Introduction
The Southern Ocean islands represent some of the most isolated and least impacted habitats on Earth, yet they are also prone to invasion by alien species (Shaw Reference Shaw, Foxcroft, Pyšek, Richardson and Genovesi2013). Due to their isolation they are often taxonomically and functionally depauperate, which is believed to reduce their biotic resistance to invasion (Vitousek Reference Vitousek1988, Reaser et al. Reference Reaser, Meyerson, Cronk, De Poorter, Eldrege and Green2007, Greve et al. Reference Greve, Steyn, Mathakutha and Chown2017). Invasive alien species represent one of the greatest threats to the native biotas on these islands (Chown et al. Reference Chown, Sinclair and Van Vuuren2008). The Prince Edward Islands (PEIs) constitute South Africa's southernmost and only sub-Antarctic territories and consist of two islands: the larger Marion Island (~270 km2) and the smaller Prince Edward Island (~45 km2; Greve et al. Reference Greve, von der Meden, Janion-Scheepers, van Wilgen, Measey, Richardson, Wilson and Zengeya2020). The introduction of alien plants to the PEIs is closely linked with their human history, whereby anthropogenic activities since the early nineteenth century and the establishment of permanent research stations have brought in propagules through clothing, outdoor equipment and particularly the importation of building materials (Gremmen & Smith Reference Gremmen and Smith1999, Greve et al. Reference Greve, von der Meden, Janion-Scheepers, van Wilgen, Measey, Richardson, Wilson and Zengeya2020). The alien plants that have established on the PEIs are predominantly of European origin and are widespread across the sub-Antarctic region, occurring on several other islands (Frenot et al. Reference Frenot, Chown, Whinam, Selkirk, Convey, Skotnicki and Bergstrom2005, Shaw Reference Shaw, Foxcroft, Pyšek, Richardson and Genovesi2013).
The PEIs are protected under the National Environmental Management: Protected Areas Act (No. 10 of 2004 NEMBA), and encompassed within this legislation is the prevention and management of invasive alien species (Department of Forestry, Fisheries and the Environment, unpubl. data 2012, Greve et al. Reference Greve, Steyn, Mathakutha and Chown2017). Most efforts have concentrated on reducing any alien plant introductions, and managing established populations has received less attention (Greve et al. Reference Greve, Steyn, Mathakutha and Chown2017). For some of the invasive alien plants (IAPs) present on the islands, eradication is no longer possible due to the extent of the invasions. Generally, complete eradication of IAPs is only successful in the very early stages of the invasion before the plant is widespread (Rejmánek & Pitcairn Reference Rejmánek, Pitcairn, Veitch and Clout2002, Genovesi Reference Genovesi2005, Renteria et al. Reference Renteria, Rouget and Visser2017). Managing species that are widespread on the PEIs has also been limited due to challenges such as inaccessibility, sensitive ecosystems and a lack of staff capacity. Much of the PEIs consist of difficult terrain, and therefore large areas are often inaccessible by foot (Bergstrom & Smith Reference Bergstrom and Smith1990). Furthermore, invasive plants such as Poa annua and Sagina procumbens are often established within habitats that support vulnerable native species, such as within the burrows and nests of seabirds and seals, and therefore any management interventions pose a risk to these co-occurring native species (Ryan et al. Reference Ryan, Smith and Gremmen2003). The PEIs have only 20–25 people occupying Marion Island at any time (Greve et al. Reference Greve, von der Meden, Janion-Scheepers, van Wilgen, Measey, Richardson, Wilson and Zengeya2020), and thus there is limited capacity to implement control programmes. Given these challenges, there has been minimal management of alien plants, and the use of conventional control methods is considered infeasible (Watkins & Cooper Reference Watkins and Cooper1971, Greve et al. Reference Greve, Steyn, Mathakutha and Chown2017).
