Overweight and obesity as outcomes of high BMI have become significant risk factors for the incidence of CVD, such as hypertension, diabetes, CHD and mortality worldwide(Reference Kim, Després and Koh1–Reference Zheng, McLerran and Rolland6). The prevalence of overweight and obesity has been increasing dramatically among Chinese adults (from 9·4 % in 1993 to 15·7 % in 2009 for overweight and from 4·0 % to 10·7 % for obesity) along with tremendous changes in diet and lifestyles(Reference Xi, Liang and He7). In 2015, the prevalence reached 5·5 % among women and 5·0 % among men for obesity and over 22 % in both sexes for overweight(Reference Afshin, Forouzanfar and Reitsma8).
Over the past few decades, dietary intake of SFA as a probable contributor to obesity prevalence has raised extensive concern and remained highly controversial throughout the world. Dietary fat intake might be related to the prevalence of overweight/obesity, but reductions in dietary fats did not result in lower body weights, which raised wide concerns about different contributions from the intakes of SFA, MUFA or PUFA(Reference Lichtenstein, Kennedy and Barrier9). The secondary analysis of observational data from PREMIER trial indicated that lower SFA intake was related to lower body weight(Reference Lin, Wang and Grambow10). However, different types of SFA also had distinct effects. Medium chain SFA (MCSFA) are a group of fatty acids presenting 6–10 saturated carbons, while long-chain SFA (LCSFA) and very long-chain SFA (VLCSFA) are defined as the class of SFA with 12–18 and > 20 carbon atoms, respectively. In general, dietary fats that are easier for oxidation may be less likely to induce obesity. Previous study reported that MCSFA were highly oxidised, but MUFA and PUFA were fairly oxidised. The extent of LCSFA oxidation declined with increasing carbon number(Reference DeLany, Windhauser and Champagne11). Thus, it seemed to be a beneficial role of MCSFA but an adverse role of LCSFA in incident overweight/obesity. Experimental trials and epidemiological studies have reported the role of SFA in energy contribution and their positive associations with body weight(Reference Lin, Wang and Grambow10,Reference de Wit, Derrien and Bosch-Vermeulen12–Reference Hariri, Gougeon and Thibault14) , whereas a few studies have found benefits from dietary medium chain TAG on body weight control(Reference Nosaka, Maki and Suzuki15,Reference Tsuji, Kasai and Takeuchi16) . However, the favourable role of MCSFA in weight loss might play a role among hypertriacylglycerolaemic and overweight Chinese subjects but not normal or obese hypertriacylglycerolaemic subjects, which may be due to the metabolic difference in energy expenditure and fat oxidation in individuals with different body weights(Reference Zhang, Liu and Wang17). To sum up, direct evidence on the associations of SFA and MCSFA intake with the incidence of overweight/obesity is still insufficient especially among Chinese population. Hence, our study aimed to examine the associations of dietary intake of total and individual SFA with the risk of overweight and obesity in the China Health and Nutrition Survey.
Materials and methods
Study population
The present study accessed the data from the China Health and Nutrition Survey in 1989–2011, an ongoing, prospective and household-based nationwide cohort study recruiting a total of over 30 000 individuals from nine provinces plus three autonomous cities across China using a multistage random cluster design for sampling(Reference Zhang, Zhai and Du18). The China Health and Nutrition Survey was devoted to examining the health and nutrition transformation of Chinese population with rapid socio-economic development, and detailed procedures have been described elsewhere(Reference Zhang, Zhai and Du18,Reference Popkin, Du and Zhai19) . Our present analysis included adults aged over 20 years at entry with a complete duration of follow-up and dietary information. After excluding individuals with stroke (n = 43), myocardial infarction (n = 26), pregnant women (n = 228) and those with BMI ≥ 24 kg/m2 or with missing height or weight at baseline or endpoint (n = 4175), the final analysis involved 8465 participants (3968 men and 4497 women) (online Supplementary Fig. S1). All the participants provided written informed consent. The study was approved by the institutional review boards at the University of North Carolina, Chapel Hill and the National Institute of Nutrition and Food Safety from the Chinese Centre for Disease Control and Prevention (UNCC-CCDC-001). This study was registered at clinicaltrials.gov as NCT03281512.
