetation. Also, the lower productivity in this mangrove type may differ from those in fringing and riverine mangroves which have clear mangrove plant zonations. Zonation based on mangrove plants is generally the result of local factors such as sediment transport, tidal inundation and nutrient availability (Bunt & Bunt, Reference Bunt and Bunt1999; Woodroffe et al., Reference Woodroffe, Horton, Larcombe and Whittaker2005; Murray, Reference Murray2006). The dominant mangrove trees in the studied location were from the genus Avicennia which was mostly found at the lower to the upper elevations. In TT and PB mangrove, the lower zone consists of higher abundance of A. aoteana. This pattern was also reported by Woodroffe et al. (Reference Woodroffe, Horton, Larcombe and Whittaker2005) and Berkeley et al. (Reference Berkeley, Perry, Smithers, Horton and Cundy2009b) in fringing mangrove of Cocoa Creek, Australia, where the upper mangrove forest of A. marina has high abundance of A. aoteana. Woodroffe et al. (Reference Woodroffe, Horton, Larcombe and Whittaker2005) suggested that A. aoteana is a useful species for sea-level indicators.
A lower number of agglutinated test species M. fusca (PB: 185 individuals/10 cm3; TT: 130 individuals/10 cm3; KSP: absent) and M. obliqua (PB: 187 individuals/10 cm3; TT: 132 individuals/10 cm3; KSP: 34 individuals/10 cm3) was found in the sediment from all sampling locations. Previous studies have also reported low amounts of M. obliqua (64–249 individuals/10 cm3) in mangrove sediments (Berkeley et al., Reference Berkeley, Perry, Smithers, Horton and Cundy2009b; Culver et al., Reference Culver, Leorri, Mallinson, Corbett and Shazili2015). The structure of the thin-shelled test of M. obliqua is known to be quickly degraded after death (Hayward et al., Reference Hayward, Scott, Grenfell, Carter and Lipps2004). Meanwhile, M. fusca is found in most intertidal areas, typically at the higher tidal elevations (Barbosa & Suguio, Reference Barbosa and Suguio1999; Horton et al., Reference Horton, Larcombe, Woodroffe, Whittaker, Wright and Wynn2003; Murray, Reference Murray2006; Gómez & Bernal, Reference Gómez and Bernal2013; Camacho et al., Reference Camacho, Maria, Moura, Connor, Scott and Boski2015; Sen et al., Reference Sen, Ghosh, Khanderao, Das, Chowdhury, Sarkar, Saha and Bhadury2015).
Only one porcelaneous test (Quinqueloculina seminula) was found in this study. The species was found only in live assemblages of PB mangroves (13 individuals/10 cm3), which indicated that the species was rare and might be transported from coastal waters by tidal propagation. Previously, Eichler (Reference Eichler2019) found that porcelaneous alien species (Quinqueloculina lamarckiana) was transported from continental shelf to mangroves of Bertioga Channel, Brazil. The species was also rarely found in living assemblages because it might have been transported from its natural habitat (Eichler, Reference Eichler2019).
Our results have shown that the agglutinated tests were mainly found in the middle to upper zones. Similarly, in temperate regions where mangrove plants have been replaced with marshes, the assemblages contain dominant agglutinated with porcelaneous test (Edwards et al., Reference Edwards, Wright and Van De Plassche2004; Scott et al., Reference Scott, Medioli and Schafer2004; Strachan et al., Reference Strachan, Finch, Hill and Barnett2015). The agglutinated tests mainly from the genera Entzia, Trochammina, Tiphotrocha and Miliammina were confined to a higher elevation area of the mean tide level (Shennan et al., Reference Shennan, Long and Horton2015).
The role of environmental parameters on species distribution
The factors that are commonly known to contribute to benthic foraminiferal abundance are salinity, temperature, nutrition, dissolved oxygen conditions, pH and type of substrate (Scott et al., Reference Scott, Medioli and Schafer2004; Culver & Horton, Reference Culver and Horton2005; Woodroffe et al., Reference Woodroffe, Horton, Larcombe and Whittaker2005; Murray, Reference Murray2006; Kemp et al., Reference Kemp, Buzas, Horton and Culver2011). However, the abundance of benthic foraminifera in mangrove environments is usually controlled by the elevation of the tidal frame (Scott et al., Reference Scott, Medioli and Schafer2004; Culver et al., Reference Culver, Leorri, Corbett, Mallinson, Shazili, Mohammad, Parham and Yaacob2013). The environments of mangrove areas, which usually have high ground temperature, high vegetation cover and high organic content, might affect the benthic foraminiferal distribution in tropical regions (Scott et al., Reference Scott, Medioli and Schafer2004; Culver & Horton, Reference Culver and Horton2005; Berkeley et al., Reference Berkeley, Perry and Smithers2009a; Culver et al., Reference Culver, Leorri, Corbett, Mallinson, Shazili, Mohammad, Parham and Yaacob2013).