Given that conventional control methods of IAPs are often not possible on the PEIs, the use of classical weed biological control (biocontrol) may offer a sustainable approach to managing IAPs. Biocontrol is the use of natural enemies, usually arthropods or pathogens, to control pest organisms (McFadyen Reference McFadyen1998). It is recognized as an important management intervention for the control of IAPs (DiTomaso et al. Reference DiTomaso, Van Steenwyk, Nowierski, Vollmer, Lane and Chilton2017, Zachariades et al. Reference Zachariades, Paterson, Strathie, van Wilgen and Hill2017, Shaw et al. Reference Shaw, Ellison, Marchante, Pratt, Schaffner, Sforza and Deltoro2018), which are serious pests of natural and agricultural ecosystems globally (Vilá & Ibáñez 2011, Van Driesche & Center Reference Van Driesche, Center, Foxcroft, Pyšek, Richardson and Genovesi2013). Natural enemies of the target IAP are sourced from the indigenous distribution, tested to ensure that they are host specific to the target plant and then released as biocontrol agents where the IAP is problematic. The biocontrol agents become a part of the new ecosystem, reducing the target IAP populations through the damage that they do through feeding on their host plants, and they then maintain the IAP populations at these lower levels permanently (McFadyen Reference McFadyen1998). Biocontrol has successfully controlled many IAP species, resulting in significant global benefits (Schwarzländer et al. Reference Schwarzländer, Hinz, Winston and Day2018, van Wilgen et al. Reference van Wilgen, Raghu, Sheppard and Schaffner2020). Biocontrol agents have been released in 152 countries, including 53 island nations (Winston et al. Reference Winston, Schwarzländer, Hinz, Day, Cock and Julien2021). South Africa is one of the most active nations in the field of biocontrol of IAPs, with 92 agent species established for the control of 66 target IAPs on the mainland (Moran et al. Reference Moran, Zachariades and Hoffmann2021); however, this management tool has not yet been extended to the country's sub-Antarctic islands. Given the lack of alternative controls and the threat of alien plants, the viability of biocontrol should be explored in this region.
Not all of the alien plants found on the PEIs will be suitable targets for biocontrol, and selection of the most appropriate targets for biocontrol will ensure that the target IAPs that are most problematic or threatening and are most likely to be effectively controlled with biocontrol are selected as priority targets. The recently developed Biological Control Target Selection (BCTS) system (Paterson et al. Reference Paterson, Hill, Canavan and Downey2021) has been used to prioritize all of the regulated alien plants on mainland South Africa, producing a ranked list of 299 alien species in the order of appropriateness as targets for biocontrol and guiding future investment into biocontrol research and implementation (Canavan et al. Reference Canavan, Paterson, Ivey, Sutton and Hill2021). The system is composed of three sections, each addressing one of the three most important characteristics of a good candidate alien plant for biocontrol. These are: 1) the importance and need for biocontrol of the alien plant, where the negative impacts/threats and the need for biocontrol over other control methods are assessed, 2) the likelihood of success, which is determined based on plant traits that have been shown to make alien plants good targets for biocontrol in the past, as well as whether the target alien plant, or its close relatives, have been controlled successfully using biocontrol elsewhere in the world, and 3) the feasibility of implementing biocontrol, where the costs and logistics of implementing a biocontrol are assessed and those that are more feasible are prioritized over others. In total, there are 13 attributes that are assessed, covering all three sections, and each attribute is given a score. These scores are then used to calculate an index which is a relative score of how appropriate each alien plant target is for biocontrol. Different attributes are weighted in the index according to how important they are in predicting biocontrol success. The logic and details of the BCTS system and how it was implemented for use on mainland South Africa are provided in three papers: Downey et al. (Reference Downey, Paterson, Canavan and Hill2021), Paterson et al. (Reference Paterson, Hill, Canavan and Downey2021) and Canavan et al. (Reference Canavan, Paterson, Ivey, Sutton and Hill2021).
In this paper, we use the BCTS system to prioritize all of the alien plants on the PEIs, which are part of South Africa but were not included in previous prioritization exercises (Canavan et al. Reference Canavan, Paterson, Ivey, Sutton and Hill2021). We then discuss and suggest ways forward for developing biocontrol programmes against the prioritized target alien plants.