Ascertainment of diet and SFA
Three-day consecutive 24-h recalls at the individual level were conducted to assess dietary intake combined with the optimisation of measuring household foods, including condiments and oils. Participants were randomly selected to be interviewed from Monday to Sunday and then were asked to report all categories of food and the proportion of each dish consumed away from and at home over the previous 24 h(Reference Zhang, Wang and Du20). A detailed description of dietary data and data collection procedure has been reported before(Reference Popkin, Lu and Zhai21). Nutrient intakes from various foods were calculated using the Chinese Food Composition Table with corresponding versions(22–24). The Food Composition Table covered the data of thirty-five fatty acids in foods, including fifteen SFA, eight MUFA and twelve PUFA, and other unmeasured or unknown fatty acids. Specifically, dietary SFA include TSFA, odd-chain SFA (the sum of undecanoic acid (11:0), tridecanoic acid (13:0), pentadecanoic acid (15:0), margaric acid (17:0) and nonadecanoic acid (19:0)), MCSFA (the sum of 6:0, 8:0 and 10:0), LCSFA (the sum of lauric acid (12:0), myristic acid (14:0), palmitic acid (16:0) and stearic acid (18:0)), VLCSFA (the sum of arachidic acid (20:0), docosanoic acid (22:0) and lignoceric acid (24:0)) and even-chain SFA (the sum of MCSFA, LCSFA and VLCSFA). Cumulative averages of total and specific SFA intakes during the follow-up were calculated and expressed as percentages of energy to represent long-term dietary SFA intake and to minimise within-individual variation. Additionally, total energy intake was calculated from Food Composition Table and validated via its association with the total energy expenditure determined by the doubly labelled water method (correlation coefficient was 0·56 (P < 0·01) for men and 0·60 (P < 0·01) for women)(Reference Yao, McCrory and Ma25). Other covariates of demographic characteristics and lifestyles were also collected, including age, sex, marital status, education level, household income, physical activity, smoking, alcohol consumption, baseline hypertension and diabetes.
Ascertainment of overweight and obesity
Standing height and body weight were measured by trained staffs following a standard protocol and instruments in each survey. BMI as the primary dependent variable was calculated as weight in kilograms divided by the square of height in metres. Participants with a BMI ≥ 24 kg/m2 and < 28 kg/m2 were determined to be overweight, while a BMI of 28 or higher was considered as obesity according to the Chinese Criteria of Weight for Adults (WS/T 428-2013)(Reference Zhai, Fang and Yu26). The follow-up duration was measured as the interval between the year at entry to the year of developing overweight/obesity or the year 2011 (the end of follow-up).
Statistical analysis
Baseline characteristics were expressed as the mean value with standard error for continuous variables with a normal distribution, while categorical variables were expressed as numbers and percentages (%) in each quartile. ANOVA (for continuous variables) and the χ 2 test (for discrete variables) were used to assess the basic features of the study population according to quartiles of SFA consumption.
We used Cox proportional hazards regression models to estimate the hazard ratio (HR) and 95 % CI of incident overweight or obesity during the follow-up by considering the first quartile of SFA intake as the reference category. In the multivariable analyses, three stepwise models were constructed to estimate the risk by adjusting for the covariates from known or suspected risk factors. Model l was adjusted for age and sex. Model 2 was further adjusted for annual household income, educational level (below high school, high school, some college, at least college or unknown), marital status (single, married, divorced or unknown), residence (urban or rural), location (north or south), physical activity (no regular activity, low to moderate activity or vigorous activity), smoking status (never, former, current or unknown), alcohol intake (abstainer or drinker), baseline hypertension (yes, no or unknown) and baseline diabetes (yes or no). The final multivariable model 3 was further adjusted for baseline BMI (kg/m2, continuous variable), intake of total energy, percentage of energy from dietary protein, PUFA, MUFA (all continuous), odd-chain SFA (for even-chain SFA) and even-chain SFA (for odd-chain SFA). In regression models, tests for trends were carried out by modelling the median values of each category as a continuous variable.
