Introduction
Microorganisms preside in every ecological niche on Earth: from the tropics to the poles, from underground mines and oil fields to the stratosphere and mountain ranges; from deserts to the Dead Sea, and hot springs to underwater hydrothermal vents (e.g. Junge et al. Reference Junge2002; Nagy et al. Reference Nagy, Perez and Garcia-Pichel2005; McCliment et al. Reference McCliment2006; Soo et al. Reference Soo2009). Microbes dominate the flux of energy and biologically important chemical cycles in the world's oceans and are estimated to have a biomass five to ten times that of all multicellular marine organisms (Pomeroy et al. Reference Pomeroy2007). The number of bacteria alone is estimated to be 1029 (Whitman et al. Reference Whitman, Coleman and Wiebe1998), which is more than the 1024 stars in the observable Universe (van Dokkum & Conroy Reference van Dokkum and Conroy2010). Considering that microbes existed on Earth ~3 Ga before the evolution of land plants (Runnegar Reference Runnegar and Schopf1992) and that animals appeared a mere 600 million years ago, the diversity and metabolic plasticity of microscopic life is not considered surprising (Staley & Gosink Reference Staley and Gosink1999). However, from a molecular perspective, this assemblage harbours much that remains unknown; not only are these cells a potential source of useful genes for medicine and biotechnology, but unravelling the taxonomic complexities of prokaryotes is considered the key to understanding the process of evolution (Pace Reference Pace1997; Pedrós-Alió Reference Pedrós-Alió2006). Microorganisms are no less relevant in the consideration of extra-terrestrial life within our Solar System and beyond. If it is assumed that abiogenesis results in cellular life – that proto-biochemistry can be considered a ‘cosmic imperative’ regardless of variation in biogenic elements (see de Duve Reference de Duve1995; Deamer & Weber Reference Deamer and Weber2010; Stüeken et al. Reference Stüeken2013) – then the prokaryotic cell is potentially a universal blueprint for life. Furthermore, if life on Earth can be considered a valid analogue, then the origin and subsequent development of any extra-terrestrial ecosystem will, to a greater or lesser extent, be characterized by its microbial community. Alternatively, if physicochemical conditions on extra-terrestrial worlds such as Mars or Europa cannot support the emergence of life, in any form, then the clues to habitability revealed by Earth's microbial consortia may be considered largely irrelevant (Dartnell Reference Dartnell2011).
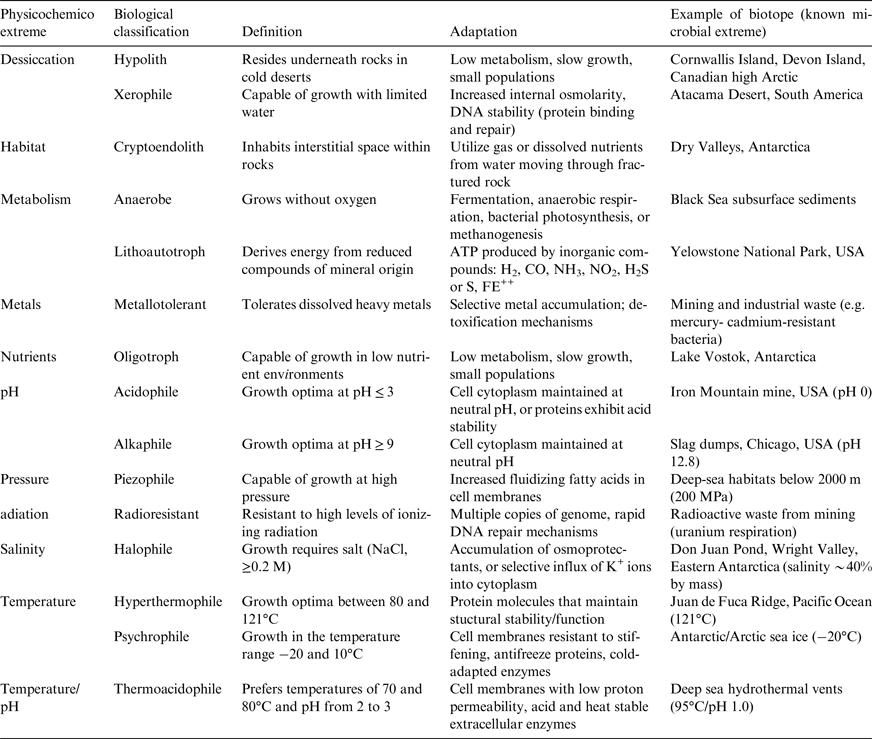
Defining the requirements and physiological limits to habitability in Earth's most extreme environments has provided a significant stimulus for the field of astrobiology (e.g. Hart Reference Hart1978; Hoyle et al. Reference Hoyle1982; Kasting et al. Reference Kasting, Whitmire and Reynolds1993; Gaidos et al. Reference Gaidos, Nealson and Kirschvink1999; Chyba & Phillips Reference Chyba and Phillips2001; Martin et al. Reference Martin, Baross, Kelley and Russell2008; McKay Reference McKay2014). Although a consensus on the origin, timing and specific location for the emergence of life on Earth is currently lacking (Lederberg Reference Lederberg1960; Davis & McKay Reference Davis and McKay1996; Chyba et al. Reference Chyba, Whitmire, Reynolds, Mannings, Boss and Russell2000), organisms that have adapted to physiological extremes are thought to provide insight into the habitability of extra-terrestrial systems (Hoover & Pikuta Reference Hoover, Pikuta, Bej, Aislabie and Atlas2009; McKay Reference McKay2014). Here, we adopt the binary definition of habitability recently coined by Cockell et al. (Reference Cockell2016) whereby a habitat is ‘an environment capable of supporting the activity of at least one known organism’. Although conservative, this construct is useful because it is not necessary to define ‘life’ and speculating on the capacities of unknown organisms is avoided. Closely coupled with the existence of habitable conditions is the extent of habitability; this provides context for the type of organisms present and the time period over which they can be sustained (Cockell et al. Reference Cockell2016). An organism that is capable of growth and reproduction within an environmental niche deemed detrimental to most life on Earth is classified as an extremophile. The biological interpretation of ‘extreme’ requires caveats (for a review see Rothschild & Mancinelli Reference Rothschild and Mancinelli2001), but the concept is particularly useful in linking adaptive responses and survival thresholds to physical (e.g. pressure, temperature and radiation) and geochemical (e.g. pH, salinity and desiccation) extremes. An overview of the key abiotic stressors, biological nomenclature and adaptive responses is provided in Table 1. Life on Earth requires two forms of energy: thermal energy for melting water and chemical energy for the maintenance and regulation of life processes (Hand et al. Reference Hand, Carlson and Chyba2007). Of these two fundamental requirements, it is the presence of liquid water that is most likely to limit habitable extra-terrestrial environments because its occurrence in our Solar System is limited (McKay Reference McKay2014). Life on Earth requires a fluid medium that dissolves molecules and facilitates the three-dimensional (3D) shape and catalytic function of enzymes (Chyba et al. Reference Chyba, Whitmire, Reynolds, Mannings, Boss and Russell2000).
Table 1. Classification and examples of extremophiles
Examples of previously unexpected microbial ecosystems that are of relevance to astrobiology include deep-sea sulphide-rich hydrothermal vents (Corliss et al. Reference Corliss1979; Spiess et al. Reference Spiess1980), deep-sea methane (CH4)- and hydrogen-rich vents (Kelley et al. Reference Kelley2001), groundwater some 2.8 km below the Earth's surface (Lin et al. Reference Lin2006), microbes dwelling within basalt rock (Stevens & McKinley Reference Stevens and McKinley1995), sediments at Challenger Deep (~10 900 m) in the Mariana Trench (Glud et al. Reference Glud2013), Lake Vida, an ice-covered Antarctic lake that has been isolated for thousands of years (Murray et al. Reference Murray2012) and Arctic cryopeg brines, which have been geologically isolated in permafrost for hundreds to millions of years (Gilichinsky et al. Reference Gilichinsky, Rivkina, Shcherbakova, Laurinavichuis and Tiedje2003; Colangelo-Lillis et al. Reference Colangelo-Lillis, Eicken, Carpenter and Deming2016). Invariably, it is members of the Archaea and Bacteria that are found in these environments, but a range of eukaryotes, polar diatoms and tardigrades for example, also exhibit robust responses to biologically challenging environments. Of particular relevance to astrobiology are polyextremophiles, organisms that are capable of tolerating more than one physiochemical extreme (Rothschild & Mancinelli Reference Rothschild and Mancinelli2001). The celestial bodies within our Solar System, which could potentially support life due to the presence of water are highlighted in Table 2, and a number of these are targets in the Ocean Worlds Exploration Program recently proposed by NASA (Anderson Reference Anderson2016). Here, we review the suite of adaptations exhibited by polyextremophiles that inhabit sea ice at Earth's polar regions. These dynamic ecosystems are among the most relevant Earth-based analogues for considering life on ice-associated ocean worlds (Deming & Eicken Reference Deming, Eicken, Sullivan and Baross2007). In a previous review, Deming & Eicken (Reference Deming, Eicken, Sullivan and Baross2007) discussed the characteristics of liquid water in ice and how they influence the abundance and activity of microbial life. The primary aim of this review is to highlight that sea-ice microbes would be capable of occupying specific niches on the moons of Europa and Enceladus.
