INTRODUCTION
Compromise to the hippocampal memory system very early in life has a marked effect on the development of episodic memory for personally experienced events, at times in the context of intact intellectual ability and at least some capacity for forming new semantic memories for general and personal factual information (Rosenbaum et al., Reference Rosenbaum, Carson, Abraham, Bowles, Kwan, Köhler and Richards2011; Vargha-Khadem et al., Reference Vargha-Khadem, Gadian, Watkins, Connelly, Van Paesschen and Mishkin1997, Vargha-Khadem, Salmond, Friston, Gadian, & Mishkin, Reference Vargha-Khadem, Salmond, Friston, Gadian and Mishkin2003). In the laboratory, this is reflected in impaired recollection of contextual information associated with studied items on tests of recall and recognition memory (Bindschaedler, Peter-Favre, Maeder, Hirsbrunner, & Clarke, Reference Bindschaedler, Peter-Favre, Maeder, Hirsbrunner and Clarke2011; Brandt, Gardiner, Vargha-Khadem, Baddeley, & Mishkin, Reference Brandt, Gardiner, Vargha-Khadem, Baddeley and Mishkin2008; Maguire, Vargha-Khadem, & Mishkin, Reference Maguire, Vargha-Khadem and Mishkin2001). The current study investigates if the spacing effect, a well-established method of ameliorating episodic memory impairment in adult populations, extends to a young adult with impaired episodic memory in relation to congenitally based abnormal development of the hippocampal system.
The hippocampus is highly vulnerable to a host of neurological conditions across the lifespan. In young children, hypoxic-ischemic events and temporal lobe epilepsy are among the most commonly known causes of significantly reduced hippocampal volume, believed to contribute to impaired episodic memory development (Cooper et al., Reference Cooper, Gadian, Jentschke, Goldman, Munoz, Pitts and Vargha-Khadem2015; Rosenbaum et al., Reference Rosenbaum, Gao, Honjo, Raybaud, Olsen, Palombo and Black2014; Vargha-Khadem et al., Reference Vargha-Khadem, Gadian, Watkins, Connelly, Van Paesschen and Mishkin1997). More recent attention has been paid to other brain regions affected in developmental amnesia, primarily to highly interconnected diencephalic structures (Dzieciol et al., Reference Dzieciol, Bachevalier, Saleem, Gadian, Saunders, Chong and Vargha-Khadem2017), with a recent report in the case H.C. of absent mammillary bodies and abnormal rotation of the hippocampus bilaterally, suggestive of a congenital origin (Rosenbaum et al., Reference Rosenbaum, Gao, Honjo, Raybaud, Olsen, Palombo and Black2014). Whether prenatal or postnatal, unlike individuals with adult-onset forms of hippocampal amnesia, developmental amnesics never acquired normal episodic memory before the onset of hippocampal volume loss. This may place them at a disadvantage in terms of opportunities to acquire learning strategies in the context of normal episodic memory. Alternatively, any disadvantage might be offset by the greater propensity for plasticity and reorganization when brain damage occurs early in life, as might have been the case in H.C., who demonstrated intact and long-term benefits of distributed practice in word-list learning (Green, Weston, Wiseheart, & Rosenbaum, Reference Green, Weston, Wiseheart and Rosenbaum2014).
A relevant finding in the memory literature is the spacing effect, referring to the robust finding that long-term retention of information is enhanced when repeated study events are distributed in time compared to when they occur in immediate succession (Ebbinghaus Reference Ebbinghaus1885; Greene, Reference Greene1989). The spacing effect has been replicated using an assortment of laboratory tests, including tests of free recall, cued recall, and recognition (Balota, Duchek, Sergent-Marshall, & Roediger, Reference Balota, Duchek, Sergent-Marshall and Roediger2006; Glenberg, Reference Glenberg1976; Shaughnessy, Zimmerman, & Underwood, Reference Shaughnessy, Zimmerman and Underwood1972) in younger and older neurotypical adults, and in memory-impaired populations (Balota et al., Reference Balota, Duchek, Sergent-Marshall and Roediger2006; Cermak, Verfaellie, Lanzoni, Mather, & Chase, Reference Cermak, Verfaellie, Lanzoni, Mather and Chase1996; Goverover, Arango-Lasprilla, Hillary, Chiaravalloti, & Deluca, Reference Goverover, Arango-Lasprilla, Hillary, Chiaravalloti and Deluca2009). Importantly, it has also been demonstrated at various developmental periods, from early infancy (Rovee-Collier, Hayne, & Colombo, Reference Rovee-Collier, Hayne and Colombo2000) and school age (Sobel, Cepeda, & Kapler, Reference Sobel, Cepeda and Kapler2011) to older adulthood (Balota et al., Reference Balota, Duchek, Sergent-Marshall and Roediger2006).