In this study, abundant hyaline test species were recovered in sediments with high sand content at the lower intertidal zone, which had higher pore-water temperature (26–32°C, mean: 29.2 ± 1.2) owing to its lower level of mangrove tree cover. However, the pore-water temperature was not at an extreme level (>32°C), therefore taphonomic loss of hyaline tests was unlikely to have occurred (Culver et al., Reference Culver, Leorri, Corbett, Mallinson, Shazili, Mohammad, Parham and Yaacob2013). Thus, pore water temperature was not regarded as a main controlling factor. Several studies have reported that increasing pore water temperatures can cause adverse effects to intertidal foraminifera (i.e. reduce locomotion, metabolism and reproduction (Buzas & Severin, Reference Buzas and Severin1993; Gross, Reference Gross2000; Wukovits et al., Reference Wukovits, Enge, Wanek, Watzka and Heinz2017; Li et al., Reference Li, Lei, Li and Jian2019; Deldicq et al., Reference Deldicq, Langlet, Delaeter, Beaugrand, Seuront and Bouchet2021). On a regional scale, cooler water temperatures are known to favour the preservation of agglutinated tests compared with warmer regions and tropical environments such as mangroves (Goldstein & Watkins, Reference Goldstein and Watkins1999; Debenay et al., Reference Debenay, Guiral and Parra2002; Berkeley et al., Reference Berkeley, Perry and Smithers2009a).
Organic matter is also documented as a key parameter that influences the distribution of benthic foraminifera in mangrove sediments. In this study, dominant agglutinated species (A. mexicana, T. inflata, M. obliqua, M. fusca) were widely distributed in the middle to upper mangrove reaches. These species showed preferences for sediments with high OM content and silty-muddy substrate, as shown in the CCA graph. The dense aquaculture activities (i.e. shrimp ponds and fish cages) in the mangrove forests might also result in high OM in the sediments, which has been reported by some authors (Chee et al., Reference Chee, Yee, Carey, Yusup and Gallagher2015; Zolkhiflee et al., Reference Zolkhiflee, Yahya and Shuib2021). In Matang mangroves, the high presence of A. mexicana, together with Haplophragmoides wilberti, was due to the enrichment of OM by leaf litter and other bioorganic substances (Satyanarayana et al., Reference Satyanarayana, Husain, Ibrahim, Ibrahim and Dahdouh-Guebas2014). These species were dominant in organic-rich sediments of shrimp ponds as reported by Debenay et al. (Reference Debenay, Marchand, Molnar, Aschenbroich and Meziane2015). Miliammina fusca has previously been reported as a dominant species in the lower mangrove zone (Scott et al., Reference Scott, Medioli and Schafer2004; Edwards & Horton, Reference Edwards and Horton2006) and at the seaward edges of marsh environments with an absence of vegetation cover (Murray & Alve, Reference Murray and Alve1999). Some studies have reported that foraminiferal species were sensitive to the variability and quality of organic matter on surface and in the deeper layer of sediments (e.g. Mojtahid et al., Reference Mojtahid, Jorissen, Lansard and Fontanier2010; Papaspyrou et al., Reference Papaspyrou, Diz, García-Robledo, Corzo and Jimenez-Arias2013; Barras et al., Reference Barras, Jorissen, Labrune, Andrald and Boissery2014; Cesbron et al., Reference Cesbron, Geslin, Jorissen, Delgard, Charrieau, Deflandre, Jézéquel, Anschutz and Metzger2016). Experimental studies on organic matter degradation have shown that the process is related to the bacterial activities present in the sediment layers causing lowering of dissolved oxygen level, consequently decreasing the number of certain foraminiferal species (usually calcareous species) by making them unable to proliferate (Duffield et al., Reference Duffield, Hess, Norling and Alve2015). Thus, further studies are required to address the variability response of species in mangrove sediments.