Table 2. Celestial bodies within the Solar System that could potentially support life due to the inferred presence of subsurface oceans
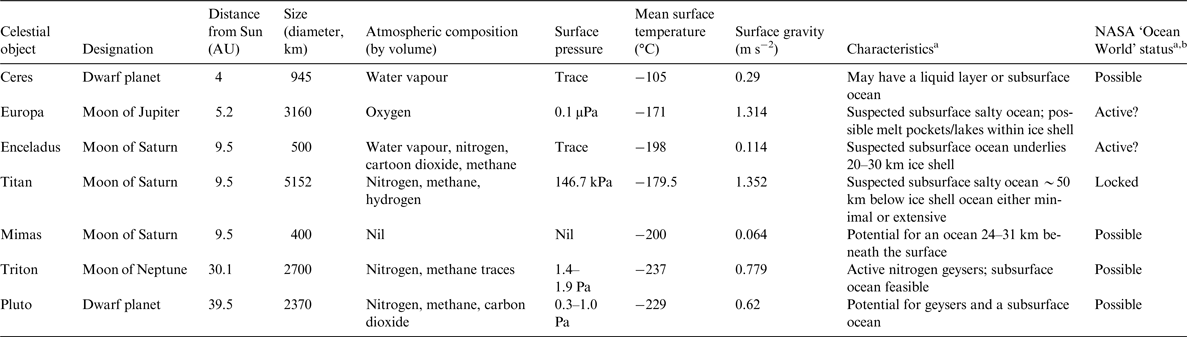
a JPL Infographics: http://www.jplnasa.gov/infographics/infographic.view.php?id=11262.
b Acitve: dynamic ocean that could support life; Possible: evidence of an ocean, biological potential unknown; Locked: trapped ocean unlikely to support life.
Sea ice
Although it is mostly an ephemeral habitat, seasonal sea ice covers up to 26 × 106 km2 of the Earth's surface (Parkinson Reference Parkinson2014) and represents one of the planet's major biomes (Thomas & Dieckmann Reference Thomas and Dieckmann2002a; Arrigo Reference Arrigo2014). Biological elements, including viruses, bacteria and microalgae are initially scavenged from the water column during ice formation, and are then confined to a labyrinth of pores and brine channels that vary in size from micrometres to several millimetres within a semi-solid freshwater matrix (Garrison Reference Garrison1991; Thomas & Dieckmann Reference Thomas and Dieckmann2002b; Arrigo & Thomas Reference Arrigo and Thomas2004). For microbial communities, the ice matrix represents a challenging physicochemical environment with oscillating gradients in temperature, salinity, pH, dissolved inorganic nutrients, as well as dissolved gas and light signatures (Mock & Thomas Reference Mock and Thomas2005). Only a subset of the initial inoculum, those bacteria and microalgae deemed to be polyextremophiles, are capable of growing within sea ice. Biological production reflects a complex relationship between physical ice dynamics, the distribution of organic and inorganic nutrients, light and ultraviolet (UV) radiation and the biological structure of the sea-ice microbial community – all of which modifies the in situ cycling of energy (Arrigo & Sullivan Reference Arrigo and Sullivan1992; Vaqué et al. Reference Vaqué2002; Stewart & Fritsen Reference Stewart and Fritsen2004). Despite the implications for being a seasonally dynamic habitat, sea-ice microbiology was considered to be in its infancy at the turn of the century (Staley & Gosink Reference Staley and Gosink1999) and significant questions still remain regarding the molecular basis for biochemical and physiological adaptation (Mock & Thomas Reference Mock and Thomas2005; Koh et al. Reference Koh2012; Ewert & Deming Reference Ewert and Deming2013; Lyon & Mock Reference Lyon and Mock2014). In 2002, the term eutectophile was introduced by microbiologist Deming (Reference Deming2002). Pertaining to eutectic, which describes the interface between solid and liquid phases of water, this term does not classify an ice-associated microbe by a single physicochemical variable, but by whatever known, and currently unknown, combination of variables influence life processes in a habitat defined by both solid and liquid phases of water. Heterotrophic bacteria and unicellular algae represent the two major eutectophilic groups within sea-ice assemblages and will be the focus of this review.
Temperature
The Earth is a cold planet and many organisms are exposed to temperatures that are permanently below 5°C (Russell Reference Russell2000; Margesin & Miteva Reference Margesin and Miteva2011; Lyon & Mock Reference Lyon and Mock2014). At sub-zero temperatures, water freezes and the resulting ice crystals can tear cell membranes; unless cells are cryopreserved using flash-freeze techniques (see Dumont et al. Reference Dumont, Marechal and Gervais2004), freezing of intracellular water is almost invariably lethal (Lorv et al. Reference Lorv, Rose and Glick2014). Despite the negative effect of low temperature on biochemical reactions, numerous organisms, in particular bacteria, yeasts, unicellular algae and fungi can successfully adapt to cold environments (Gerday et al. Reference Gerday2000; Gerday Reference Gerday2013). Most are either psychrotolerant (capable of growth close to the freezing point of water; fastest growth occurs at >20°C) or psychrophilic (fastest growth occurs at ≤15°C; growth is not possible >20°C) (Cavicchioli et al. Reference Cavicchioli2002) with generation times that range from 2 h to 10 days (Gerday et al. Reference Gerday2000). Here the term psychrophile is used in a generic sense to describe all microorganisms capable of growth in cold environments. In Arctic and Antarctic marine habitats, seawater and sediment temperatures can drop to approximately −2°C; within sea-ice internal fluids typically range from −2 to −30°C (Ewert & Deming Reference Ewert and Deming2013). The lowest temperature recorded for active in situ photosynthesis by sea-ice algae is currently −10°C (Ralph et al. Reference Ralph2005). Although the tolerance of cold-adapted bacteria appears to be highly variable, in vitro heterotrophic activity has been observed at temperatures as low as −33°C (Bakermans & Skidmore Reference Bakermans and Skidmore2011). The cold completely permeates microorganisms in these environments and all components of the cell – membranes and transport systems, intracellular solutes, nucleic acids and proteins – must be suitably adapted (Cavicchioli et al. Reference Cavicchioli2002; Morgan-Kiss et al. Reference Morgan-Kiss2006). The physiological and ecological success of psychrophiles is thought to reflect an ability to sense temperature change in the environment (Margesin & Miteva Reference Margesin and Miteva2011). In bacteria, the sensor transduces the signal to the genome, subsequently up-regulating genes whose products are associated with cold adaptation (Shivaji & Prakash Reference Shivaji and Prakash2010). This includes regulating membrane fluidity, maintaining protein synthesis, producing cold-acclimation proteins and facilitating freeze tolerance or avoidance mechanisms (Feller & Gerday Reference Feller and Gerday2003; Gerday Reference Gerday2013). Given sufficient time, the metabolic plasticity of psychrophiles facilitates acclimation to ranging polar temperatures (Mock & Hoch Reference Mock and Hoch2005).
Membrane fluidity
The ability to retain a functional lipid bilayer is an important low-temperature requirement because cell membranes control the transport of nutrients and metabolic waste products in and out of the cell (Thomas & Dieckmann Reference Thomas and Dieckmann2002a; Lyon & Mock Reference Lyon and Mock2014). The functional state of this bilayer is a liquid-crystalline phase, but decreased temperatures induce a gel–phase transition and a dramatic loss of membrane properties. Importantly, the temperature at which this occurs is dependent on the lipid composition of the membrane (Feller & Gerday Reference Feller and Gerday2003). In order to maintain fluidity, organisms utilize a combination of changes in fatty acid composition, including polyunsaturated, short-chain, branched or cyclic fatty acids (White et al. Reference White, Wynn-Williams and Russell2000; Mock & Thomas Reference Mock and Thomas2005). One of the well-documented responses, the increase in polyunsaturated fatty acids (PUFAs), has been observed in polar bacteria (Nichols et al. Reference Nichols1999; Russell & Nichols Reference Russell and Nichols1999) diatoms (Torstensson et al. Reference Torstensson2013), dinoflagellates (Thomson et al. Reference Thomson2004) and chlorophytes (Chen et al. Reference Chen, He and Hu2012). Increases in polyunsaturated bonds promote a looser packing of lipids, which decreases the gel–phase transition (Lyon & Mock Reference Lyon and Mock2014). Although the sensory and signal pathways involved in PUFA synthesis are unknown in polar microalgae (Lyon & Mock Reference Lyon and Mock2014), membrane fluidity is connected with optimal photosynthesis at low temperature, specifically the correct folding of membrane-associated proteins, which form the photosynthetic electron transport chain (Morgan-Kiss et al. Reference Morgan-Kiss2006).