In subdivision and secondary analyses, we divided TSFA into odd-chain SFA and even-chain SFA. Then, even-chain SFA were further subdivided into MCSFA including 6:0, 8:0 and 10:0, LCSFA and VLCSFA to assess associations of SFA with different carbon numbers with overweight or obesity. We also conducted subgroup analyses to examine the interactions stratified by age (< 50 years or ≥ 50 years), sex (male or female), location (north or south), urban site (yes or no), smoking status (never, former or current), alcohol intake (abstainer or drinker), education level (below great high school or great high school and above), physical activity (low activity, moderate activity or vigorous activity), household income (below median or above median) and history of hypertension (yes or no). P for interaction was tested by the likelihood-ratio test. In the sensitivity analyses, we excluded participants with extreme BMI (< 15 kg/m2) or extreme energy intake (< 2092 (500) or > 16736 (4000) kJ/d (kcal/d)) at baseline to minimise their influences and excluded participants with only the first 2 years of follow-up to observe if the results changed substantially.
Statistical analyses were performed using SAS (version 9.4). A two-sided P value of less than 0·05 was considered significant for all analyses.
Results
Baseline characteristics
The baseline characteristics of the participants from China Health and Nutrition Survey are presented in Table 1. At baseline, participants with higher SFA intake were more likely to be elderly, single and current drinkers, to reside in southern urban areas, to have higher levels of education and they also tended to be wealthier, as indicated by overall higher annual household income. These participants were also engaged in less physical activity and less likely to have a history of hypertension. For dietary and nutritional states, generally, they consumed higher energy content. In addition, participants with higher SFA consumption were apt to consume more red meat, poultry, fruit and total fats, whereas they consumed less carbohydrate and protein.
Table 1. Baseline participant characteristics according to SFA intake (n = 8465)
(Numbers and percentages; mean values and standard errors)