Ease of administration and universality add to the appeal of distributed practice as a tool for memory enhancement, as it can be implemented in various rehabilitation settings for populations with memory disorders (Balota et al., Reference Balota, Duchek, Sergent-Marshall and Roediger2006; Cermak et al., Reference Cermak, Verfaellie, Lanzoni, Mather and Chase1996; Green et al., Reference Green, Weston, Wiseheart and Rosenbaum2014), and also contribute to the retention of information in educational programs (Kapler, Weston, & Wiseheart, Reference Kapler, Weston and Wiseheart2015; Pashler et al., Reference Pashler, Bain, Bottge, Graesser, Koedinger, McDaniel and Metcalfe2007; Sobel et al., Reference Sobel, Cepeda and Kapler2011) and in the workplace (Kim, Wong Kee You, Wiseheart, & Rosenbaum, under review). Thus, efforts have turned to optimizing the spacing effect in a multitude of real-world applications, including improving memory in memory-impaired populations (Goverover, Arango-Lasprilla, et al., Reference Goverover, Arango-Lasprilla, Hillary, Chiaravalloti and Deluca2009; Goverover, Hillary, Chiaravalloti, Arango-Lasprilla, & DeLuca, Reference Goverover, Hillary, Chiaravalloti, Arango-Lasprilla and DeLuca2009; Sohlberg, Ehlhardt, & Kennedy, Reference Sohlberg, Ehlhardt and Kennedy2005). Although the benefit of distributed practice on memory has been investigated extensively in varied populations, only a few studies have focused on hippocampal amnesic patients. Those that do included individuals who developed amnesia as adults, and none to our knowledge investigated differences in performance as a function of spacing schedule (expanding vs. equal-interval), which might lead to different memory benefits (discussed below).
Cermak et al. (Reference Cermak, Verfaellie, Lanzoni, Mather and Chase1996) investigated the effects of massed versus distributed practice on recognition and recall performance in a group of individuals with adult-onset amnesia due to Korsakoff’s syndrome or encephalitis. Target words were repeated five times, with either five intervening items (lag 5 condition), or zero intervening items (lag 0 condition). Thus, these two conditions differed both in terms of the number of intervening items and the corresponding time delay associated with the presentation of the intervening items. The results indicated that amnesic patients benefited from spaced repetition as much as the healthy control participants on both the recognition and recall tasks. This experiment suggests that the spacing effect can be supported in amnesic individuals with medial temporal lobe (MTL) damage that includes the hippocampus.
This finding is at odds, however, with past functional magnetic resonance imaging (fMRI) research showing that the magnitude of activity and connectivity in the MTL was greater during repeated study of items after a long versus short delay, and that MTL connectivity following a long delay was predictive of subsequent paired-associate recall performance (Vilberg & Davachi, Reference Vilberg and Davachi2013). Other studies have also shown reduced repetition suppression in MTL regions, including parahippocampal gyrus and both the left and right posterior hippocampus, when repetitions are spaced compared to massed in recognition (Xue et al., Reference Xue, Mei, Chen, Lu, Poldrack and Dong2011) and continuous recognition (Brozinsky, Yonelinas, Kroll, & Ranganath, Reference Brozinsky, Yonelinas, Kroll and Ranganath2005) paradigms.
In the context of implementing the spacing effect, an important question is how, or according to what schedule, should repeated study events be distributed? Distributed practice can be spaced out according to an equal-interval schedule, as used in the study by Cermak and colleagues (Reference Cermak, Verfaellie, Lanzoni, Mather and Chase1996) described above, or an expanding schedule (Balota et al., Reference Balota, Duchek, Sergent-Marshall and Roediger2006; Karpicke & Roediger, Reference Karpicke and Roediger2007, Reference Karpicke and Roediger2010). An equal-interval schedule involves equally spread out study events, whereas an expanding spacing schedule involves gradually increasing the intervals between each subsequent exposure to the study item. These spacing conditions can be contrasted with a massed study schedule, in which repeated study events of a given item occur in immediate succession.
Mixed findings have been reported in the literature regarding whether an equal-interval schedule or expanding schedule is more beneficial for retention. Whereas some studies do not show a difference between the two types of spacing schedules (e.g., Balota et al., Reference Balota, Duchek, Sergent-Marshall and Roediger2006; Carpenter & DeLosh, Reference Carpenter and DeLosh2005; Cull, Reference Cull2000; Karpicke & Bauernschmidt, Reference Karpicke and Bauernschmidt2011), other studies have demonstrated a larger benefit from expanding over equal-interval spacing schedules in specific contexts (Gerbier & Keonig, Reference Gerbier and Koenig2012; Karpicke & Roediger, Reference Karpicke and Roediger2007; Nakata, Reference Nakata2015), and yet other studies have indicated benefits of schedules where spacing was decreased with each subsequent study session (Küpper-Tetzel, Kapler, & Wiseheart, Reference Küpper-Tetzel, Kapler and Wiseheart2014). However, no known studies have examined whether people with developmental amnesia benefit more from an expanding relative to an equal-interval spacing schedule. Moreover, to our knowledge, only one study has even investigated the spacing effect in a developmental amnesic person (Green et al., Reference Green, Weston, Wiseheart and Rosenbaum2014, who studied H.C.).
