Alpha-lactalbumin (α-LA) is a protein constituent found in all mammalian milk (Lönnerdal & Lien, Reference Lönnerdal and Lien2003) that plays a role in the regulation of lactose synthesis (Hill & Brew, Reference Hill and Brew1975). Beta-lactoglobulin (β-LG) is a protein constituent found in bovine milk but not in human milk and is known to bind to retinoid, long-chain fatty acids, and steroids (Kontopidis et al., Reference Kontopidis, Holt and Sawyer2004). Processing of milk (pasteurization, high temperature treatments) may give rise to structural and functional alterations in these whey proteins (Inagaki et al., Reference Inagaki, Kawai, Xijier, Fukuoka, Yabe, Iwamoto and Kanamaru2017; Liu et al., Reference Liu, Grosvenor, Li, Wang, Ma, Clerens, Dyer and Day2019). The significance of such changes for the intestinal health of the consumer is unknown, but we have previously shown that commercial bovine α-LA containing oligomeric molecules exhibits potent cytotoxicity to IEC-6 cells, which are part of the rat intestinal crypt cell line, whilst the native monomer was not cytotoxic (Xu et al., Reference Xu, Sugiura, Nagaoka and Kanamaru2005a). Furthermore, we attempted to alter bovine α-LA using 2,2,2-trifluoroethanol (TFE), widely used in studies as a reagent to denature the native conformation of peptides and proteins (Buck, Reference Buck1998; Naeem et al., Reference Naeem, Iram and Bhat2015). Bovine α-LA denatured with 30% TFE also strongly inhibited IEC-6 cells’ growth (Xu et al., Reference Xu, Sugiura, Nagaoka and Kanamaru2005b). There is a need to understand whether denatured whey proteins produced during processing of milk could be a risk factor for compromised intestinal health.
In this study, we investigated the effects of native and denatured whey proteins α-LA and β-LG on human Caco-2 cells, which differentiate into polarized intestinal epithelial cells. Additionally, we report on the safety and influence of ingested native and denatured bovine milk proteins in two suckling mouse models, healthy and human rotavirus (HRV)-infected gastrointestinal disease. HRV is the major viral pathogen leading to severe diarrhea in infants and young children (Esona & Gautam, Reference Esona and Gautam2015).
Materials and methods
Materials
All chemicals used in this study were of reagent grade. The bovine α-LA and β-LG were purchased from Davisco Foods International, Inc. (Eden Prairie, MN). Bovine lactoferrin was purchased from Merck (Darmstadt, Germany). HRV MO strain (serotype G3P[8]) was propagated in MA104 cells, and a virus titer was determined using the fluorescent cell focus-forming unit (FCFU) assay as described previously (Ebina et al., Reference Ebina, Tsukada, Umezu, Nose, Tsuda, Hatta, Kim and Yamamoto1990).
Cell line
IEC-6 cells, which are part of the crypt cell line derived from the small intestines of rats, and Caco-2 cells, which are part of the human colon adenocarcinoma cell line, were obtained from the American Type Culture Collection (Rockville, MD). Both types of cells were maintained in Dulbecco's modified Eagle's medium (DMEM; ICN Biomedicals, Aurora, OH), supplemented with 10% fetal calf serum (FCS), and cultured in an incubator with a humidified atmosphere and 5% CO2 at 37°C.
TFE treatment
The purification of native α-LA and β-LG was performed according to methods described in the online Supplementary file, Purification of native α-LA and β-LG. The obtained proteins were treated with TFE as previously described by Xijier et al. (Reference Xijier, Mori, Fukuoka, Cairangzhuoma, Inagaki, Iwamoto, Yabe and Kanamaru2012) with slight modifications. The native form was dissolved in 25 mM Tris-HCl buffer (pH 8.0) containing 25% (v/v) TFE at a concentration of 0.5 mg/ml, filtered through a 0.22 μm filter, and incubated at 37°C for 2 d in a slowly rotating (4 rpm) test tube on a Versatile Tube Rotator (Miltenyi Biotec, Gladbach, Germany). The solution was dialyzed using distilled water and then lyophilized.