Cold-adapted enzymes
Most of the chemical reactions that occur in living organisms are catalysed by enzymes (Gerday Reference Gerday2013). Because microbes are organisms with variable internal temperatures, in cold environments enzymes must overcome the inhibiting effects of low kinetics, specifically the freezing of molecules and decreased rates of catalysis (Casanueva et al. Reference Casanueva2010; Lyon & Mock Reference Lyon and Mock2014). The molecular adaptation of enzymes to compensate for reduced reaction rates is considered a critical feature of cold-adapted microbes (Russell Reference Russell1997; Gerday et al. Reference Gerday2000; Morgan-Kiss et al. Reference Morgan-Kiss2006). These enzymes are characterized as having a high catalytic efficiency at low temperatures, high degrees of thermolability and increased structural flexibility for better substrate access (Thomas & Dieckmann Reference Thomas and Dieckmann2002a; Feller & Gerday Reference Feller and Gerday2003; Siddiqui & Cavicchioli Reference Siddiqui and Cavicchioli2006; Struvay & Feller Reference Struvay and Feller2012). Numerous enzymes have now been characterized and high activity/low stability appears to underlie a general principle of activity-stability trade-off in cold-adapted enzymes (Siddiqui & Cavicchioli Reference Siddiqui and Cavicchioli2006; Collins et al. Reference Collins, Margesin, Schinner, Marx and Gerday2008). Relative to the enzymes found in thermophilic cells, cold-adapted enzymes exhibit (a) an optimum temperature that is displaced towards low temperatures by as much as 30°C, (b) catalytic efficiency that is close to the apparent optimum and (c) rapid inactivation at temperatures >25°C (Russell Reference Russell2000; Gerday Reference Gerday2013). Cold-adapted proteins are produced with relatively minor changes in amino acid sequences and no dramatic differences in 3D structure, but they can be up to ten times more active at low and moderate temperatures (Feller Reference Feller2003; Margesin & Miteva Reference Margesin and Miteva2011; Gerday Reference Gerday2013). For example, cold-adapted proteases are produced by the psychrophilic bacterium Colwellia psychrerythraea 34H (Huston et al. Reference Huston, Krieger-Brockett and Deming2000), but the adaptation does not appear to correlate with a unique set of genes (Methé et al. Reference Methé2005). Furthermore, genome analysis of C. psychrerythraea predicts that a significant percentage of the enzymes associated with protein and peptide degradation are localized external to the cytoplasm. Other extracellular compounds will be discussed subsequently, but the capacity to synthesize cold-adapted degradative enzymes has important implications for astrobiology because of the like requirement for substrate modification in cold environments. Interestingly, ribulose-1,5-bisphosphate carboxylase/oxygenase (RUBISCO), one of the key enzymes associated with photosynthesis, is not modified by psychrophilic microalgae; instead, these organisms attempt to compensate by increasing intracellular enzyme concentration (Devos et al. Reference Devos1998). The reason why polar microalgae cannot modify RUBISCO remains unknown, but the energetic cost of production appears to significantly constrain carbon fixation in Antarctic diatoms (Young et al. Reference Young2015a). Applications for cold-adapted enzymes in biotechnology are being explored (Cavicchioli et al. Reference Cavicchioli2002; Marx et al. Reference Marx2007; Struvay & Feller Reference Struvay and Feller2012).
Cryoprotectants
Cellular freezing, as it occurs on Earth, induces the formation of cytoplasmic ice crystals, which leads to osmotic imbalance and cellular damage. Cold-loving organisms prevent this by utilizing ‘chemical chaperones’, compatible solutes such as polyols, polyamines, sugars and amino acid derivatives, which function as freeze protection molecules. The function of these compounds is to prevent the denaturation and aggregation of proteins and reduce the freezing point, thereby maintaining high in vivo enzymatic activity (Shivaji & Prakash Reference Shivaji and Prakash2010; Lyon & Mock Reference Lyon and Mock2014). Of particular interest is the amino acid derivative dimethylsulfoniopropionate (DMSP), which is produced in high concentrations by sea-ice algae for reasons that are not entirely clear. DMSP may play a role in stabilizing enzymes against cold-induced denaturation (Nishiguchi & Somero Reference Nishiguchi and Somero1992), but there is also evidence to suggest that it acts as a grazing deterrent via its cleavage to acrylate (Mock & Thomas Reference Mock and Thomas2005; Fredrickson & Strom Reference Fredrickson and Strom2009), and, due to a rapid reaction with the hydroxyl radical (OH), it may also serve as an antioxidant system by actively scavenging intracellular OH− (Sunda et al. Reference Sunda2002). Interestingly, the intracellular concentration of DMSP is influenced by light, nutrients and pH in many algal taxa (Marlin & Kirst Reference Marlin and Kirst1997), while its enzymatic cleavage product dimethylsulphide (DMS) contributes significantly to the global sulphur cycle (Welsh Reference Welsh2000; Arrigo & Thomas Reference Arrigo and Thomas2004; Kloster et al. Reference Kloster2006; Sievert et al. Reference Sievert, Kiene and Schulz-Vogt2007).
Some psychrophiles produce antifreeze or ice-binding proteins (IBPs), which are characterized by their capacity to cause a temperature difference in the melting and freezing of ice (Celik et al. Reference Celik2013). These proteins effectively modify ice crystal structure and inhibit recrystallization of ice within the cell (Gilbert et al. Reference Gilbert2004; De Maayer et al. Reference De Maayer2014). Exposure to low temperatures elicits the up- or down-regulation of a significant number of genes in psychrophiles, a process termed the cold-shock response (Casanueva et al. Reference Casanueva2010). Although a mechanistic understanding of this processes is lacking, the genes prominently up-regulated in cold-adapted organisms code for cold-shock proteins that regulate a variety of cellular processes, including transcription, translation, protein folding and membrane fluidity (Hébraud & Potier Reference Hébraud and Potier1999; Phadtare Reference Phadtare2004).
Extracellular compounds
When exposed to sub-zero temperatures, it is important for ice-associated microbes to maintain an aqueous external environment. Effective micro-habitat modifiers produced within the ice matrix include IBPs and extracellular polymeric substances (EPS), which act as cryoprotectants (Mock & Thomas Reference Mock and Thomas2005). Also known as ice-active substances, IBPs (and EPS) enhance brine retention by inhibiting ice growth and recrystallization in the immediate vicinity of the cell (Krembs et al. Reference Krembs, Eicken and Deming2011; Davies Reference Davies2014). This improves the habitability of the ice by preventing injury during freezing (Raymond & Knight Reference Raymond and Knight2003), trapping and protecting pockets of saline water within brine channels (Krembs et al. Reference Krembs, Eicken and Deming2011) and potentially facilitating the attachment of cells to adjacent ice crystals (Raymond & Kim Reference Raymond and Kim2012; Ulig et al. Reference Ulig2015). To date, all sea-ice algae that have been tested (diatoms, prymnesiophytes, prasinophytes and chlorophytes) have been shown to produce IBPs (Lyon & Mock Reference Lyon and Mock2014), and phylogenetic analysis suggests that the required genes were obtained through the horizontal gene transfer of bacterial IBPs (Raymond & Kim Reference Raymond and Kim2012; Davies Reference Davies2014; Raymond Reference Raymond2014). The EPSs produced by sea-ice microbes (known also as extracellular polysaccharide substances; both abbreviated as EPS) are gel-like muscilages composed of complex organic macromolecules that have a high surface area comprising carbohydrates (both mono- and polysaccharides), amino acids and proteins (Decho Reference Decho1990; Underwood et al. Reference Underwood2010). Because different bacterial and algal strains produce EPS with varying physical and chemical structures, the behaviour of these microbial exudates in aqueous solution is complex (Krembs et al. Reference Krembs, Eicken and Deming2011). The term refers to a diverse range of polysaccharides and ancillary compounds (Ewert & Deming Reference Ewert and Deming2013) and there has been significant ambiguity in defining and also analysing EPS. However, the presence of EPS within the sea-ice matrix highlights a significant adaptive response to a range of stressors. Similar to IBPs, these polymers modify brine channel habitability (Krembs et al. Reference Krembs, Eicken, Junge and Deming2002, Reference Krembs, Eicken and Deming2011; Underwood et al. Reference Underwood2010, Reference Underwood2013), but they also provide a viscous media that keeps extracellular enzymes in the vicinity of the cell and are likely to facilitate cell adhesion and motility within brine channels (Krembs & Deming Reference Krembs, Deming, Margesin, Schinner, Marx and Gerday2008). Additional advantages of microbial EPS production include protection from toxic heavy metals (Ozturk & Aslim Reference Ozturk and Aslim2010) and the prevention of desiccation in a high-saline environment (Thomas & Dieckmann Reference Thomas and Dieckmann2002a; Aslam et al. Reference Aslam, Cresswell-Maynard, Thomas and Underwood2012a; Steele et al. Reference Steele, Franklin and Underwood2014).