Q, quartile.
* P trend values were analysed by ANOVA for continuous variables or the χ 2 test for categorical variables.
Total SFA and subdivisions of SFA intake and risk of overweight/obesity
A total of 3171 incident overweight/obesity cases were identified, including 1649 women and 1522 men, during a median 11 years of follow-up. The intake of TSFA had a null association with the risk of overweight/obesity in the age- and sex-adjusted model (model 1, P trend = 0·15). After further adjusting for demographic and dietary covariates, the association was still unchanged (P trend = 0·19 and 0·35 in model 2 and model 3, respectively). The intake of even-chain SFA among 8465 participants accounted for a larger proportion of TSFA (almost 99 %) than odd-chain SFA consumption. Similarly, odd-chain SFA and even-chain SFA consumption were not associated with the risk of overweight/obesity after adjustment for multiple confounders (Table 2).
Table 2. Associations between total SFA and subtypes of total SFA intake and overweight and obesity in the CHNS (n = 8465)
(Hazard ratios and 95 % confidence intervals)*
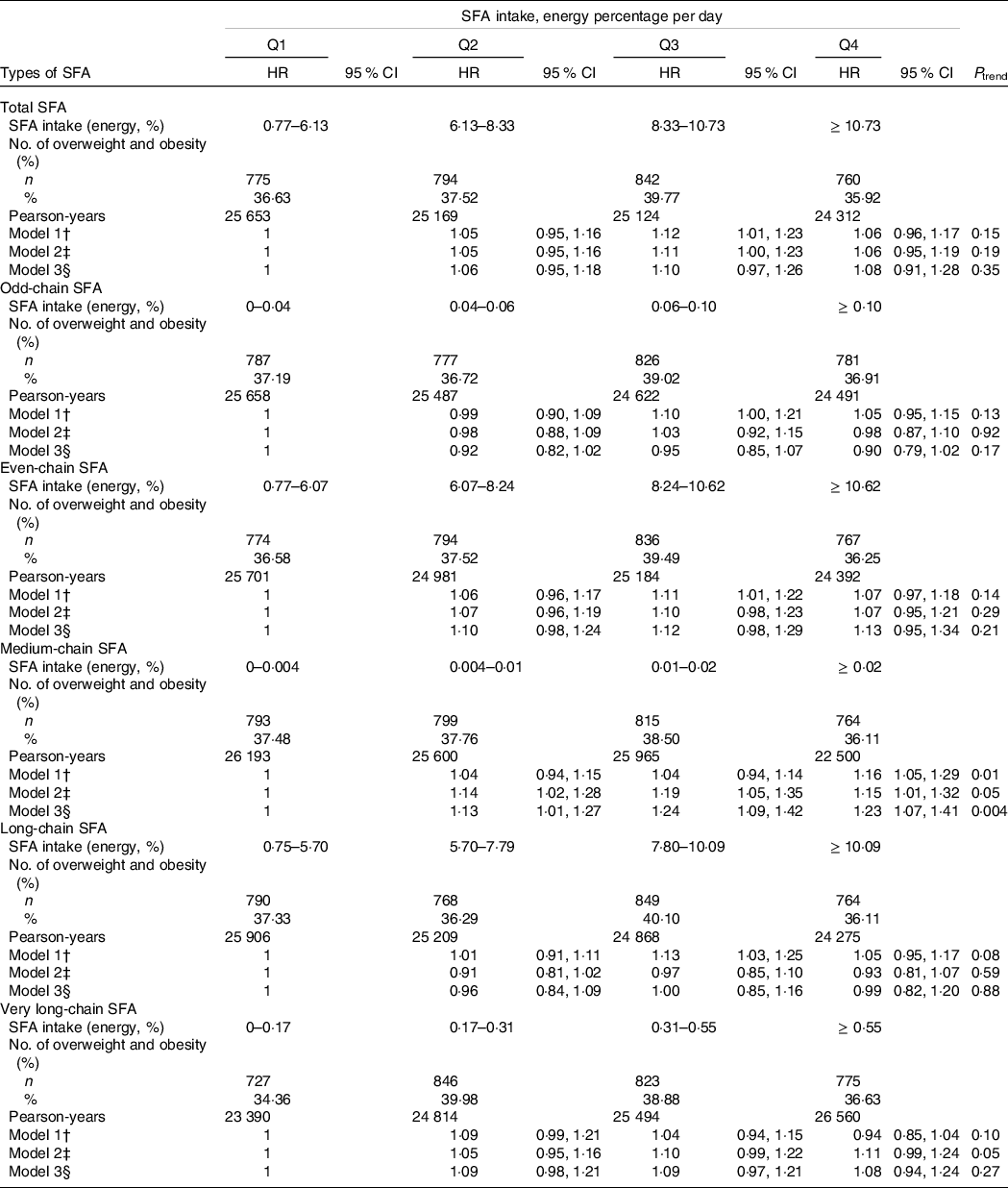
HR, hazard ratio; Q, quartile.
* Time-dependent Cox proportional hazards regression models were used to assess the HR (95% CI) of overweight and obesity by quartiles of total, odd-chain, even-chain, medium-chain, long-chain and very long-chain SFA intakes.
† Model 1 was only adjusted for age and sex.
‡ Model 2 was further adjusted for quartiles of household income, educational level (below high school, high school, some college, at least college or unknown), marital status (single, married, divorced or unknown), residence (urban or rural), location (north or south), physical activity (no regular activity, low or moderate activity or vigorous activity), smoking status (never, former, current or unknown), alcohol intake (abstainer or drinker), baseline hypertension (yes, no or unknown) and baseline diabetes (yes or no).
§ Model 3 was further adjusted for baseline BMI, intake of total energy, percentage of energy from dietary protein, PUFA, MUFA, odd-chain SFA (for even-chain SFA) and even-chain SFA (for odd-chain SFA).