In the first of two experiments, Green and colleagues (Reference Green, Weston, Wiseheart and Rosenbaum2014) assessed the impact of distributed practice on H.C.’s memory performance in a free recall, verbal learning paradigm. The results showed that the largest spacing lag (24 intervening items between repeated study events) increased H.C.’s recall performance by 20% compared to the massed (lag 0) condition. The second experiment used a multi-day, paired-associate, learning-to-criterion, verbal learning spacing paradigm, in which memory was assessed after 1 week. H.C. performed 40% better in the spaced review, compared to the massed, condition. However, H.C. took four times as many trials to reach criterion compared with controls, suggesting that her spacing effect was achieved via abnormal means and might not be reproduced at this magnitude without intensive training conditions. That H.C.’s training was labourious compared to controls on the paired-associate, learning-to-criterion task reported in Green et al. (Reference Green, Weston, Wiseheart and Rosenbaum2014) may relate to the abovementiond finding that the spacing effect is associated with MTL activity in the context of paired-associate recall (Vilberg & Davachi, Reference Vilberg and Davachi2013).
The aim of the present study was to further investigate the spacing effect in developmental amnesia by continuing to profile the impact spacing has on H.C.’s memory performance. To complement the previous investigation on the spacing effect in H.C. (Green et al., Reference Green, Weston, Wiseheart and Rosenbaum2014), the present study investigated whether an expanding or equal-interval spacing schedule would result in better memory performance in H.C. A second aim of the present study was to determine the impact of spacing, if any, on H.C.’s memory performance in the context of a continuous recognition paradigm. Although H.C. has benefited from spacing on tests of free recall and paired-associate recall, fMRI evidence of hippocampal activation associated with the spacing effect in continuous recognition (Brozinsky et al., Reference Brozinsky, Yonelinas, Kroll and Ranganath2005) suggests that she might not benefit in this instance. Given the potential of the spacing effect as an intervention technique for individuals with compromised episodic memory in relation to hippocampal system compromise, it is important to know when it will be effective.
METHODS
Design
The study had a two (group: H.C. vs. controls) × three (spacing schedule: massed, equal-interval, expanding) mixed factorial design. Both groups were presented with English nouns, as described further below, and target words were presented six times according to one of the three spacing schedules, the independent variable. The spacing schedules were adapted from a previous study by Balota et al. (Reference Balota, Duchek, Sergent-Marshall and Roediger2006) that investigated the spacing effect in healthy young and older adults, as well as individuals with dementia of the Alzheimer’s type. In the study by Balota et al., targets in each of the spacing conditions (massed, equal-interval, and expanding) were initially presented three times in immediate succession to help ensure that all participant groups successfully encode the words. Then, after the third presentation of the target, the spacing schedules differed. Here we used the same approach of presenting targets three times in immediate succession in each of the spacing conditions to help ensure that H.C. and control participants successfully encoded the words.
Following Balota et al., a learning-to-criterion requirement was not implemented in the present study. After the third presentation of the target, the spacing schedules differed. In the massed schedule, the target was presented three more times in immediate succession. Thus, the first presentation of the target was followed by five massed repetitions (massed: 0-0-0-0-0). The equal-interval schedule consisted of the initial presentation of the target, followed by two massed repetitions, and then three equally spaced repetitions with three intervening events (equal interval: 0-0-3-3-3). The expanded schedule consisted of the initial presentation of the target, followed by two massed repetitions, and then three expanding repetitions with one, three, and five intervening events (expanded: 0-0-1-3-5). The dependent variable was participants’ free recall and recognition performance, as described further below.
Materials
The word pool used in the current study consisted of 175 one-syllable nouns taken from the MRC Psycholinguistic Database (Coltheart, Reference Coltheart1981), with imageability and Kucera-Francis frequency (Kucera & Francis, Reference Kucera and Francis1967) ratings of 432 to 667 and 1 to 967, respectively. The nouns were randomly assigned to one of five lists, with each list having a total of 35 unique words. In each list, 8 words served as primacy buffers and 8 words served as recency buffers. Each study list consisted of 9 target words: 3 target words were presented under each of the 3 spacing schedules. Targets were counterbalanced across participants. The remaining 10 words in the list served as filler words included throughout the study list to ensure the proper spacing of the items.