Salinity
Closely related to sub-zero temperature tolerance is the physiological challenge of salt stress and the adaptive responses elicited by halophiles. Because salinity within the ice matrix is a function of temperature, microbes constrained to brine channels near the upper surface of the ice can be exposed to saline concentrations >200‰ (Kottmeier & Sullivan Reference Kottmeier and Sullivan1988; Arrigo & Sullivan Reference Arrigo and Sullivan1992; Mock Reference Mock2002), while the onset of ice melt rapidly exposes cells to freshwater lenses where the salinity can be as low as 0‰ (Thomas & Dieckmann Reference Thomas and Dieckmann2002a). In addition to these extreme seasonal events, both the salinity and interstitial brine volume fraction can fluctuate over timescales of a few hours to several days (Ewert & Deming Reference Ewert and Deming2014), which necessitates a dynamic response from ice-associated microbes. Physiological activity is challenged by both hyper- and hyposalinity, and in sea-ice algae this ranges from reduced photosynthetic efficiency and capacity (Ryan et al. Reference Ryan, Ralph and McMinn2004; Ralph et al. Reference Ralph2007; Steele et al. Reference Steele, Franklin and Underwood2014) to enzyme damage and cell lysis (Ewert & Deming Reference Ewert and Deming2014). Although sea-ice algae can be physiologically active at saline concentrations ranging from 10 to 80‰, maximum photosynthesis occurs at salinities between 30 and 50‰ (Arrigo & Sullivan Reference Arrigo and Sullivan1992; Ryan et al. Reference Ryan, Ralph and McMinn2004; Ralph et al. Reference Ralph2007). Sea-ice bacteria appear to be more tolerant of ambient salt concentrations given that sustained metabolic activity has been observed at 20–70‰ while maintaining an incubation temperature of −1.8°C (Martin et al. Reference Martin, Hall and Ryan2009).
Compatible solutes
High osmolarity of the brine phase imposes two distinct constraints on microbial life: impaired protein function which, if exacerbated, can lead to protein precipitation, and increased osmotic pressure, which can result in dehydration and a reduction in cell volume (Krell et al. Reference Krell2007; Ewert & Deming Reference Ewert and Deming2013). In response to highly saline conditions, osmolytes, including inorganic ions and organic solutes (e.g. proline, mannitol and glycine betaine) are accumulated or synthesized within the cell (Thomas & Dieckmann Reference Thomas and Dieckmann2002a; Ewert & Deming Reference Ewert and Deming2014). Conversely, hyposaline shock can be alleviated by releasing osmolytes to the external environment. In general, the accumulation of compatible solutes in halotolerant and halophilic organisms assists with the maintenance of turgor pressure, cell volume and intracellular electrolytes – all of which are important for cell proliferation (Roberts Reference Roberts2005). However, these mechanisms are energetically expensive and the disturbance of cellular homeostasis due to the influx or efflux of ions can result in a temporary decline in growth and cell division (Krell et al. Reference Krell2007). Fluctuating water potential is also a stimulus for the production of bacterial EPS (Chang & Halverson Reference Chang and Halverson2003; Chang et al. Reference Chang2007; Ozturk & Aslim Reference Ozturk and Aslim2010), but only recently has a similar response been demonstrated in sea-ice algae (Aslam et al. Reference Aslam2012b; Steele et al. Reference Steele, Franklin and Underwood2014; Ugalde et al. Reference Ugalde2014). Microscale environmental buffering with EPS provides a hydrated environment around the cell, which is highly beneficial and likely to be strongly selected for following initial ice formation (Krembs & Deming Reference Krembs, Deming, Margesin, Schinner, Marx and Gerday2008). Indeed, the regional significance of EPS within sea ice is such that it can be accurately predicted from physical-biogeochemical models that use ice thickness, salinity and algal biomass as core variables (Underwood et al. Reference Underwood2013).
Light
Ice-associated microbes are exposed to a highly variable light regime, from extremely low seasonal irradiance at the bottom of the ice matrix to doses of both visible and UV-B radiation near the ice surface that can cause physiological damage (Martin et al. Reference Martin, Hall and Ryan2009; McMinn & Martin Reference McMinn and Martin2013; Arrigo Reference Arrigo2014). Because of high light attenuation by sea ice and the presence of surface snow, midday summer irradiance beneath the ice can be as low as 1–2% of the incident surface irradiance (Cota Reference Cota1985; McMinn et al. Reference McMinn, Ashworth and Ryan1999a). Low light stress is therefore common in photosynthetic microbes. However, the capacity for sea-ice algae to harvest light is remarkable; active photosynthesis has been observed at irradiances <0.5 µE m−2 s−1, or 0.01% of incident surface irradiance (Palmisano & Sullivan Reference Palmisano and Sullivan1983), and complete photosynthetic saturation has been demonstrated at <10 µE m−2 s−1 in some Antarctic taxa (McMinn et al. Reference McMinn, Martin and Ryan2010). By increasing intracellular pigment concentrations and producing accessory photosynthetic pigments (e.g. fucoxanthin and chlorophyll c), acclimation to low light can be achieved on a timescale of hours (McMinn et al. Reference McMinn, Ryan and Gademann2003; McMinn & Martin Reference McMinn and Martin2013). In diatoms, this reflects the capacity to densely pack pigments and associated binding proteins and modify the structure of thylakoid membranes (Lyon & Mock Reference Lyon and Mock2014). In the model microalgal taxon Fragilariopsis cylindrus, even a small reduction in ambient irradiance (2 µE m−2 s−1 versus 15 µE m−2 s−1) elicits a response whereby pigment concentrations are doubled and specific chloroplast PUFAs are produced which enhance thylakoid fluidity and the velocity of electrons within photosynthetic reaction centres (Mock & Kroon Reference Mock and Kroon2002). Remarkably, algal cells growing within brine channels retain the capacity for acclimation to relatively high light (up to 350 µE m−2 s−1) (Ralph et al. Reference Ralph2005). The strategy for minimizing light-induced cellular damage (photoinhibition) includes non-photochemical quenching mechanisms, specifically the diatoxanthin–diadinoxathin xanthophyll cycle, which effectively limits the amount of energy that can reach photosynthetic reaction centres. Interestingly, photosynthetic performance at increased irradiance is negatively influenced by both in situ temperature and salinity (Maxwell et al. Reference Maxwell1994; Ralph et al. Reference Ralph2005, Reference Ralph2007).
UV radiation
Despite the attenuation by snow and sea ice, microbes can be exposed to in situ UV radiation, including biologically harmful UV-B wavelengths (280–320 nm). Estimates vary greatly, but between 0.3 and 13% of the surface flux is transmitted through to the bottom of the ice matrix (Trodahl & Buckley Reference Trodahl and Buckley1990; Perovich Reference Perovich1993; Belzile et al. Reference Belzile2000; Ryan et al. Reference Ryan2012). Since the 1980s, significant research effort has been devoted to understanding the seasonal increase in UV radiation in polar regions (e.g. Ryan Reference Ryan1992; McMinn et al. Reference McMinn, Ashworth and Ryan1999a; Thomas & Dieckmann Reference Thomas and Dieckmann2002a). While it appears that stratospheric ozone depletion has had a minimal effect on the annual microbial biomass generated in Antarctic coastal regions (McMinn et al. Reference McMinn, Heijnis and Hodgson1994; Ryan et al. Reference Ryan2012), at the organismal level UV-B can inhibit photosynthesis in sea-ice algae by impeding photosystem II. In addition, this wavelength band can either directly damage DNA, alter biomolecules, inactivate biochemical activities (e.g. alteration of protein synthesis), or induce the formation of reactive oxygen species (ROS), which requires an energetically expensive antioxidant response (Mallick & Mohn Reference Mallick and Mohn2000; Meador et al. Reference Meador2002; Häder et al. Reference Häder2015). Perhaps the most notable photoprotection measure exhibited by ice-associated algae is the production of mycosporine-like amino acids (MAAs). The most common suite of MAAs comprises porphyra-334 and/or shinorine together with palythine; these small secondary metabolites act as a chemical sunscreen by absorbing UV-B and dissipating the energy as heat (Arrigo & Thomas Reference Arrigo and Thomas2004; Arrigo Reference Arrigo2014). There appear to be no comparable adaptive mechanisms in sea-ice bacteria and rapid exposure to increased UV-B radiation, typical during the ice melt phase, significantly reduces metabolic activity (Martin et al. Reference Martin, Hall and Ryan2009).