Methodology
The list of alien plants was taken from Greve et al. (Reference Greve, Steyn, Mathakutha and Chown2017), as it contains the most up-to-date records of introduced plant species. Twenty-four alien plants have been recorded at some point on the PEIs. The alien plants have been categorized according to their invasive status, including species that were introduced but never naturalized (transient), eradicated or have remained localized in their distribution, despite long periods of establishment (Greene & Greene Reference Greene and Greene1963, Gremmen & Smith Reference Gremmen and Smith1999, le Roux et al. Reference le Roux, Ramaswiela, Kalwij, Shaw, Ryan and Treasure2013). For the prioritization assessment, Ochetophila trinervis was excluded as evidence has shown that it arrived through natural dispersal (by vagrant birds) and therefore should not be considered an alien species (Kalwij et al. Reference Kalwij, Medan, Kellermann, Greve and Chown2019). All other alien species were included regardless of whether the species is no longer present as a threat to the PEIs. This is to account for species that may become a threat in the future through reintroduction, have undetected populations or are in a lag phase of the invasion process (Crooks & Soulé Reference Crooks, Soulé, Sandlund, Schei and Viken1999). Seventeen alien plant species are currently established on the PEIs, all of which occur on Marion Island and only three of which occur on Prince Edward Island (Greve et al. Reference Greve, von der Meden, Janion-Scheepers, van Wilgen, Measey, Richardson, Wilson and Zengeya2020).
The BCTS system was applied to the alien plants using the methods of Canavan et al. (Reference Canavan, Paterson, Ivey, Sutton and Hill2021). Two changes were required to the BCTS system to account for the characteristics of the PEIs. Firstly, the attribute ‘1B - geographic distribution’ was modified. This attribute was intended to reflect the potential geographical range of alien plants across South Africa. However, given the restricted size of the PEIs, the attribute was modified to reflect how widespread alien plants are on the islands based on appropriate distribution measures. The scoring of this attribute was modified to align with the established classification categories from Gremmen (Reference Gremmen1971), which outline the species distributions of the alien plants. Secondly, the attribute ‘2D - habitat stability’ was removed. This attribute assesses whether or not an alien plant establishes in unstable habitats (i.e. cultivated land and improved pastures). Biocontrol in these habitats has been found to have a reduced likelihood of success due to the disturbance levels. Given that the PEIs are protected Special Nature Reserves, prohibiting any form of cultivation, this attribute is not relevant. From this, 12 attributes were applied to the 23 alien plants using the BCTS system (Table I).
Table I. The 12 attributes used from the Biological Control Target Selection system grouped into three sections (see details in Table S1).

Results
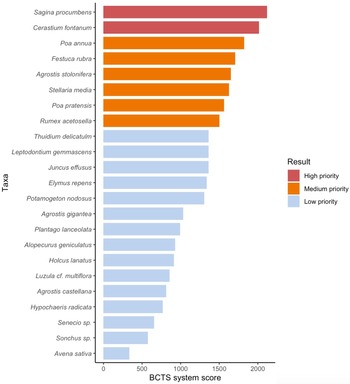
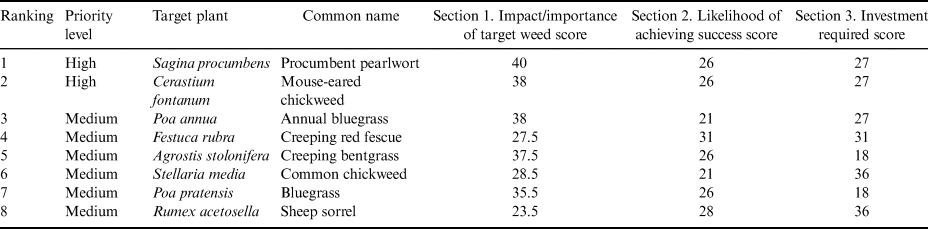
The final score from the BCTS system ranked the alien plants according to their suitability for biocontrol (Fig. 1; see scoring and rationale in Table S1). Two alien plants can be considered high-priority targets as they had the highest BCTS score – S. procumbens and Cerastium fontanum (score > 2000; Fig. 1 & Table II). Six alien plants had relatively high BCTS scores (> 1500, but < 2000) due to a number of attributes that would make them suitable targets such as high impacts and widespread distributions (Fig. 1). However, these were assigned as medium-priority targets based on certain attributes that are likely to make biocontrol more challenging compared to the top two alien plants, such as the presence of native congeners or taxonomic complications (see full details in the rationale in Table S1). Fifteen alien plants scored < 1500 and were assigned as low priority. Implementing biocontrol of these species is likely to produce relatively fewer benefits and to pose greater challenges. None of the alien plants have been targeted for biocontrol elsewhere and thus all would need substantial investment in research to encompass all aspects of such a programme.