We also assessed the relationship between the intake of SFA with different carbon numbers and the risk of overweight/obesity (Table 2). MCSFA consumption was positively associated with a higher risk of overweight/obesity after adjustment for age and sex. In the multivariate-adjusted model (model 2), the HR across the quartiles of MCSFA intake were 1·14 (95 % CI 1·02, 1·28), 1·19 (95 % CI 1·05, 1·35) and 1·15 (95 % CI 1·01, 1·32) (P trend = 0·05). Furthermore, the positive association of MCSFA and the risk of overweight/obesity was stronger in the fully adjusted model (model 3). The HR of overweight/obesity was 1·23 (95 % CI 1·07, 1·41) by comparing the highest quartile with the reference (non-consumers). Multivariate-adjusted HR were 1·13 (95 % CI 1·01, 1·27) and 1·24 (95 % CI 1·09, 1·42) for MCSFA intake, which accounted for 0·004–0·01 and 0·01–0·02 % of energy, respectively (P trend = 0·004). No significant association was observed for LCSFA consumption in the age- and sex-adjusted model. Although in the third quartile the HR and 95 % CI were above 1, a null association was detected comparing the group with the highest LCSFA consumption with the reference group (HR was 1·05 (95 % CI 0·95, 1·17); P trend = 0·08). No relationship remained in the multivariate-adjusted models (P trend = 0·59 and 0·88, respectively). Similar to the association of LCSFA and overweight or obesity, VLCSFA intake did not play a role in increasing or lowering the risk of overweight/obesity. In model 3, there were no apparent changes in HR across the increasing quartiles of VLCSFA consumption (HR: 1·09 (95 % CI 0·98, 1·21) for 0·17–0·31 % of energy, 1·09 (95 % CI 0·97, 1·21) for 0·31–0·55 % of energy and 1·08 (95 % CI 0·94, 1·24) for the highest consumption; P trend = 0·27).
Individual SFA intake and risk of overweight/obesity
We also conducted secondary analyses to further observe the significant association for MCSFA intake (Table 3). Although the consumption of 6:0 was related to 32 % increase in the risk of overweight/obesity in the age- and sex-controlled model (HR was 1·32 (95 % CI 1·14, 1·53) when comparing the highest category with non-consumers; P trend < 0·001), the positive association disappeared, and a protective role existed instead in the multivariate-adjusted models. In the fully adjusted model, participants in the highest category of 6:0 consumption had 32 % lower risk of overweight/obesity than non-consumers (HR: 0·68, 95 % CI 0·56, 0·84; P trend < 0·001), whereas a positive association between 10:0 intake and overweight/obesity was detected. The intake of 10:0 was associated with 3 % and 13 % higher risk of overweight/obesity along with increasing consumption (model 1; HR: 1·03 (95 % CI 0·94, 1·12) for 0·006–0·013 % of energy and 1·13 (95 % CI 1·04, 1·23) for the highest category; P trend = 0·01). After adjustment for all other potential cofounders, the detrimental association of 10:0 in relation to the risk of overweight/obesity was strengthened. Participants with the highest consumption (10:0 intake ≥ 0·013 % of energy per day) had 25 % higher risk of overweight/obesity (HR: 1·25; 95 % CI 1·10, 1·42; P trend < 0·001), whereas no relationship between 8:0 and the risk of overweight/obesity was observed in any model (all P trend > 0·05).
Table 3. Associations between subtypes of medium-chain SFA intake and overweight and obesity in the CHNS (n = 8465)
(Hazard ratios and 95 % confidence intervals)*

C, category; HR, hazard ratio.
* Time-dependent Cox proportional hazards regression models were used to assess the HR (95 % CI) of overweight and obesity by categories of subtypes of medium-chain SFA, including 6:0, 8:0 and 10:0.
† Model 1 was only adjusted for age and sex.
‡ Model 2 was further adjusted for quartiles of household income, educational level (below high school, high school, some college, at least college or unknown), marital status (single, married, divorced or unknown), residence (urban or rural), location (north or south), physical activity (no regular activity, low or moderate activity or vigorous activity), smoking status (never, former, current or unknown), alcohol intake (abstainer or drinker), baseline hypertension (yes, no or unknown) and baseline diabetes (yes or no).
§ Model 3 was further adjusted for baseline BMI, intake of total energy, percentage of energy from dietary carbohydrates, protein, PUFA, MUFA, odd-chain SFA (for even-chain SFA) and even-chain SFA (for odd-chain SFA).