Dark survival
The term dark survival was first coined by Antia (Reference Antia1976) and is used to describe the retention of viability in photoautotrophs without growth during exposure to darkness. Polar marine phytoplankton need to survive almost total darkness for up to 4 months of the year (Peck Reference Peck2005; McMinn et al. Reference McMinn, Martin and Ryan2010), but remarkably little is known about the physiological and biochemical mechanisms required for dark survival (McMinn & Martin Reference McMinn and Martin2013; Lyon & Mock Reference Lyon and Mock2014). Some taxa survive by producing cysts or other resting forms but in Antarctica this is limited to a few species of diatoms (Chaetoceros spp.) and a number of dinoflagellates (Taylor & McMinn Reference Taylor and McMinn2002). Mixotrophy, which combines both phototrophic and heterotrophic modes of energy acquisition, has been observed in ice-associated nanoflagellates, but remains somewhat ambiguous because of species-specific differences in resource requirements and variable feeding behaviours (Moorthi et al. Reference Moorthi2009; Paterson & Laybourn-Parry Reference Paterson and Laybourn-Parry2012). Perhaps more intriguing is that fact that facultative heterotrophy, itself is often only turned on by long periods of darkness (Legrand et al. Reference Legrand, Graneli and Carlson1998), remains unsubstantiated as a dark survival strategy for sea-ice diatoms (Bunt & Lee Reference Bunt and Lee1972; Horner & Alexander Reference Horner and Alexander1972). In situ metabolism of radio-labelled organic substrates by Arctic taxa appears to be negligible and only two studies have ever demonstrated the capacity for Antarctic diatoms to incorporate glucose and exogenous amino acids into proteins and other complex organic polymers, and even this only accounted for a maximum of 0.3% of the total carbon requirement (Palmisano & Sullivan Reference Palmisano and Sullivan1983; Rivkin & Putt Reference Rivkin and Putt1987). What has been clearly demonstrated is a remarkable capacity for maintenance metabolism; under non-photosynthetic conditions survival by microalgae does not require a significant drawdown of stored energy products such as mono- and polysaccharides (Martin et al. Reference Martin2012; McMinn & Martin Reference McMinn and Martin2013). How this is achieved at a molecular level remains unknown.
Dissolved gases
Physiological adaptations that sustain photosynthesis at high O2 and low CO2 within the confines of an alkaline environment are critically important (Arrigo & Thomas Reference Arrigo and Thomas2004; McMinn et al. Reference McMinn, Pankowski and Delfatti2005, Reference McMinn2014). Because of the limited exchange with the underlying water column, there are a number of dramatic chemical changes that take place within the ice matrix. With respect to inorganic chemistry, precipitation of the metastable mineral ikaite (CaCO3.6H2O) is currently of interest because of implications for the sea-ice-driven carbon pump, global carbon cycle and possibly even tropospheric ozone concentrations (Dieckmann et al. Reference Dieckmann2008; Rysgaard et al. Reference Rysgaard2014). Carbonate chemistry dynamics are also influenced by biological activity, which significantly influences the in situ concentration of dissolved gases (O2, CO2) and pH (Thomas & Dieckmann Reference Thomas and Dieckmann2002b). The seasonal increase in pH is correlated with in situ photosynthetic carbon assimilation and the depletion of dissolved inorganic carbon (Thomas & Papadimitriou Reference Thomas, Papadimitriou, Thomas and Dieckmann2003). With the exception of microbial mat communities, macrophytes and sea grasses, hyperoxia is rare in marine systems and sea-ice microbes are uniquely exposed to some of the highest dissolved oxygen concentrations on the planet (McMinn et al. Reference McMinn, Pankowski and Delfatti2005). As a result, photosynthetic performance and growth is compromised in ice-associated microalgae at elevated oxygen concentrations. This reflects the trend observed in all plants, whereby the competitive effect of O2 on RUBISCO (photorespiration) and generation of extra toxic oxygen species disrupts metabolism. Oxygen can accept electrons from various biomolecules resulting in the production of ROS such as superoxide (O2 −), hydrogen peroxide (H2O2), singlet oxygen (lO2) and hydroxyl (•OH) radicals (Foyer et al. Reference Foyer, Lelandais and Kunert1994; Rothschild & Mancinelli Reference Rothschild and Mancinelli2001). These toxic photochemical products are capable of damaging cellular components such as D1 proteins within photosynthetic reaction centres as well as nucleic acids. In response, sea-ice diatoms are known to produce high-activity antioxidative enzymes, such as catalase, glutathione peroxidase and glutathione reductase, which are capable of scavenging ROS (Thomas & Dieckmann Reference Thomas and Dieckmann2002a; Arrigo & Thomas Reference Arrigo and Thomas2004). However, other than their positive identification and presumed mode of action, very little is currently known about how sea-ice organisms utilize these enzymes to cope with oxidative stress (Thomas & Dieckmann Reference Thomas and Dieckmann2002a; Janknegt et al. Reference Janknegt2008). In addition to intracellular ROS formation, extracellular accumulation is common within the brine channel network. Most notable is H2O2 which also forms photochemically in the upper regions of the ice and can reach concentrations up to 0.1 µM (King et al. Reference King2005). In some diatom genera, the antioxidative defence response includes a commensalism with epiphytic bacteria, which consume the H2O2 produced during photosynthesis (Schriek Reference Schriek2000; Hünken et al. Reference Hünken, Harder and Kirst2008). Despite the fact that shelf waters surrounding Antarctica are effective sinks for rising anthropogenic carbon emissions – a process which may in fact facilitate faster rates of photosynthesis and growth – the direct effect of CO2 on phytoplankton physiology remains poorly understood (Young et al. Reference Young2015b).
For microbes confined to the sea-ice matrix the situation is significantly different; in spring and summer photosynthetic activity significantly influences sea-ice CO2–carbonate chemistry (Thomas & Papadimitriou Reference Thomas, Papadimitriou, Thomas and Dieckmann2003; Delille et al. Reference Delille2007; McMinn et al. Reference McMinn2014). Although the number of assessments carried out within natural ice in both the Arctic and Antarctic remains limited, the available data describe an in situ trend of very low pCO2 (<100 µatm) and high pH (>8.6) during spring months (see Bates et al. Reference Bates2014). However, this dynamic is complex and unpredictable because it reflects both the physical conditions (e.g. temperature, salinity, ice thickness, ikaite formation/dissolution) and biological processes (e.g. primary production and respiration) (Bates et al. Reference Bates2014). Limited experimental work with microalgae present near the surface of the ice has shown that if cells are incubated at a constant pH of ~8.0, then an increase in CO2 availability results in a growth increase of approximately 20%. However, if microbes acclimated to this region of the ice are exposed to decreasing pH (< ~7.2), then CO2 at high concentrations has been shown to reduce growth rates by almost 50% (McMinn et al. Reference McMinn2014) (Fig. 1).
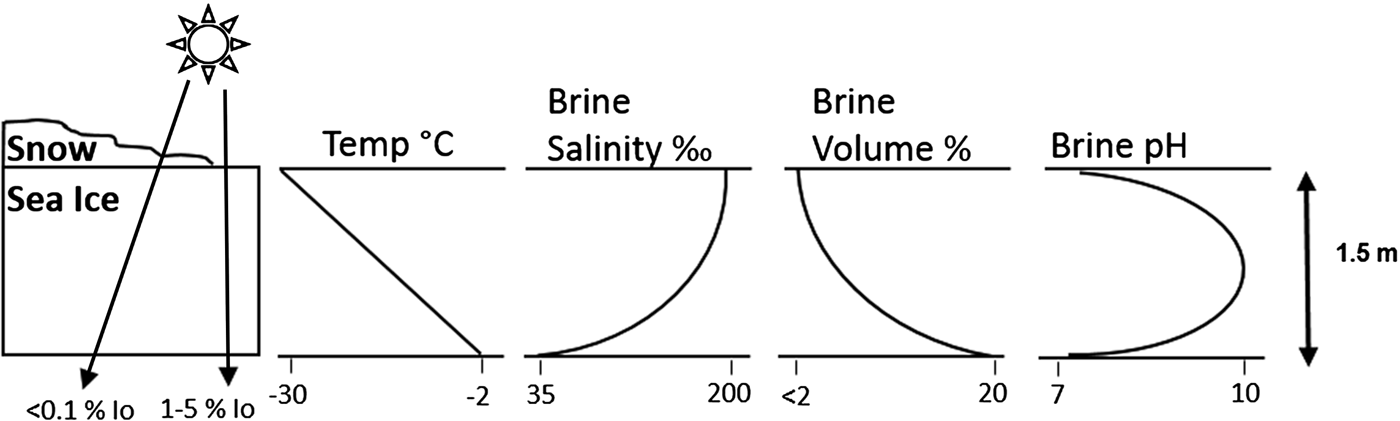
Fig. 1. Schematic representation of the physicochemical gradients that occur within annual polar sea ice. Note that snow cover significantly reduces incident irradiance (I 0). In situ carbonate chemistry dynamics are complex and highly variable; the trend in pH is shown to indicate the range of microbial niches present in sea ice due to gradients in physicochemical conditions. Adapted from Eicken (Reference Eicken1992), Thomas & Dieckmann (Reference Thomas and Dieckmann2002a) and Hare et al. (Reference Hare2013).