Other agglutinated species such as E. macrescens and T. inflata are known to have better preservation potential and ability to withstand taphonomic signatures compared with other benthic foraminiferal species (Ozarko et al., Reference Ozarko, Patterson and Williams1997; Berkeley et al., Reference Berkeley, Perry, Smithers, Horton and Taylor2007). However, the occurrence of E. macrescens in this study was rare because the foraminiferal assemblages were only taken on the surface sediments. The species was thought to be an infaunal type, suggesting that it may have moved downcore (Scott et al., Reference Scott, Medioli and Schafer2004; Culver et al., Reference Culver, Leorri, Corbett, Mallinson, Shazili, Mohammad, Parham and Yaacob2013; Strachan et al., Reference Strachan, Finch, Hill and Barnett2015). Meanwhile, T. inflata and A. mexicana thrived in areas with higher OM, especially in the dense mangroves of KSP. Similar conditions have also been reported in marshes of the USA and North Island, New Zealand (Murray, Reference Murray2006).
Foraminiferal test dissolution occurs when the pH range is below optimal conditions, which range from pH 6.5–7.2 (Murray, Reference Murray2006). Previous study reported that decalcification of hyaline species Ammonia beccarii in normal marine salinity occurred when pH decreased below pH 7.5 (Le Cadre et al., Reference Le Cadre, Debenay and Lesourd2003). In mangrove forests, pH decreases owing to the decomposition of leaf litter by bacteria in the sediments, might also explain the decrease of calcareous tests (Debenay & Guillou, Reference Debenay and Guillou2002).
The pH recorded at the sampling sites was between 6.4–7.8 (7.1 ± 0.3). In general, pH variations within the intertidal region are higher than those in other marine environments due to freshwater input either from rainwater or river runoff (Erskian & Lipps, Reference Erskian and Lipps1977; Debenay et al., Reference Debenay, Guiral and Parra2002, 2006; Scott et al., Reference Scott, Medioli and Schafer2004) and calcareous tests are known to be sensitive to pH fluctuations (Phleger & Bradshaw, Reference Phleger and Bradshaw1966; Saraswat et al., Reference Saraswat, Kouthanker, Kurtarkar, Nigam and Linshy2011; Weinmann et al., Reference Weinmann, Goldstein, Triantaphyllou and Langer2021). Owing to pH fluctuations, several studies have applied benthic foraminifera as an indicator for ocean acidification (Haynert et al., Reference Haynert, Schönfeld, Schiebel, Wilson and Thomsen2013; Schmidt et al., Reference Schmidt, Kucera and Uthicke2014; Kawahata et al., Reference Kawahata, Iguchi, Inoue, Iwasaki, Kuroyanagi and Suzuki2019). At lower pH values, benthic foraminifera require substantial energy to re-calcify their tests in order to survive (Woodroffe et al., Reference Woodroffe, Horton, Larcombe and Whittaker2005). Similarly, abundance of agglutinated foraminifera has been reported at pH levels ranging from pH 6.2–6.6 with absence of calcareous foraminifera in vegetated mangroves at Sandy Creek, Queensland (Woodroffe et al., Reference Woodroffe, Horton, Larcombe and Whittaker2005).
Comparison with other mangrove forest
Penang Island assemblages contain high numbers of agglutinated test species with 17 species. The agglutinated species in the present study were similar to those found in mangrove forests such as in Kapar, Selangor and Matang, Perak (Satyanarayana et al., Reference Satyanarayana, Husain, Ibrahim, Ibrahim and Dahdouh-Guebas2014). Meanwhile in Setiu, Terengganu, Culver et al. (Reference Culver, Mallinson, Corbett, Leorri, Rouf, Shazili, Yaacob, Whittaker, Buzas and Parham2012, Reference Culver, Leorri, Corbett, Mallinson, Shazili, Mohammad, Parham and Yaacob2013) identified a high number of hyaline test species (46 species). Particularly, Setiu wetland is a fringing mangrove swamp where saltwater intrusion is higher compared with Penang Island mangroves. A higher range of salinity (>30 PSU) was known to increase the number of calcareous tests species (Hayward et al., Reference Hayward, Scott, Grenfell, Carter and Lipps2004; Horton & Murray, Reference Horton and Murray2007; Culver et al., Reference Culver, Mallinson, Corbett, Leorri, Rouf, Shazili, Yaacob, Whittaker, Buzas and Parham2012, Reference Culver, Leorri, Mallinson, Corbett and Shazili2015, 2019). Despite that, laboratory experiments on two calcareous species (Ammonia tepida and Haynesina germanica) have found that fluctuation in salinity had smaller impact on food uptake (Lintner et al., Reference Lintner, Biedrawa, Wukovits, Wanek and Heinz2020). Since the range of salinity recorded in this study was not as high as that in other studies, the number of calcareous tests recovered was low (<10 species).