Fig. 1. The Biological Control Target Selection (BCTS) system score for the 23 alien plants assessed (see further details in Table S1). The species highlighted in red represent high priority plants that based on the attributes scored are considered feasible biocontrol targets in the Prince Edward Islands. The species highlighted in orange represent medium priority targets and species in blue represent low priority targets.
Table II. List of priority target plants for biological control in the Prince Edward Islands based on the Biological Control Target Selection system (see details in Table S1).
Discussion
There are 23 alien plants recorded on the PEIs. Six of the species on Marion Island and three on Prince Edward Island have become established and have spread over substantial distances from their sites of introduction (Greve et al. Reference Greve, Steyn, Mathakutha and Chown2017) and are considered invasive (sensu Richardson et al. Reference Richardson, Pyšek, Rejmánek, Barbour, Panetta and West2000). Using the BCTS system, it was determined that two of these invasive species, S. procumbens and C. fontanum, should be considered high priorities for biocontrol. A further six species have attributes that make them less favourable for biocontrol; however, they remain threats to the PEIs and should be considered as potential targets in the future, but only after the higher-priority species have been targeted.
S. procumbens and C. fontanum have established across both islands and are considered too widespread and abundant to be effectively controlled using physical control methods and herbicides (Gremmen & Smith Reference Gremmen and Smith1999, Ryan et al. Reference Ryan, Smith and Gremmen2003). The species occur across Marion Island and have recently spread into Prince Edward Island. If left unmanaged, these species threaten Prince Edward Island's pristine state as they can cause rapid transformation in both disturbed and undisturbed habitats (Gremmen & Smith Reference Gremmen and Smith1999, Ryan et al. Reference Ryan, Smith and Gremmen2003, Mukhadi Reference Mukhadi2011, Twala et al. Reference Twala, Janion-Scheepers, le Roux and Greve2018). S. procumbens is extremely difficult to manage due to the occurrence of large numbers of long-lived seeds, with up to 200 000 seeds m2 (N.J.M. Gremmen, unpubl. data Reference Ryan, Smith and Gremmen2003). Similarly, C. fontanum is also able to produce high numbers of seeds and reproduces throughout most of the year (Mukhadi Reference Mukhadi2011). Biocontrol initiated on these priority species could include seed-feeding agents. Seed-feeders can reduce the invasive potential of a plant by limiting both seed banks and dispersal (Dennill & Donnelly Reference Dennill and Donnelly1991, Shoba & Olckers Reference Shoba and Olckers2010).
Four of the medium-priority species - Agrostis stolonifera, Festuca rubra, Poa pratensis and P. annua - are grasses and are the most abundant alien plants on the PEIs (Greve et al. Reference Greve, Steyn, Mathakutha and Chown2017). The Poaceae family is the greatest contributor of non-native species to the sub-polar environment (Frenot et al. Reference Frenot, Chown, Whinam, Selkirk, Convey, Skotnicki and Bergstrom2005). The invasive grasses have all had recorded impacts yet did not rank as high-priority species. The species each have attributes that make their suitability for biocontrol less favourable than the top-priority species. For example, A. stolonifera is known to hybridize with Agrostis castellana and Agrostis capillaris (Belanger et al. Reference Belanger, Meagher, Day, Plumley and Meyer2003), of which A. castellana is present as an IAP on the island. The potential for hybridization may result in hybrid genotypes co-occurring on the island, and this would present challenges to effective biocontrol as agents are less likely to feed on hybrid populations (Paterson et al. Reference Paterson, Hill, Canavan and Downey2021). The invasive P. annua had a reduced BCTS score due to the presence of a native congener in the PEIs, Poa cookii. While this is unlikely to stop a biocontrol programme, it is a factor that would add time and costs to such a programme (Paterson et al. Reference Paterson, Hill, Canavan and Downey2021). Finding a host-specific agent for P. annua is likely to be more challenging as they may feed on the related congener, and therefore more extensive host specificity studies would be required. Grasses have not been considered good targets for biocontrol in the past; however, recent evidence suggests that they may be equally as likely to be successful targets as other plant species (Sutton et al. Reference Sutton, Canavan, Day, den Breeyen, Goolsby and Cristofaro2019). There have, however, been relatively few grasses targeted for biocontrol, so it is difficult to predict success, and none of the species present on the PEIs have been considered for biocontrol elsewhere in the world. These factors are likely to result in the requirement for greater investment in biocontrol programmes compared to the top-priority species. However, given their impacts, biocontrol should still be considered if resources are available and the higher-priority species already have sufficient resources allocated to them.