Nutrients
Along with the biological components, the incorporation of dissolved inorganic nutrients, including nitrate, ammonium, phosphate, silicate and trace metals such as iron takes place during initial ice formation. Zones of high biological production are governed by microbial-community succession and the re-supply of nutrients, which is highly variable with respect to vertical stratification within the ice matrix (McMinn et al. Reference McMinn, Ashworth and Ryan1999a, Reference McMinnb; Fritsen et al. Reference Fritsen2001). In contrast to what might be expected, in situ nutrient concentrations generally remain high and are seldom growth-limiting for biological assemblages. This is particularly the case at the ice/water interface where there is continuous nutrient exchange with the underlying seawater (Thomas & Papadimitriou Reference Thomas, Papadimitriou, Thomas and Dieckmann2003; Arrigo Reference Arrigo2014). In the strict absence of biological activity, the concentration of nutrients is proportional to brine salinity and can therefore be exceptionally high in the coldest regions that are found near the upper surface of the ice (Werner et al. Reference Werner, Ikavalko and Schunemann2007). Despite the recycling of organic matter by bacteria which promotes the accumulation of phosphate and ammonium, the seasonal assimilation of nutrients by microalgae in regions of the matrix that are isolated from nutrient re-supply strongly influence the nutrient:salinity relationship (e.g. Dieckmann et al. Reference Dieckmann1991; Riedel et al. Reference Riedel2007) and this can lead to localized nutrient exhaustion (McMinn et al. Reference McMinn1999b; Fritsen et al. Reference Fritsen2001), particularly during the period just prior to ice melt, which coincides with the highest in situ microbial biomass (Lizotte & Sullivan Reference Lizotte and Sullivan1992; Günther et al. Reference Günther, Gleitz and Dieckmann1999). In general, the supply and production of nutrients is greater than the apparent capacity for utilization within the sea-ice system (Fritsen et al. Reference Fritsen2001; Thomas & Papadimitriou Reference Thomas, Papadimitriou, Thomas and Dieckmann2003). This is significant because sea-ice algae and bacteria appear to be unable to sequester either inorganic substrates or organic compounds with decreasing temperature because of lowered substrate affinity (Nedwell Reference Nedwell1999; Pomeroy & Wiebe Reference Pomeroy and Wiebe2001). Higher substrate concentrations are typically required at the lower end of a species optimum growth temperature, and growth inhibition can be reversed if higher substrate concentrations become available (Thomas & Dieckmann Reference Thomas and Dieckmann2002b).
The potential for life in extra-terrestrial ice-covered oceans
The current search for extraterrestrial life is the search for life as we know it; life comprised of organic molecules in liquid water (Chyba et al. Reference Chyba, Whitmire, Reynolds, Mannings, Boss and Russell2000). However, conditions beyond the protective confines of Earth's atmosphere are likely to challenge any form of life – space is a nutritional wasteland with respect to water and organic compounds and is subject to extreme cold, solar wind, galactic radiation, space vacuum and negligible gravity (Chyba & Phillips Reference Chyba and Phillips2001; Rothschild & Mancinelli Reference Rothschild and Mancinelli2001; Horneck et al. Reference Horneck, Klaus and Mancinelli2010). As a field of research, microbial ecology has always been juxtaposed between scales – the requirement to resolve life processes at the cellular level, while at the same time qualifying, and preferably quantifying, the contribution of microbes to global biogeochemistry (Azam & Malfatti Reference Azam and Malfatti2007). At the astronomical scale, this challenge is amplified significantly. Despite the growing list of exoplanets and the prospect for life on suspected ocean world's, targeting ‘cosmic life rafts’ within the vastness of space is far from trivial. However, initial efforts have been highly productive with respect to the survival of model microbial strains that have been exposed to the effects of radiation, desiccation and vacuum environments using ground-based spaceflight analogues, space shuttle missions and the space stations (see Horneck et al. Reference Horneck, Klaus and Mancinelli2010 for an excellent review). Much of this work has focused on the survival of spores exposed to space vacuum and Solar UV. Extending the survival envelope to other celestial objects is, for the time being, a theoretical undertaking, but the adaptive capacity of ice-associated eutectophiles is such that much of Europa and Enceladus can be considered plausible habitats for Earth-like life.
The satellites of the outer Solar System are highly variable. While most moons are composed of rock and ice, rock that is separated from ice due to tidal-based heating that leads to the formation of clearly defined liquid oceans is only a feature on some moons (Schubert et al. Reference Schubert2010). With respect to exobiology, Jupiter's moon Europa and Saturn's moon Enceladus may be considered the most favourable modern habitats for complex organic chemistry and possibly life. Potential ecological niches on both moons comprise the ice layer, brine oceans and seafloor environments (Cottin et al. Reference Cottin2015).
Europa
Spectroscopic analysis coupled with observations of the geology and magnetic field measurements reveal that below the icy exterior is an ocean that is in direct contact with the moon's rocky seafloor (Chyba & Phillips Reference Chyba and Phillips2002). In principle, interior heat is released from the moon's core, which may contribute to organic processes that are comparable with the hydrothermal vent systems on Earth (Cottin et al. Reference Cottin2015). The exact thickness of Europa's icy exterior and ocean depth remains unknown. Estimates for the outer shell range from ~3 to >30 km (Greenburg et al. Reference Greenburg2000; Kerr Reference Kerr2001; Hand & Chyba Reference Hand and Chyba2007; Schmidt et al. Reference Schmidt2011) but mounting evidence suggests that ocean water reaches the moon's surface via tidally driven stress fractures (Schmidt et al. Reference Schmidt2011; Hand & Brown Reference Hand and Brown2013; Roth et al. Reference Roth2014). The formation of these features in the crust are likely to be unpredictable, but models infer both their persistence for tens of thousands of years and the potential for daily inundation of seawater in the temperature range of −4 to 0°C (Greenburg et al. Reference Greenburg2000; Melosh et al. Reference Melosh2004). With respect to habitability, temperature and the chemical composition of the ocean are important considerations, not least because of their influence on the depth of the ice–water interface. At present, the salt concentration and composition of the subsurface ocean remains poorly constrained; magnesium (Mg2+) should be the dominant cation and sulphate (SO4 2−) the dominant anion, but the relative abundance of sodium chloride (NaCl) and the bulk salinity have yet to be resolved. Europa's ocean could be a ~100 km deep source of nearly fresh water or an ocean with a NaCl concentration close to the point of saturation (Hand & Chyba Reference Hand and Chyba2007). Remarkably, a recent whole-moon model simulation of the thermal evolution of Europa predicts a relatively well mixed ocean with a salt concentration of ~35 ppt, essentially comparable to oceans on Earth (Travis et al. Reference Travis, Palguta and Schubert2012). The brines that infiltrate the ice-cover exterior could be high in magnesium sulphate (MgSO4), sodium sulphate (Na2SO4) and sodium carbonate (Na2CO3), but also potentially sulphuric acid. With respect to the surface of Europa, significant reservoirs of oxygen have been spectrally detected in the form of H2O2, sulphuric acid (H2SO4) and carbon dioxide (CO2); molecular oxygen and ozone are inferred on the basis of Europa's tenuous oxygen atmosphere (Gaidos et al. Reference Gaidos, Nealson and Kirschvink1999). Important biogenic materials such as sulphur may be continually ejected and transported from Io to Europa, however bombardment by energetic charged particles in the Jovian magnetosphere is significant and no known organism could live within ~10 cm of the surface (Greenburg et al. Reference Greenburg2000).
Enceladus
One of the defining characteristics of Saturn's moon Enceladus is the cryovolcanism, which results in plumes of vapour and icy particles being ejected into space from the moon's South Pole region (Schmidt et al. Reference Schmidt2008; Postberg et al. Reference Postberg2009; Porco et al. Reference Porco, DiNino and Nimmo2014). The gas plume is primarily dominated by water vapour, but also contains approximately 5% CO2, 1% CH4, 1% ammonia (NH3) and small quantities of heavier hydrocarbons and organic molecules. The plume-associated particles are, for the most part water ice, but also contain dust in the form of salt-rich ice grains, specifically NaCl, and silicon-rich, nanometre-sized particles, both of which provide compelling evidence for a subsurface ocean (Postberg et al. Reference Postberg2009, Reference Postberg2011; Hsu et al. Reference Hsu2015). Although many details remain unclear, the favoured mechanism for the formation and maintenance of a liquid layer on Enceladus is tidal heating driven by the moons eccentric orbit (Spencer & Nimmo Reference Spencer and Nimmo2013; Porco et al. Reference Porco, DiNino and Nimmo2014). Until recently, this body of water was thought to comprise a regional south polar sea, but a recent interpretation of data collected by the Cassini spacecraft infers the presence of a global subsurface ocean (Thomas et al. Reference Thomas2015). The relation of the ocean to both the inner core and outer shell of the moon have yet to be defined, but modelling carried out by Thomas et al. (Reference Thomas2015) suggests that the ocean is 26–31 km thick, underlying an ice crust that is 21–26 km thick. At the south polar region of Enceladus, the ice thickness is predicted to be ≤13 km. Analysis of the plume has provided significant insight into the interior of Enceladus; because silica colloids aggregate and precipitate quickly at high ionic strength, silica nanoparticles can be used to probe the pH, salinity and water temperature at the bottom of the ocean, while the micrometre-sized dust grains infer composition and thermal processes at the ice–water interface and in the vent complex (Spencer & Nimmo Reference Spencer and Nimmo2013). At the core–ocean interface, the temperature could be >90°C with a pH >8.5 (Hsu et al. Reference Hsu2015). However, estimates for the subsurface ocean pH vary significantly; Hsu et al. (Reference Hsu2015) derived values of 8.5–10.5 and a salinity of <40 ppt from plume nanoparticle analysis, while a thermodynamic model of carbonate speciation produced by Glein et al. (Reference Glein, Baross and Waite2015), which was linked to plume analysis of CO2 and carbonate salts, alludes to a ‘soda ocean’ (pH ~ 11–12). This latter interpretation is highly significant because of the inference to serpentinization, a water–rock reaction that leads to the generation of H2. Whether this is a contemporary geothermal dynamic remains unclear (Sekine et al. Reference Sekine2015), but H2 is a geochemical fuel that can support both the abiotic and biological synthesis of organic molecules (Glein et al. Reference Glein, Baross and Waite2015). The temperature gradient across the subsurface ocean remains equally speculative with estimates ranging from −3 to −23°C (Nimmo et al. Reference Nimmo2007; Parkinson et al. Reference Parkinson2008). Within the ice shell of Enceladus, specifically at the plume source-water surface, the temperature is predicted to be ~0°C, pH ~ 8.5–9 and salinity >5 ppt (Postberg et al. Reference Postberg2009). As is the case with Europa, direct measurements of liquids may be possible in the future, but drilling missions will be complex and costly (Dachwald et al. Reference Dachwald2014).