Cell proliferation assay (water-soluble tetrazolium-1 method)
The water-soluble tetrazolium-1 (WST-1) assay was performed as previously described by Inagaki et al. (Reference Inagaki, Kawai, Xijier, Fukuoka, Yabe, Iwamoto and Kanamaru2017) using a cell counting kit (Dojindo, Kumamoto, Japan). Briefly, cell suspensions containing 10% FCS were seeded into 96-well plates at a concentration of 2.0 × 104 cells/well. After pre-incubation for 5 h or 24 h, cells were incubated for a certain period in DMEM supplemented with 1% FCS in the absence or presence of the sample. Cell growth was detected using WST-1 assay, and absorbance was monitored at 450 nm.
Measurement of transepithelial electrical resistance
Caco-2 cell suspensions were seeded into 12 mm i.d. Transwell® inserts (Millicell-HA, 0.45 μm pore size; Merck) in 24-well plates at a concentration of 6.0 × 104 cells/well, and 600 μl and 400 μl of DMEM supplemented with 10% FCS was added to the basolateral and apical compartments, respectively. The cells were then cultured at 37°C in a humidified atmosphere of 5% CO2. The culture medium was replaced on alternate days. The samples were dissolved in 1% FCS medium at a concentration of 10 mg/ml and were added to the inserts. The outer well was also replaced with 10% FCS medium. The exact conditions employed in each individual experiment are described in more detail in the figure legends. Electrodes were placed on the upper and lower chambers, and the transepithelial electrical resistance (TER) was measured using Millicell-ERS equipment (Merck).
HRV-infected diarrheal mouse model
The assay was conducted as previously described by Inagaki et al. (Reference Inagaki, Yamamoto, Cairangzhuoma, Xijier, Yabe, Uchida, Kawasaki, Nakagomi, Nakagomi, Minamoto and Kanamaru2013). Briefly, BALB/c mice that were 14 d pregnant were purchased from Japan SLC (Hamamatsu, Japan) and gave birth shortly thereafter. HRV MO strain (1.7 × 105 FCFU/50 μl) was used to inoculate the 5 d old mice. After virus inoculation, the milk sample was orally administered by gavage three times at 24, 48, and 72 h post-inoculation (hpi). Milk samples contained native and TFE-treated bovine α-LAs and β-LGs, as well as bovine lactoferrin. Test mice were given milk sample (2.5 mg) dissolved in 50 μl of phosphate-buffered saline (PBS) (pH 7.2). Control mice were given 50 μl of PBS. Stools were characterized using a three-point ranking system (diarrhea index, DI) every 24 h after virus inoculation: (1) normal brown formed stool or no stool; (2) soft orange stool; (3) liquid yellow stool. The care and experimental procedures were reviewed and approved by the Animal Care and Use Committee, Gifu University, Japan.
Statistical analyses
The statistical analyses of the in vitro and in vivo experiments were conducted using Tukey's and Dunnett's multiple tests, respectively. Data are presented as means ± standard deviation.
Results
Effect of the native and TFE-treated β-LGs on undifferentiated Caco-2 cells
First, we characterized the native and TFE-treated β-LGs according to their molecular properties using chromatography and SDS-PAGE (online Supplementary Figs 1A and B). Then, we examined the effect of the native and TFE-treated β-LGs on cell growth for undifferentiated Caco-2 cells using the WST-1 method. The native β-LG promoted cell proliferation, whereas the TFE-treated β-LG significantly inhibited cell growth (Figs 1a and b). These observations are similar to the results obtained using IEC-6 cells (online Supplementary Figs 1C and D).