The BCTS system has shown that both S. procumbens and C. fontanum have favourable attributes for biocontrol; however, the unique characteristics of the PEIs' habitats present both positive and challenging factors in developing biocontrol programmes. The PEIs have only 22 native species (Chau et al. Reference Chau, Mtsi, Münbergová, Greve, le Roux and Mairal2020), and for the high-priority species none of the native species are congeners. This greatly improves the chances of finding host-specific agents as specialist natural enemies that are used as biocontrol agents are unlikely to feed outside of the genus of their host plant (Pemberton Reference Pemberton2000). In addition, the plant list for host specificity testing can be reduced to only include PEI natives, thus reducing time and costs (Paterson et al. Reference Paterson, Hill, Canavan and Downey2021). Lastly, it is probable that there is sufficient capacity to release and monitor agents due to the presence of a permanent research station that is staffed by scientists. These are all factors that are advantageous logistically and would improve the feasibility and speed with which new biocontrol agents could be developed.
The most challenging factor will probably be finding biocontrol agents that can tolerate the PEIs' environmental conditions. While biocontrol has been implemented successfully in climates with freezing conditions (e.g. in Alberta, Canada; McClay Reference McClay, Moran and Hoffman1996, De Clerck-Floate & Schwarzländer Reference De Clerck-Floate and Schwarzländer2001, Van Hezewijk et al. Reference Van Hezewijk, Bourchier and De Clerck-Floate2010), agents have never been released in areas with such chronically low temperatures and limited thermal variation as in the PEIs. Biocontrol agents will rely on a certain combination of time and temperature to reach development (Jarošík et al. Reference Jarošík, Honěk, Magarey and Skuhrovec2011); therefore, the cool summer months may present an insufficient sum of effective temperatures or degree-days to complete life cycles for most arthropods. It may be beneficial to prioritize biocontrol agents that are closely related to the arthropods that are already established on the PEIs, as related species often have similar thermal requirements (Jarošík et al. Reference Jarošík, Honěk, Magarey and Skuhrovec2011). There are only a few arthropod families that are established in the PEIs, with mites and springtails being the numerically dominant species and flies (Diptera) and beetles (Coleoptera) being the most common insects (Barendse & Chown Reference Barendse and Chown2001, Chown & Convey Reference Chown and Convey2016). A further 15 terrestrial insects have been introduced to the PEIs and have naturalized (Greve et al. Reference Greve, Steyn, Mathakutha and Chown2017). Sub-Antarctic arthropods are often found to have generalist eurythermal characteristics, flexible ecophysiological traits (Slabber et al. Reference Slabber, Worland, Leinaas and Chown2007, Renault et al. Reference Renault, Leclerc, Colleu, Boutet, Hotte and Colinet2022), stress selection traits such as low reproductive investment and unusually long life cycles (Barendse & Chown Reference Barendse and Chown2001, Haupt et al. Reference Haupt, Sinclair and Chown2014). Arthropods in the Antarctic region often do not complete their life cycles in one season, and different stages are exposed to different thermal conditions, suggesting selection for highly thermally plastic genotypes (Bahrndorff et al. Reference Bahrndorff, Lauritzen, Sørensen, Noer and Kristensen2021). These factors could guide pre-release assessments for any potential biocontrol agents so that their thermal acclimation responses could be tested to determine whether they have the phenotypic flexibility to survive and develop on the PEIs. The prospects of finding such biocontrol agents are promising, particularly in light of the already-established alien arthropods. For example, a recently introduced alien parasitoid species, Aphidius matricariae, has naturalized on the PEIs from what is believed to be a single gravid female (Slabber Reference Slabber2005). This species has been used as a biocontrol agent for aphid pests elsewhere and has adapted to feed on the introduced alien aphid Rhopalosiphum padi (Slabber Reference Slabber2005). Despite the harsh conditions present on the PEIs, introduced alien arthropods can establish if they exhibit the necessary adaptive traits.