Participants
H.C. is a right-handed woman who was 27 years old at the time of testing. She was born prematurely in gestational week 32 and was assumed to have experienced hypoxia soon after birth (Olsen et al., Reference Olsen, Palombo, Rabin, Levine, Ryan and Rosenbaum2013; Rosenbaum et al., Reference Rosenbaum, Gao, Honjo, Raybaud, Olsen, Palombo and Black2014), resulting in impaired episodic memory that was first noted when she was 4 years of age. Analysis of MRI scans taken in 2012 indicates relatively focal changes to the hippocampus and structures closely connected to it (Olsen et al., Reference Olsen, Palombo, Rabin, Levine, Ryan and Rosenbaum2013), which has been found in other cases of developmental amnesia (Dzieciol et al., Reference Dzieciol, Bachevalier, Saleem, Gadian, Saunders, Chong and Vargha-Khadem2017). H.C.’s hippocampal volume is reduced by 30% bilaterally, and this reduction is generally consistent across subfields. However, a more recent study suggests that H.C.’s memory impairment and neuroanatomical findings may be congenital in origin. A detailed examination of H.C.’s hippocampal memory system displayed agenesis of the mammillary bodies, rerouting of the fornices, and hippocampal malrotation (Rosenbaum et al., Reference Rosenbaum, Gao, Honjo, Raybaud, Olsen, Palombo and Black2014). The hippocampal malrotation entails incomplete infolding of the hippocampus, appearing abnormally rounded in shape. The absence of mammillary bodies is significant because the mammillary bodies begin to form in fetal development at 9–10 weeks, and hippocampal rotation begins at 13 weeks (Rosenbaum et al., Reference Rosenbaum, Gao, Honjo, Raybaud, Olsen, Palombo and Black2014). H.C. has been studied extensively and her memory impairments are well-documented. Her neuropsychological profile is displayed in Table 1.
Table 1 Neuropsychological profile of H.C.
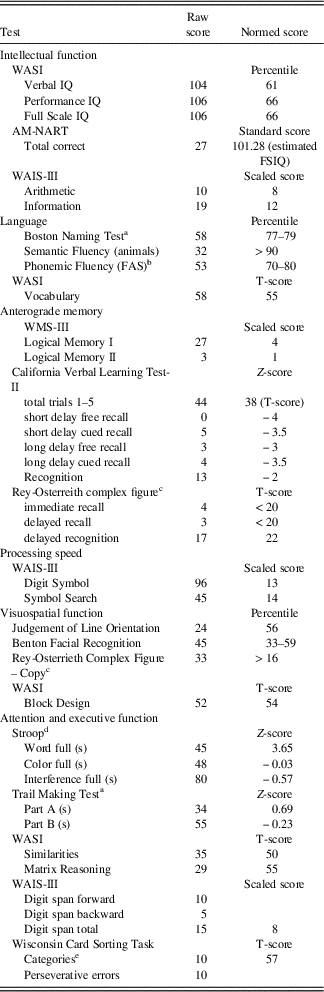
Note. AM-NART, American National Adult Reading Test; WASI, Wechsler Abbreviated Scale of Intelligence; WAIS-III, Wechsler Adult Intelligence Scale–III. Additional results of neuropsychological testing reported in Rosenbaum et al. (Reference Rosenbaum, Carson, Abraham, Bowles, Kwan, Köhler and Richards2011), Hurley et al. (2012), and Rabin et al. (2012).
a Spreen & Strauss (1998).
b Tombaugh, Kozak, & Rees (1996)
c Meyers & Meyers (1996).
d In-house unpublished normative data.
e Heaton et al. (1993).
H.C. demonstrates impaired episodic recollection and intact familiarity processes (Rosenbaum et al., Reference Rosenbaum, Carson, Abraham, Bowles, Kwan, Köhler and Richards2011), and has difficulty remembering personal and public events. Unlike control participants and individuals with Korsakoff syndrome in whom mammillary bodies are reduced (Cermak, Butters, & Moreines, Reference Cermak, Butters and Moreines1974), H.C.’s anterograde recognition memory performance does not appear to benefit from semantic encoding of verbal study material. H.C.’s memory performance does, however, benefit from distributed, compared to massed, repetition (Green et al., Reference Green, Weston, Wiseheart and Rosenbaum2014), as described above. She has also learned to compensate for her impaired episodic memory by using devices to help her remember events and goals. H.C. completed high school and 2 years of college, and has successfully held several jobs. In the present study, H.C.’s performance was compared to that of 10 healthy control participants who were matched to her in terms of age and education (7 females; mean age, 25.2 years; SD, 1.13; range, 24–27 years; mean education, 15.2 years; SD, 1.13, range; 13–16 years). Participants were fluent in English and had no known history of psychiatric or neurological illness. All participants gave written informed consent and received monetary compensation for their participation, as approved by the Baycrest and York University ethics committees.
Procedure
Each participant took part in one experimental session. Throughout the session, participants were seated in front of an LCD monitor connected to a computer. The experimenter was seated near the participant in a position where they were able see the LCD monitor and use the keyboard to record the participants’ verbal responses. At the start of the session, both written and verbal instructions were given for the experimental task, and participants completed a practice run to ensure that they understood the task before starting the experiment.