Subgroup and sensitivity analyses
In subgroup analyses that adjusted for the same covariates in Tables 2 and 3, our main findings that no significant association existed between TSFA consumption and the risk of overweight/obesity were generally unchanged (online Supplementary Table S1). No pronounced difference appeared between TSFA intake and the risk of overweight/obesity in men and in women (P for interaction = 0·04). In addition, a protective role of TSFA intake in the risk of overweight/obesity was observed among elderly participants (≥ 50 years) compared with young participants (P for interaction = 0·01). Additionally, a detrimental effect of higher TSFA intake appeared in participants who dwelled in urban areas compared with participants who dwelled in rural areas (P for interaction = 0·02). No significant effect modification was observed for location (north or south), smoking status, alcohol consumption, education level, physical activity, income or history of hypertension (all P for interaction > 0·05). In sensitivity analyses, exclusion of cases with a follow-up duration of less than 2 years or those with extreme BMI (< 15 kg/m2) or extreme energy intake (< 2092 (500) or > 16736 (4000) kJ/d (kcal/d)) did not materially change the main findings (online Supplementary Table S2).
Discussion
We found that TSFA consumption was not associated with the risk of overweight or obesity. However, higher MCSFA intake was related to 23 % increased risk in the multivariate-adjusted model, wherein 6:0 was associated with a lower risk and 10:0 was associated with a higher risk of overweight/obesity. To our knowledge, this was the first study to assess longitudinally the associations of dietary SFA intake with the risk of overweight/obesity among nationwide Chinese population.
A series of previous studies have shown adverse effects of TSFA intake on various health-related outcomes. One of our studies found that SFA were positively associated with total and CVD mortality among 521 120 participants aged 50–71 years from the National Institutes of Health-American Association of Retired Persons Diet and Health Study(Reference Zhuang, Zhang and He27). In a special population with a high risk of CVD from the PREvención con DIeta MEDiterránea study, higher SFA intake was associated with 81 % (HR: 1·81; 95 % CI 1·05, 3·13) higher risk of CVD(Reference Guasch-Ferré, Babio and Martínez-González28). However, in this study, no association with the risk of overweight/obesity was detected for TSFA intake. A number of established studies focused on the relationship between TSFA intake and overweight/obesity-relevant outcomes, such as cardiometabolic diseases, cancers and type 2 diabetes (T2D)(Reference Engin29), and supported our findings. Furthermore, the results from the European Prospective Investigation into Cancer and Nutrition-Netherlands cohort suggested that TSFA intake was not related to T2D risk and that the association depended on the types and food sources of SFA(Reference Liu, van der Schouw and Soedamah-Muthu30). MCSFA, a subtype of SFA, seem to prevent depositing fat. The beneficial role of MCSFA in the incidence of obesity has been unravelled in some experimental studies. The absorption of MCSFA was quick through vein to the liver(Reference Decker31). MCSFA were rapidly oxidised without requiring carnitine palmitoyltransferase, which would not lead to excessive fat deposition(Reference DeLany, Windhauser and Champagne11). In addition, these fatty acids could increase the expenditure of energy and induce satiety(Reference Hill, Peters and Swift32). Besides, the interaction may occur between MCSFA intake and gut microbiota. MCSFA could lower obesity risk by the increase of Bacteroidetes and the decrease of Firmicutes and Proteobacteria in gut of mice(Reference Machate, Figueiredo and Marcelino33). However, decanoic acid (10:0) was found to have adverse effect on human trophoblasts by inducing oxidative stress and mitochondrial dysfunction(Reference Yang, Lim and Bazer34). Thus, individual MCSFA may have different associations with the incidence of overweight/obesity. MCSFA are present in dietary oil sources such as coconut oil, palm kernel oil, goat milk and sheep milk and often occur in their dietary form as medium-chain TAG (MCT)(Reference Žáček, Bukowski and Mehus35). Long-chain TAG are the forms of storing fat for LCSFA in the body. A systematic review and meta-analysis of randomised controlled trials with a duration of more than 3 weeks among healthy adults suggested that replacing long-chain TAG with MCT in the diet could induce a small reduction in body weight (–0·51 kg; 95 % CI –0·80, –0·23 kg; P < 0·001; I 2 = 35 %)(Reference Mumme and Stonehouse36). Animal experiments also showed the efficacy of MCT in suppressing body fat accumulation, insulin resistance, and the inflammatory response(Reference Geng, Zhu and Xie37) and the potentially synergistic effect of MCT with fish oil on reduced CVD risk(Reference Kondreddy, Anikisetty and Naidu38). The benefits of MCT from MCSFA intake seemed to support the view that MCT-enriched diets are feasible for body weight control or overweight/obesity-related disease improvement. However, we found a positive relationship between MCSFA