Another important challenge to consider is the structural simplicity of these sub-Antarctic island ecosystems. Invertebrates constitute the only herbivores and detritivores on the PEIs, and the assemblages are characterized by few herbivores and predators and a high number of decomposers (Vernon et al. Reference Vernon, Vannier and Trehen1998, Chown et al. Reference Chown, McGeoch and Marshall2002). Most ecosystems are far more complex, with food webs encompassing hundreds to thousands of species, connected through multiple links of various strengths (Polis et al. Reference Polis and Strong1996). The introduction of biocontrol agents to this new region poses risks of direct and indirect effects on the PEIs. The direct effects are considered in biocontrol programmes through host specificity testing; however, assessing indirect effects is often not carried out due to the complexity involved (Todd et al. Reference Todd, Pearce and Barratt2021). For the PEIs, there is an opportunity to encompass risk assessments of the indirect effects due to the simple food webs that exist. Risk assessments could help identify the potential consequences of introducing the biocontrol agents by predicting how they might interact within food webs and considering how to reduce these risks (Todd et al. Reference Todd, Pearce and Barratt2021). The agents may become superabundant in the PEIs when encountering an abundant food source in the target weed with little competition and no natural enemies (Pearson & Callaway Reference Pearson and Callaway2003), and thus they may become a new food source for native species. The consequences of these food web interactions will need to be considered to determine whether there is potential for them to restructure community interactions (Pearson & Callaway Reference Pearson and Callaway2003). Yet consideration of these potential effects needs to be gauged against the risks posed by the IAPs if left unmanaged (Downey & Paterson Reference Downey and Paterson2016).
The PEIs are one of the last relatively untouched habitats left within South African territory, and alien plants, if left unmanaged, have the potential to alter their habitats and to affect ecosystem processes and function. Yet control of these species has been negligible due to both difficulty in the implementation of control methods and a lack of information required to prioritize and plan control efforts (le Roux et al. Reference le Roux, Ramaswiela, Kalwij, Shaw, Ryan and Treasure2013). The use of the BCTS system will help to direct conservation funding on the PEIs to the alien plants in most need of control. While biocontrol on the PEIs would represent a new climatic zone for the release of agents, this should not deter biocontrol research. Thermal tolerance testing is an established protocol within biocontrol and can predict the likelihood of a biocontrol agent being climatically suitable prior to release, so the release of ineffective agents will be avoided. A successful biocontrol programme on any of the high- or medium-priority species identified in this study would bring significant benefits to the PEIs and would provide a permanent and environmentally friendly solution to mitigating the negative impacts of the IAPs. South Africa is a world leader in weed biocontrol and has the supportive legislation and research capacity (Ivey et al. Reference Ivey, Hill and Zachariades2021) to carry out safe and effective biocontrol research for the PEIs.
Financial support
We acknowledge the funding from the South African Working for Water (WfW) programme of the Department of Forestry, Fisheries and the Environment: Natural Resource Management Programmes (DFFE: NRMP). Funding was also provided by the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation (NRF) of South Africa.
Disclaimer
Any opinions, finding, conclusions or recommendations expressed in this material are those of the authors and the NRF does not accept any liability in this regard.
Author contributions
KC and IDP conceptualized the study. KC: data curation, visualization, writing - original draft preparation. IDP: supervision, writing - original draft preparation. Both authors revised the draft manuscript and approved the final manuscript.
Supplemental material
A supplemental table will be found at https://doi.org/10.1017/S0954102023000135