For H.C., the session consisted of five study/test cycles, which each corresponded to one of the five word lists. To avoid ceiling effects in controls and increase the difficulty of this task, the session consisted of one study/test cycle, which included all five words lists. Each study/test cycle consisted of a study phase and test phase. During the study phase, participants performed a continuous recognition task, with stimuli presented using E-Prime software (Version 1.1, www.neurobs.com). Words were presented sequentially in black uppercase letters on a white background for 4 s. After the presentation of each word, a question screen was presented with the statement, “Has this word previously been shown?”, in black font on a white background. This question was presented for 15 s, followed by an instruction to verbally state with a “yes” or ‘no” response if the word had been seen previously. The experimenter then entered “Y” for a “yes” response and “N” for a “no” response using the keyboard. A black fixation cross of 0.5 s was placed in between all words and question screens. Figure 1 depicts the temporal sequence of the task.
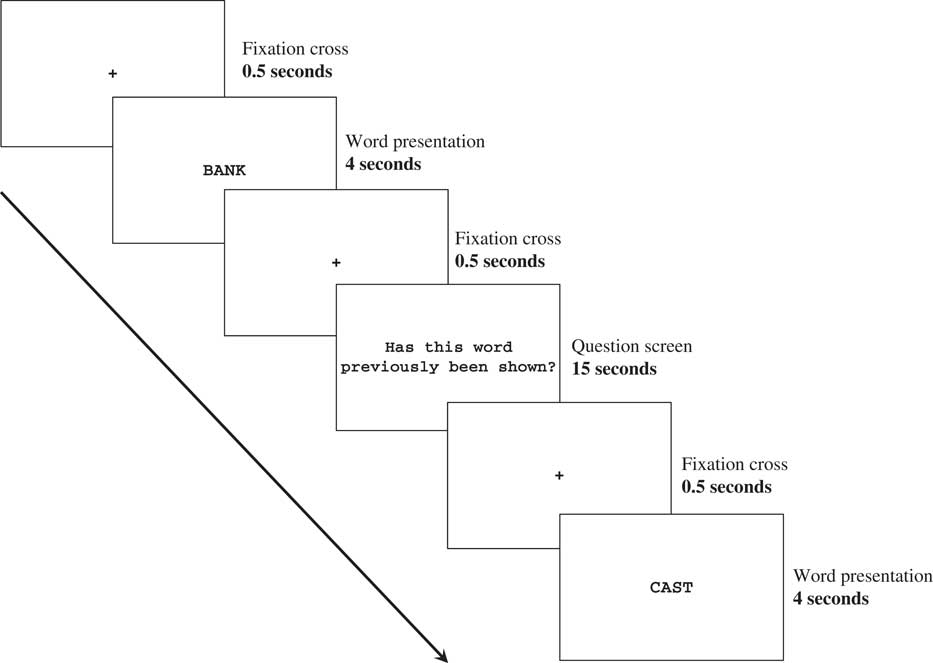
Fig. 1 Temporal sequence of the study phase and the recognition memory test.
During the test phase, participants were first tested on free recall and given the equivalent of 1 min per study list to recall as many words as they could from the study list. Thus, whereas H.C. was given 1 min during each of the five study/test cycles, controls were given 5 min for the 1 study/test cycle they completed. Next, participants were given a recognition test, which required them to judge whether they had seen the word during the study phase. Similar to the study phase, words were presented sequentially in black uppercase letters on a white background for 4 s. After the presentation of each word, a question screen was presented with the statement, “Has this word previously been shown?”, in black on a white background. This question was presented for 15 s, followed by an instruction to verbally state with a “yes” or “no” response if the word had been seen previously. The experimenter then entered “Y” for a “yes” response and “N” for a “no” response using the keyboard. For each of H.C.’s 5 study/test cycles, the words presented during the recognition test consisted of the 9 targets in the corresponding list and six distractors. Controls were tested on recognition using the same overall number of targets and distractors presented to H.C.: 45 targets and 30 distractors. The entire experiment lasted approximately 60 min.
Analyses
Participants’ free recall performance was scored according to the proportion of words that were correctly recalled at test. Recognition performance was assessed by deriving hit rate minus false alarm rate scores. The recall and recognition data were analyzed separately using repeated-measures analyses of variance (ANOVAs), with spacing schedule as the sole factor. A post hoc modified t test, designed for testing a single case against a control group of small to moderate size (Crawford & Howell, 1998), was then used to compare H.C.’s memory performance to matched controls.
RESULTS
Free Recall
H.C.’s free recall performance (proportion correct) was at floor, as seen in Figure 2. She recalled one, two, and zero target words from the massed, equal-interval, and expanding spacing schedules, respectively. Since H.C. demonstrated floor effects for free recall, her data were not submitted to any statistical analyses. Free recall performance for healthy control participants is also presented in Figure 2, which shows that recall was highest for the expanding spacing schedule (M=.240; SD=.147) followed by the equal interval (M=.220; SD=.114) and massed spacing (M=.187; SD=.151) schedules, respectively. However, the results of a repeated-measures ANOVA conducted on the control data did not reveal a significant effect of spacing [F(2,18)=.787; p=.47].