Although the foraminiferal species found were mostly similar to those reported in other studies in Malaysian mangrove forests, three newly recorded species were recovered in the present study. These species were identified as A. perlucida, E. neosimplex and E. sandiegoense. These hyaline test species have been reported in coastal environments worldwide (Murray et al., Reference Murray, Whittaker and Alve2000; Murray, Reference Murray2006; Sugawara et al., Reference Sugawara, Minoura, Nemoto, Tsukawaki, Goto and Imamura2009; Debenay, Reference Debenay2012). In this study, A. perlucida was found in all three mangrove locations, while E. neosimplex was only found in TT and PB mangroves. The hyaline test species, E. sandiegoense was only recovered in PB sediments. The rare occurrences of A. perlucida, E. neosimplex and E. sandiegoense in living assemblages from sampling locations were possibly due to tests being transported from their natural habitation by tidal movement and flood events (Mendes et al., Reference Mendes, Gonzalez, Dias, Lobo and Martins2004; Debenay & Luan, Reference Debenay and Luan2006; Raposo et al., Reference Raposo, Clemente, Figueiredo, Vilar, Lorini, Frontalini, Martins, Belart, Fontana, Habib and Laut2018).
In general, spatial and seasonal variabilities are due to the interaction between fauna, reproduction strategy, predation and food sources (Hayward et al., Reference Hayward, Grenfell, Sabaa, Kay and Clark2011; Scott et al., Reference Scott, Mudie and Bradshaw2011; Buzas et al., Reference Buzas, Hayek, Jett and Reed2015). In this study, numerous burrows found in the sediments (especially in lower to middle zones) indicated that there was intense bioturbation by macrofauna such as fiddler crabs. As a result, the fragile tests of smaller benthic foraminifera, particularly agglutinated tests were mostly destroyed (Debenay et al., Reference Debenay, Guiral and Parra2002; Debenay & Parra, Reference Debenay and Parra2004; Perry et al., Reference Perry, Berkeley and Smithers2008). On the other hand, the rare occurrence of a calcareous hyaline test genus such as Elphidium indicated weak eutrophic conditions and stratification of water column (Alve, Reference Alve2003).
Overall, the distribution of benthic foraminiferal tests in this study was influenced by the environmental parameters of Penang Island mangrove forests, particularly the OM content and sediment type. The distribution pattern of benthic foraminiferal tests showed microhabitat preferences at different intertidal zones, with hyaline tests dominating the lower zone assemblages and agglutinated tests in the middle to the upper zones. This zonation pattern is valuable in determining the potential consequences of habitat degradation, which is particularly prevalent in mangrove environments.
Conclusions
The distribution pattern of benthic foraminifera tests showed microhabitat preferences in which hyaline tests (A. aoteana, Elphidium neosimplex and E. hispidulum) were highly abundant in the lower mangrove zone, while agglutinated tests (A. mexicana and Trochammina inflata) were abundant in the middle to upper mangrove zones. The distribution pattern observed was mainly due to the influence of OM content and particle size in the sediments. The tests distribution pattern of benthic foraminifera in Penang Island showed that the number of agglutinated tests species was higher than the hyaline and porcelaneous tests, which commonly occurred in mangrove environments. The data from this study will provide a baseline for future monitoring of environmental changes in the mangrove area on Penang Island.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315422001072
Acknowledgements
We thank Mr Johari Othman from the Electron Microscope Unit, School of Biological Sciences, USM for his technical assistance with the SEM images. Thanks also to Steve Culver and Fabrizio Frontalini for helping with species identification.
Financial support
This work was supported by a RUI grant from Universiti Sains Malaysia (1001/PBIOLOGI/8011054).