intake and excess body weight. Previous studies reported that the effects of MCT in stimulating insulin secretion as well as anabolism-related processes and inducing hypertriacylglycerolaemia might counteract its protective roles. In addition, the pros or cons of MCT depended on the composition, including energy intake, nature of ingredients, MCT/long-chain TAG ratio, octanoate/decanoate ratio and duration of the regimen(Reference Bach, Ingenbleek and Frey39). More importantly, the threshold that a high energy level (approximately 50 %) of MCT should be reached to achieve body weight loss was impractical in human nutrition(Reference Max, Bach and Pallier40,Reference Bray, Lee and Bray41) . Additionally, in comparison with nondiabetic controls with no family history of T2D, a larger post-prandial triglyceridaemia response, as an independent risk factor for CVD, was found among relatives of diabetic patients who were fed a MCSFA-rich meal (mostly supplied by 80 g coconut oil)(Reference Pietraszek, Hermansen and Pedersen42). Established studies mainly focused on the associations for higher intake of TSFA and subtypes of SFA, including LSFA and VLSFA; however, limited studies in relation to 6:0, 8:0 and 10:0 as subdivisions of MCSFA and health-related outcomes were conducted. A cross-sectional study that utilised data from the National Health and Nutrition Examination Survey (2003–2006) showed that higher intakes of 6:0, 8:0 and 10:0 were all significantly associated with a higher mortality (P = 0·008, 0·019 and 0·027, respectively)(Reference Jayanama, Theou and Godin43). Nevertheless, we found an inverse relationship between 6:0 and 10:0 intakes and the risk of overweight/obesity, and no association existed for 8:0 intake. This result may be due to divergent food sources of 6:0, 8:0 and 10:0. The main natural sources of 6:0 are dairy products, such as whole-fat milk powder, yogurt, cream and butter, whereas 10:0 is principally derived from coconut oil. Dairy product consumption has raised wide controversy in recent decades because of the SFA content. Dairy products were seemingly able to reduce premeal appetite and food intake in a randomised, unblinded, crossover design, which meant that dairy products were likely to lose weight(Reference Vien, Fard and El Khoury44). Prospective cohort studies consistently suggested that yogurt consumption contributes to reduced adiposity indexes and the metabolic syndrome risk(Reference Sayon-Orea, Martínez-González and Ruiz-Canela45). Furthermore, a meta-analysis of mainly cross-sectional studies indicated that the pooled OR of obesity via comparing the highest category with the lowest category of total dairy product consumption were 0·54 (95 % CI 0·38, 0·77) for children, 0·75 (95 % CI 0·69, 0·81) for adults and 0·74 (95 % CI 0·68, 0·80) for both, and an increment of 200 g/d milk consumption was associated with 16 % lower risk of obesity (OR: 0·84 (95 % CI 0·77, 0·92))(Reference Wang, Wu and Zhang46). In addition, the favourable roles of higher dairy product consumption, yogurt in particular, in the prevention of T2D as well as overall and CVD mortality were also detected(Reference Gijsbers, Ding and Malik47–Reference Farvid, Malekshah and Pourshams49). For the relationship between coconut oil and obesity and related outcomes, an experimental study observed that weight gain of obese rats increased with the supplementation of virgin coconut for 30 d(Reference Ströher, de Oliveira and Martinez-Oliveira50). Moreover, a pooled result of 21 interventional studies showed that coconut oil intake resulted in higher levels of LDL-cholesterol, HDL-cholesterol and total cholesterol, which are lipid profile markers linked to a higher risk of cardiometabolic diseases, compared with other vegetable oil consumption(Reference Neelakantan, Seah and van Dam51). Additionally, a structured literature review concluded that no available data supported the value of coconut oil consumption in increasing satiety and weight loss(Reference Santos, Howell and Earnest52). Conversely, previous randomised and double-blind clinical trials have pointed to beneficial effects of coconut oil on cardiovascular health, including eliciting favourable changes in insulin sensitivity and CVD risk-associated parameters and reducing abdominal obesity(Reference Assunção, Ferreira and dos Santos53,Reference Korrapati, Jeyakumar and Putcha54) . However, these experiments were conducted in the background of a balanced diet or hypoenergetic diet characterised by increased consumption of fruits and vegetables, reduced simple carbohydrates and animal fat consumption as well as reduction or elimination of alcohol consumption and smoking, plus moderate physical activity. Given that the cause of overweight or obesity is a positive energy balance in which energy intake is greater than energy expenditure, the observed benefits could not be ascribed to coconut oil intake, while the overall benefits of regular physical activity, a balanced diet and lifestyles were overlooked(Reference Hebden, O’Leary and Rangan55–Reference Cureau, Sparrenberger and Bloch57). Accordingly, further clinical trials conducted among participants without diet or lifestyle intervention or advice are warranted.