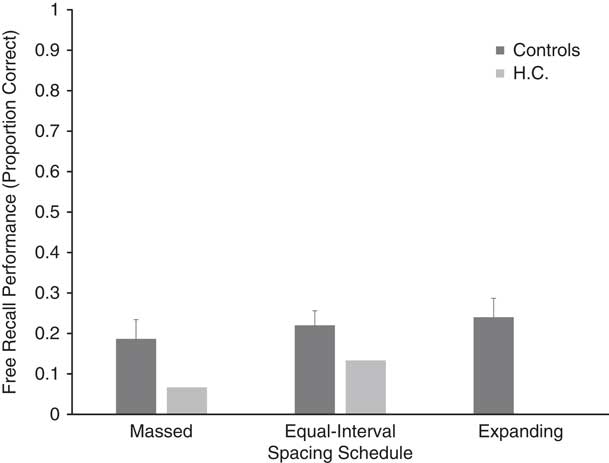
Fig. 2 Free recall performance (proportion correct) for HC and controls. Error bars=standard error.
Recognition
Figure 3 displays corrected recognition scores (hit rate - false alarm rate) for H.C. and healthy control participants. Recognition performance for the healthy controls was lowest in the massed spacing schedule (M=.610; SD=.179), followed by the equal-interval (M=.703; SD=.193) and expanding (M=.710; SD=.166) spacing schedules, respectively. The results of the repeated-measures ANOVA conducted on the control data indicated a significant effect of spacing [F(2,18)=5.999; p=.01; η p 2=.4]. Bonferroni-corrected post hoc tests showed a significant difference between massed and equal-interval schedules (p=.029), and between massed and expanding schedules (p=.035). A significant difference was not detected between equal-interval and expanding conditions (p=1).
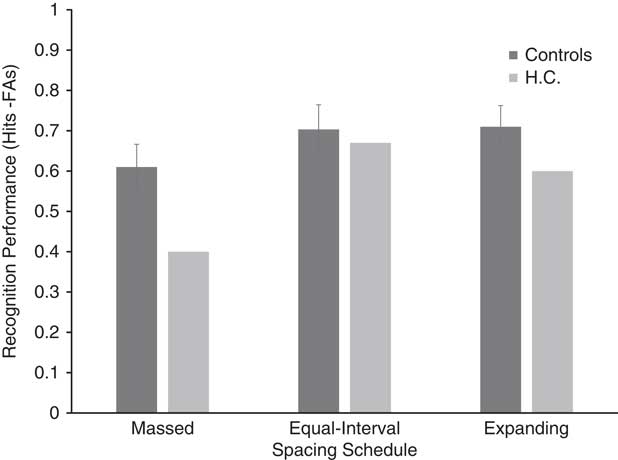
Fig. 3 Corrected recognition performance (hit rate – false alarm rate) for HC and controls. Error bars=standard error.
H.C.’s recognition performance was lowest in the massed spacing schedule, followed by the expanding and equal-interval spacing schedules, respectively. Although the results shown in Figure 3 may seem suggestive of disproportionate differences between H.C. and the control group in the massed spacing schedule compared to the two spacing schedules, the results of the modified Crawford t test did not reveal any significant differences between H.C.’s recognition performance compared to that of controls across the three spacing schedules [massed t(10)=1.123; p=.288; equal-interval t(10)=0.164; p=.872; and expanding t(10)=0.633; p=.541].
DISCUSSION
The present study provides evidence of a spacing effect in the developmental amnesic person H.C., whereby recognition of studied words benefited when they were repeated with intervening words between repetitions (spaced) compared to when they were repeated in immediate succession (massed). These results are in line with and extend those reported in previous studies (Balota et al., Reference Balota, Duchek, Sergent-Marshall and Roediger2006; Green et al., Reference Green, Weston, Wiseheart and Rosenbaum2014) by demonstrating a spacing effect in H.C. using a continuous recognition paradigm, which is associated in fMRI with reduced repetition suppression within the hippocampus/MTL when repetitions are spaced compared to massed (Brozinsky et al., Reference Brozinsky, Yonelinas, Kroll and Ranganath2005). Although a clear spacing effect was seen in both healthy controls and a developmental amnesic person, neither showed a difference in benefit between equal-interval and expanding schedules, which is in line with past studies using similar study designs (Balota et al., Reference Balota, Duchek, Sergent-Marshall and Roediger2006; Cull, Reference Cull2000; Carpenter & DeLosh, Reference Carpenter and DeLosh2005; Logan & Balota, Reference Logan and Balota2008).
However, there is some evidence in the literature suggesting that the preferred spacing schedule (expanding vs. equal-interval) may vary based on retention interval (Karpicke & Roediger, Reference Karpicke and Roediger2007; Logan & Balota, Reference Logan and Balota2008), study task (Gerbier & Koenig, Reference Gerbier and Koenig2012; but also see Cull, Reference Cull2000), and the amount of spacing separating early retrieval attempts, whereby expanded retrieval benefits are more likely to occur for nonsystematic expansion (e.g., 0-1-6-8-10) compared to systematic expansion (e.g., 0-2-4-6-8) when there is an increased likelihood of successful early retrieval events (Maddox, Balota, Coane, & Duchek, Reference Maddox, Balota, Coane and Duchek2011). Future research should continue to investigate whether these factors impact the benefit of distributed practice on memory in both amnesic and neurotypical populations.