Europa and Enceladus are both compelling targets for astrobiological exploration. The inferred presence of liquid oceans that have persisted over geological timescales is intriguing, even if they actually prove to be a common occurrence in our Solar System (Chyba & Hand Reference Chyba and Hand2001; Stevenson et al. Reference Stevenson2015). Life as we know it is expected to be absent on the surface of Europa and Enceladus, precluded by ionizing radiation and extremely low temperatures. Hydrothermal vents may or may not exist (Chyba Reference Chyba2000; Hsu et al. Reference Hsu2015) and photosynthesis is not possible under ice that is kilometres thick. Six elements in the Periodic Table are ubiquitous in the macromolecules of life on Earth: C, H, N, O, P and S; availability of these biogenic elements is tightly coupled with habitability in the microbial domains of life (Cockell et al. Reference Cockell2016). With respect to the relative abundance of CHNOPS on the moons of Saturn and Jupiter, very little is known (Chyba & Phillips Reference Chyba and Phillips2001, Reference Chyba and Phillips2002), although various pathways have been proposed whereby chemical energy is made available in the form of disequilibrium concentrations of redox reactants produced on the surface of both Europa (e.g. Gaidos et al. Reference Gaidos, Nealson and Kirschvink1999; Chyba Reference Chyba2000; Chyba & Hand Reference Chyba and Hand2001; Chyba & Phillips Reference Chyba and Phillips2001) and Enceladus (Parkinson et al. Reference Parkinson2008). The only known form of carbon on Europa's surface is CO2, but the radiolytic chemistry that results from bombardment by energetic electrons and ions trapped within the Jovian magnetic field has shown, at least experimentally, that carbon could be partitioned into reservoirs of CO2, CO and H2CO3 with an approximate ratio of [5 : 1 : 1], respectively (Hand et al. Reference Hand, Carlson and Chyba2007). On Enceladus, deposits of H2O2, NH3 and CO2 are evident, which appear to originate from the fraction of plume material that is not lost to space (Parkinson et al. Reference Parkinson2008). Validating sources of free energy on either moon remains challenging (Gaidos et al. Reference Gaidos, Nealson and Kirschvink1999; Chyba & Phillips Reference Chyba and Phillips2002), as is qualifying possible metabolic life processes or ecosystem dynamics. However, celestial bodies that are in all probability hostile to life in general, may still harbour biologically permissive domains.
The fracture networks in the icy exterior of both moons appear to actively exchange material from the oceanic subsurface through to the outer exterior of the ice, a process which could reflect the presence of brines at shallow depths over diapiric plumes of relatively warm ice. Whether tidally driven water migrates diurnally through fissures to the surface remains controversial (Chyba & Phillips Reference Chyba and Phillips2001); however, the recently proposed theory that Europa's ice shell comprises a mobile plate tectonic-like system overlying warmer convecting ice (Kattenhorn & Prockter Reference Kattenhorn and Prockter2014) infers dramatic subduction events. This dynamic has important implications for both fluid transport and the possibility of a microbial ‘stasis zone’. Microbes present in more favourable regions such as the ice/water interface or subsurface ocean that were forced upwards and exposed to near-surface temperatures would conceivably flash-freeze. This process of vitrification does not occur naturally on Earth, but it is highly relevant to astrobiology because of the potential for extreme cold to induce a state of suspended animation, similar to the process of cryopreservation. The prospect that this process could be reversed – a return to metabolic activity correlated with tectonic processes – is highly speculative, but it is also intriguing. Importantly, future missions to icy moons might not require that probes drill through tens of kilometres of ice to gain an insight into the potential biology of subsurface oceans. Stress-related fissures in the ice are complex, and for the time being our understanding of the geophysics is based largely on theoretical modelling. For example, tidal-tectonic dynamics and crustal recycling models for Europa infer that fractures are transient features that only remain active for 104–106 years (Greenburg et al. Reference Greenburg2000). While transient near-surface liquid water environments that permit life are considered feasible (Gaidos & Nimmo Reference Gaidos and Nimmo2000; Greenburg et al. Reference Greenburg2000), there is limited data for either moon with which to accurately resolve fracture network topography, specifically ice permeability and brine volume (but see Kargel et al. Reference Kargel2000). Whether biologically available nitrogen, sulphur and phosphorus are present is unknown. Availability of these elements requires that they are present in the rocky core and that convective mechanisms drive strong subsurface circulation. In the absence of hydrothermal vent activity that might facilitate the exchange of elements or even support seafloor ecosystems, a fundamental requirement for the presence of life in a subsurface ocean would be the significant surface-to-ocean transfer of oxidants or organics (Chyba Reference Chyba2000; Chyba & Hand Reference Chyba and Hand2001; Hand et al. Reference Hand, Carlson and Chyba2007). Clearly, the physiological demands on an in situ microbial consortia would be exceptional, regardless of whether fracture zones provided a point of origin for life, or ephemeral habitats reflecting the passive transport of organisms from the ocean below.
The potential for significant tidal-driven movement of fluids through the fracture network is an important point of difference in the comparison with Earth's sea-ice ecosystem. Additional caveats include habitat scale (metres versus kilometres) and localized physicochemical variability (isolated brine channels versus fissures with unknown dimensions). Pressure is also an important consideration. At the ice/water interface on Europa this is likely to be between 84 and 205 MPa (Kargel et al. Reference Kargel2000), but this is well within the range of pressure resistance that cellular life can survive (see Vanlindt et al. Reference Vanlindt2011). In very general terms, the lower biological temperature threshold of approximately −20°C would allow microbial life to exist at the ice/water interface on Europa and Enceladus and extend upwards into the overlying ice, perhaps by as much as 5 km (Nimmo & Manga Reference Nimmo, Manga, Pappalardo, McKinnon and Khurana2009). In accordance with known biology, microbes would not be able to maintain membrane fluidity in significantly colder regions of the ice and the production of cold-adapted enzymes and cryoprotectants would be impaired. If fracture topography dictates fine-scale thermal isolation and chloride salts are abundant, then temperatures below −20°C would result in the formation of hypersaline brines exceeding 200‰ and limit the microbial capacity for extracellular buffering. However, sea-ice microbes could tolerate the salinity regime that spans the ice/water interface and approximately 5 km of ice. The assumption here is that salinity is governed by temperature; while bulk salinity could be similar to Earth's oceans, the relative abundance of salts comprising the reservoirs on both moons has yet to be resolved and interpretation of chemical gradients is speculative. Indeed, some models for Europa infer a process of continued acidification that may have formed an ocean of eutectic sulphuric acid unlikely to be conducive to any known form of life (Kargel et al. Reference Kargel2000) (Fig. 2).