The strengths of our study included the prospective design and long duration of follow-up. We also used cumulative average intakes of nutrients to better understand a long-term diet instead of baseline dietary intake only. Additionally, we comprehensively considered the relationship between the intakes of individual SFA with different numbers as well as lengths of carbon chains and the risk of overweight/obesity. In addition, we carried out sensitivity analyses to examine the stability of our results and found similar findings. However, our study also had some limitations. First, although dietary data from enrolled participants were collected from 3-d consecutive 24-h recalls combined with a weighing method, measurement errors were still inevitable, which might dilute true associations between specific SFA intake and risk of overweight/obesity. Second, we could not adjust for trans-fatty acid intake because of the lack of data in our study. Nonetheless, the overall trans-fatty acid consumption in China was not very high during the study period(Reference Piernas and Popkin58–Reference Wang, Zhai and Zhang60) and might not significantly affect the documented findings. Third, given that only a small number of participants (n = 118) developed obesity, we considered the overall risk of overweight/obesity as an outcome instead of separating them as two outcomes, which would have provided more implications on human health in relation to non-normal BMI. Fourth, a causal relationship could not be established because of the observational nature of our study, and residual confounding was still possible even after full multivariable adjustment. Last, our participants were all from a homogeneous ethnic group, which might restrict the generalisation of our findings despite extensive evidence among Chinese population.
In summary, we did not find a significant association between TSFA intake and a higher risk of overweight/obesity, whereas a positive association was observed for MCSFA consumption. Higher consumption of 6:0 was related to a lower overweight/obesity risk; however, a positive relationship was detected for 10:0 consumption. Limitation of 10:0 consumption might be conducive to the prevention of overweight/obesity. Increasing the intake of dairy product-sourced 6:0, such as yogurt and milk, may be protective for developing overweight/obesity. More future studies are needed to investigate the effects of specific subcategories of SFA on obesity-related outcomes in China and other countries.
Acknowledgements
This research uses data from the China Health and Nutrition Survey (CHNS). We are grateful for research grant funding from National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, R01 HD30880; P2C HD050924), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, R01 DK104371), the NIH Fogarty D43 TW009077 for financial support for the CHNS data collection and analysis files since 1989, the China-Japan Friendship Hospital, Ministry of Health for support for CHNS 2009, Chinese National Human Genome Center at Shanghai since 2009, and Beijing Municipal Center for Disease Prevention and Control since 2011. The authors also thank the National Institute for Nutrition and Health, China Center for Disease Control and Prevention.
This study was supported by the Zhejiang Provincial National Natural Science Foundation of China (grant number LR18C200001).
J. J. designed the study; F. W., L. M., Y. Z., X. C., P. Z., W. W. and J. W. provided statistical expertise; F. W. and L. M. wrote the manuscript; J. J. had full access to all of the data in the study, and took responsibility for the integrity of the data and the accuracy of the data analysis; and all authors conducted the research, contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content, and read and approved the final manuscript.
The authors declare that they have no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114521002890