The finding that H.C., and amnesic participants in general, demonstrate a spacing effect lends itself to interesting discussion of one of the leading account of this effect: the study-phase retrieval hypothesis. According to this hypothesis, repeated occurrences of a target automatically serve as retrieval cues for its previous occurrence, which is assumed to be critical for repeated practice to be effective (Raaijmakers, Reference Raaijmakers2003, Thios & D’Agostino, Reference Thios and D’Agostino1976). The benefit of repetition is elevated further when retrieval is successful in spaced conditions, because the spacing between repetitions places greater demands on retrieval processes compared to when the repetitions are massed. This desirable retrieval difficulty is assumed to benefit the memory trace more compared to when retrieval is easier, partly because it is hypothesized to slow the rate of forgetting (Rovee-Collier, Reference Rovee-Collier1995; Toppino & Gerbier, 2014). Thus, according to the study-phase retrieval account, one might expect that hippocampal amnesic participants would benefit less than controls from study-phase retrieval, due to retrieval difficulty demonstrated by the former group. However, as discussed further below, it is possible that spaced repetition engages strategic retrieval processes that may support longer-lasting representations in memory and are upheld by the prefrontal cortex (PFC), which is intact in these patients.
The spacing effect demonstrated by H.C. in the present study may have been supported by intact MTL regions. Whereas past studies have shown perirhinal cortex to be engaged during item recognition, the hippocampus, fornix, and mammillary bodies have been implicated in recall (Aggleton & Brown, Reference Aggleton and Brown1999, Tsivilis et al., Reference Tsivilis, Vann, Denby, Roberts, Mayes, Montaldi and Aggleton2008). Despite the absence of H.C.’s mammilliary bodies and anterior fornices as well as atrophy of the anterior thalamic nuclei bilaterally and hippocampal malrotation/volume loss, her perirhinal cortex, parahippocmpal cortex, and entorhinal cortex remain intact (Olsen et al., Reference Olsen, Palombo, Rabin, Levine, Ryan and Rosenbaum2013). Thus, it is not completely surprising that H.C.’s free recall performance was at floor, whereas her recognition performance was comparable to that of control participants across each of the spacing conditions (massed, expanding, equal-interval). The results of the present study aligns with the study-phase retrieval hypothesis, in that in contexts that are more conducive to H.C.’s successful retrieval (e.g., recognition tests), spaced versus massed repetitions are more beneficial for subsequent memory performance.
Interestingly, however, H.C. previously showed a spacing effect in the context of a free recall paradigm (Green et al., Reference Green, Weston, Wiseheart and Rosenbaum2014), which may seem inconsistent with H.C.’s floor performance on free recall in the present study. This apparent discrepancy may be due to differences in the paradigms used in the two studies. Green and colleagues (Reference Green, Weston, Wiseheart and Rosenbaum2014) had participants encode a series of words in a list, allotting 1.5 s of study time per word. In the current study, participants encoded a series of words in a list with a time allowance of 4 s per word. Additionally, immediately after the presentation of each word, participants judged whether they had already seen the word in the list. Thus, the amount of time that transpired by the end of a study list, as well as the encoding task itself, differed across both studies. Another major difference between the two studies was the used spacing schedules: whereas the current study used massed, equal-interval, and expanding spacing schedules, with each target repeated five times, Green et al. (Reference Green, Weston, Wiseheart and Rosenbaum2014) used lags of 0, 1, 6, and 24 intervening items, with each target presented twice under one of the four lag conditions. Together, these paradigm differences may help account for H.C.’s seemingly inconsistent free recall performance across the two studies.
One might also wonder whether testing participants on free recall before recognition impacted the results of the latter test. The relation between recall and recognition has been studied extensively, and although it was once believed that recognition is automatic and independent of recall processes, past studies have shown that recall and recognition are related and associated with comparable retrieval processes in healthy and amnesic populations (Haist, Shimamura, & Squire, Reference Haist, Shimamura and Squire1992; Tulving & Thomson, Reference Tulving and Thomson1971). However, these findings should be considered in the context of the abovementioned findings that specific brain structures have been differentially associated with recall and recognition (Aggleton & Brown, Reference Aggleton and Brown1999, Tsivilis et al., Reference Tsivilis, Vann, Denby, Roberts, Mayes, Montaldi and Aggleton2008).
Moreover, the phenomenon of “recognition failure of recallable words” (Tulving & Thomson, Reference Tulving and Thomson1973) demonstrates that it is possible for successful recall to be paired with unsuccessful recognition of a target item. Thus, although recall and recognition seem to be associated with comparable retrieval processes, the neural findings, combined with demonstrated dissociations between recall and recognition performance, suggest that these forms of retrieval do not overlap entirely. It is not clear whether and to what extent the preceding free recall test impacted the results of the recognition test in the present study, particularly in light of the finding that H.C. showed floor effects for recall.