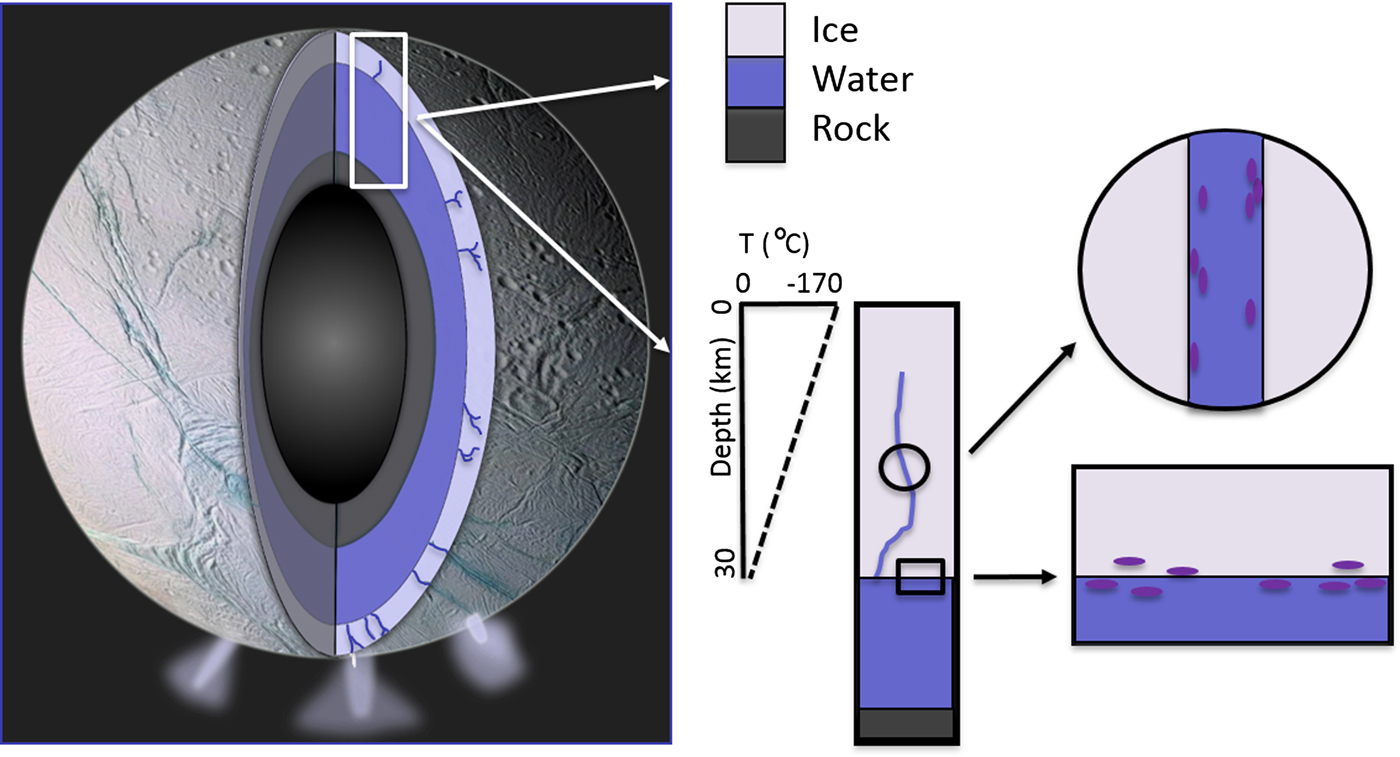
Fig. 2. Conceptual model of an ice fissure and the ice/water interface on Europa or Enceladus. These represent two ecological niches that Earth-based eutectophiles could potentially inhabit.
Proposed metabolic pathways for subsurface ecosystems on Europa include methanogenesis, sulphur reduction and iron oxide reduction (Gaidos et al. Reference Gaidos, Nealson and Kirschvink1999; Chyba Reference Chyba2000; Cockell et al. Reference Cockell2016). Sulphur reduction is of particular relevance because recent analysis of 16S rRNA genes from ice-associated bacteria infers that this process, although previously unknown, could be taking place within sea ice (Bowman Reference Bowman2015). A subsurface ecology driven by the reaction HCHO + O2 → H2O +CO2 is of interest because neither photosynthesis nor hydrothermal vent activity is required to sustain life (Chyba & Phillips Reference Chyba and Phillips2001). On one level, any emphasis on biological production that accounts for ecosystem processes approaching the ‘planetary scale’ is compelling. For example, substantive estimates infer that ~109 mol y−1 equivalent molecular oxygen (O2) could reach Europa's ocean through radiolysis and ice shell recycling, which in principle could produce ~108–109 g y−1 of biomass (Chyba & Hand Reference Chyba and Hand2001). From a microbial perspective, this approach is somewhat misleading. The constraints on inhabiting extreme environments on Earth illustrates that although microbial assemblages are often capable of growing quickly, as physiological thresholds are approached resource dependency increases. Because these habitats are often characterized by multiple physicochemical stressors, limitations to growth are exacerbated at physiological extremes, which leads to patchy spatial and temporal distributions. The sea-ice ecosystem is a particularly useful exemplar: there is a finite temporal window for photosynthetic metabolism within spatially confined brine channels that have a finite concentration of nutrients and limited capacity for gas exchange. When viewed in this context, life in extreme environments is inherently resource limited. Conversely, organisms in these environments are inherently resourceful as evidenced by their capacity for rapid opportunistic growth. Biologically available elements notwithstanding, the light required by microalgae for photosynthesis is so minimal that several authors have alluded to the possibility of light-driven metabolism associated with tidal dynamics and ice fissures on Europa (Gaidos & Nimmo Reference Gaidos and Nimmo2000; Greenburg et al. Reference Greenburg2000). However due to the process of vitrification described earlier, this is considered by most to be theoretically impossible.
We bring this review to a close by highlighting the fact that there are other metabolic pathways associated with the sea-ice ecosystem that do not require Solar radiation, all other factors being permissive of life. Because gene expression remains underexplored and a defined framework that links microbial community function with biogeochemical drivers is lacking, Bowman (Reference Bowman2015) recently used a technique called metabolic inference to screen 16S rRNA gene libraries to reveal metabolic plasticity in ice-associated prokaryotes (see Bowman & Ducklow Reference Bowman and Ducklow2015). This novel tool for identifying metabolic pathways has ranging implications for astrobiology. In Table 3, we cross-reference pathways of high biogeochemical interest with the relative abundance of bacterial genera in sea ice (Bowman Reference Bowman, Rosenberg, DeLong, Lory, Stackebrandt and Thompson2013), and provide a simplistic indicator for whether Europa and Enceladus could support the biogeochemistry. It is important to note that the last two columns of Table 3 only refer to the requirement for a specific metabolic reactant being present and that few other requirements for the pathways, much less the organisms, are met. It will be necessary to qualify each pathway under controlled laboratory conditions, but the identification of new targets for metabolic profiling represents a significant step in framing the adaptive capacity of sea-ice microbes to multi-stressor environments.
Table 3. Pathways of biogeochemical interest for Europa and Enceladus identified by metabolic inference of ice-associated bacteria
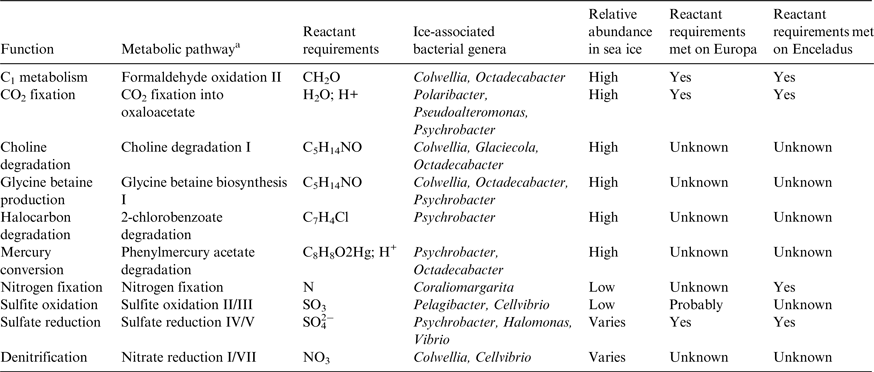
a Pathway names as described by the MetaCyc ontology.
Adapted from Bowman (Reference Bowman, Rosenberg, DeLong, Lory, Stackebrandt and Thompson2013, Reference Bowman2015) and Cockell et al. (Reference Cockell2016).
Conclusions
The search for life beyond Earth requires a robust definition of the physical and chemical boundaries for Earth-based extremophiles, and for prebiotic chemistry to account for the original synthesis of life (Dartnell Reference Dartnell2011). Because of these constraints on known biology and the sheer magnitude of the Universe, habitability of any celestial object will only be confirmed by showing inhabitation (McKay Reference McKay2014). Although far from prophetic, this statement by McKay (Reference McKay2014) in describing the availability and relative importance of common elements in the Universe applies equally well to the fact that no definite limits for life on Earth have been established for any given extreme (Harrison et al. Reference Harrison2013; Cottin et al. Reference Cottin2015). Furthermore, the response of extremophiles to multiple physiological stressors currently impedes the search for extra-terrestrial life because we lack a mechanistic framework that links the capacity for biological adaptation with environmental variability (Dartnell Reference Dartnell2011; Harrison et al. Reference Harrison2013). Equally challenging, is the requirement to characterize the theoretical limits for supporting biological processes that are distinct from the limits imposed on Earth-based analogues. While this may initially seem idiosyncratic, an important transitional step will be to undertake experimental work across multiple physicochemical extremes and couple this with research that contrasts the limits of Earth's biosphere with extra-terrestrial environments of interest (Harrison et al. Reference Harrison2013). Viewed in this context, environments that are not usually encountered on Earth – or even ever – can be framed within the context of hypotheses for testing habitat variability and the potential distribution of life in the Universe (Cockell Reference Cockell2014). The value of the sea-ice ecosystem to astrobiology is the in situ gradient of physicochemical extremes. This habitat represents the closest Earth analogue to the likely fracture network on Europa and Enceladus and eutectophiles are highly relevant biological reference organisms for facilitating further research and planning for future exploration to these moons.
Acknowledgements
We gratefully acknowledge the anonymous reviewers for their insightful and constructive comments, which improved the manuscript significantly. We also thank Fraser Kennedy for assistance with graphic design.