Another explanation for the spacing effect demonstrated by H.C. could be related to neocortical compensatory mechanisms, through which H.C. achieved similar levels of recognition performance as control participants but via alternative means. Past fMRI work has shown that Jon, another person with developmental amnesia who experienced 50% volume loss in his hippocampi, demonstrated increased activity in the same brain regions as control participants during a retrieval task, in addition to regions that were not activated in the healthy control participants (Maguire et al., Reference Maguire, Vargha-Khadem and Mishkin2001). Similarly, H.C. has also demonstrated activity in several extra-hippocampal brain regions to a greater extent than that found in control participants on tasks that required remembering and imagining (Rabin, Olsen, Gilboa, Buchsbaum, & Rosenbaum, Reference Rabin, Olsen, Gilboa, Buchsbaum and Rosenbaum2016). However, functional connectivity between these extra-hippocampal and hippocampal regions did not differ between H.C. and control participants. Thus, it is difficult to draw conclusions about compensatory mechanisms.
One could speculate, however, on the importance of cognitive control processes, such as those relating to strategic retrieval in the spacing effect. Of interest, past fMRI studies have shown that reduced repetition suppression in the left PFC leads to better subsequent memory performance (Callan & Schweighofer, 2010; Wagner, Maril, & Schacter, 2000). Along these lines, it could also be the case that the spacing effect that Cermak and colleagues (Reference Cermak, Verfaellie, Lanzoni, Mather and Chase1996) showed in adult-onset amnesics was supported by prefrontally mediated strategic retrieval processes that remained intact in these patients. Although speculative, it is possible that MTL structures work together with the PFC to support the spacing effect, and that the PFC supports the effect when hippocampal function is compromised.
A general theoretical framework, referred to as Working With Memory (WWM), describes the contribution of the frontal lobes to strategic memory processing (Moscovitch, Reference Moscovitch1992; Moscovitch & Winocur, Reference Moscovitch and Winocur1992). Generally, the frontal lobes are thought to enrich the memory trace and facilitate memory processes. For example, there is evidence that the frontal lobes keep track of time with respect to temporal order effects (McAndrews & Milner, Reference McAndrews and Milner1991; Milner, Petrides, & Smith, Reference Milner, Petrides and Smith1985; Moscovitch & Melo, Reference Moscovitch and Melo1997) and enahnce information with semantic organization during encoding (McAndrews & Milner, Reference McAndrews and Milner1991). Additionally, the dorsolateral PFC has been implicated in setting the goals of the retrieval task and beginning the retrieval search process, thereby establishing a retrieval mode (Lepage, Ghaffar, Nyberg, & Tulving, Reference Lepage, Ghaffar, Nyberg and Tulving2000; Rugg &Wilding, Reference Rugg and Wilding2000) by preparing an individual to engage in retrieval. Moreover, the posterior ventromedial PFC and frontal pole are thought to signal acceptance and rejection, respectfully, of a signal resulting from an activated memory trace based on an intuitive “felt rightness” (Moscovitch & Winocur, 2002). Although interactions between MTL and PFC cannot be determined based on a behavioural study of a single case, it is an important direction for future patient research.
The present study provides additional insight into the spacing effect in H.C., a young adult with developmental amnesia. Along with the many benefits afforded by case studies (for discussion see Rosenbaum, Gilboa, & Moscovitch, Reference Rosenbaum, Gilboa and Moscovitch2014), there are additional drawbacks, including limitations in terms of the generalizability of the results due to the small sample size. Another potential limitation of the present study is the use of the modified Crawford t test to compare H.C.’s performance to that of controls, as it has been shown to be conservative (Crawford & Howell, 1998), making it more prone to Type II errors. An alternative approach would have been to conduct a 2 (group: H.C., controls) × 3 (spacing condition) between-within repeated measures ANOVA using H.C.’s recognition scores from each of the five lists she completed to calculate a mean and variance values. Alternatively, H.C.’s overall mean score could have been paired with the variance of the control group to conduct the ANOVA. However, this would lead to the limitation of assuming that H.C.’s variance is equivalent to that of the control group. Yet another approach, described in Green et al. (Reference Green, Weston, Wiseheart and Rosenbaum2014), is to resample H.C.’s data to create an artificial group. The corresponding limitation, however, would be the lack of independent observations. Future research should be conducted to test whether the findings of the present study are replicable in other (adult) developmental amnesics.
ACKNOWLEDGMENTS
We are grateful to H.C. and her family for their continued contributions to memory research. This research was supported by grants from the Canadian Partnership for Stroke Recovery and National Sciences and Engineering Research Council of Canada to R.S.R. and a fellowship from the Canadian Partnership for Stroke Recovery to A.S.N.K. The authors declare no conflicts of interest.