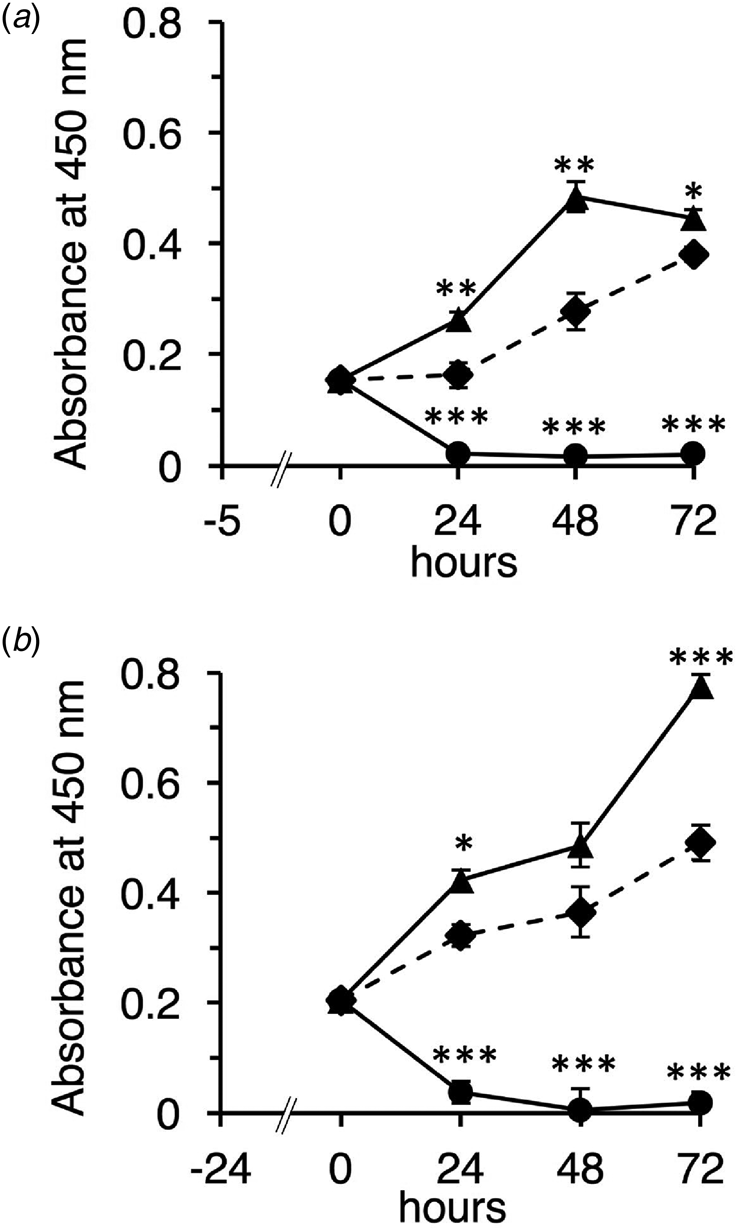
Fig. 1. Effect of the native and TFE-treated β-LGs on undifferentiated Caco-2 cells. Cells were seeded and incubated for 5 or 24 h in DMEM supplemented with 10% FCS (a, 5 h pre-culture; b, 24 h pre-culture). After pre-culture, cells were added with DMEM supplemented with 1% FCS in the absence of sample (control, diamonds and dotted line) or presence of 10 mg/ml of native β-LG (triangles and solid line), or presence of 10 mg/ml of the TFE-treated β-LG (circles and solid line), then incubated for 24, 48, and 72 h. Cell growth was detected using WST-1 assay, and absorbance was monitored at 450 nm. Each measurement was performed in quadruplicate, and each value represents the mean ± sd. ***P < 0.001, **P < 0.01, and *P < 0.05 indicate significant difference from the respective control.
Effect of the native and TFE-treated β-LGs on differentiating Caco-2 cells
Next, we investigated the effect of the native and TFE-treated β-LGs on differentiating Caco-2 cells using TER measurement. The TER value was used as a differentiation index that indicates the tight junction's integrity and barrier function (Srinivasan et al., Reference Srinivasan, Kolli, Esch, Abaci, Shuler and Hickman2015). In preliminary experiments we confirmed that the TER value gradually increased depending on the growth term and reached and maintained a constant value of ~350 Ω cm2 at day 15 and later (data not shown). Since this value could represent differentiated Caco-2 cells (Srinivasan et al., Reference Srinivasan, Kolli, Esch, Abaci, Shuler and Hickman2015), we measured the TER value every 3 d for 12 d.
As shown in Figure 2a, the native β-LG significantly promoted TER value on day 6. The value continued to increase gradually thereafter, suggesting that the native β-LG prompts the integrity of the tight junction. By contrast, the TFE-treated β-LG strongly inhibited the formation of the Caco-2 cell monolayer. Similar observations have been made for α-LA (data not shown).
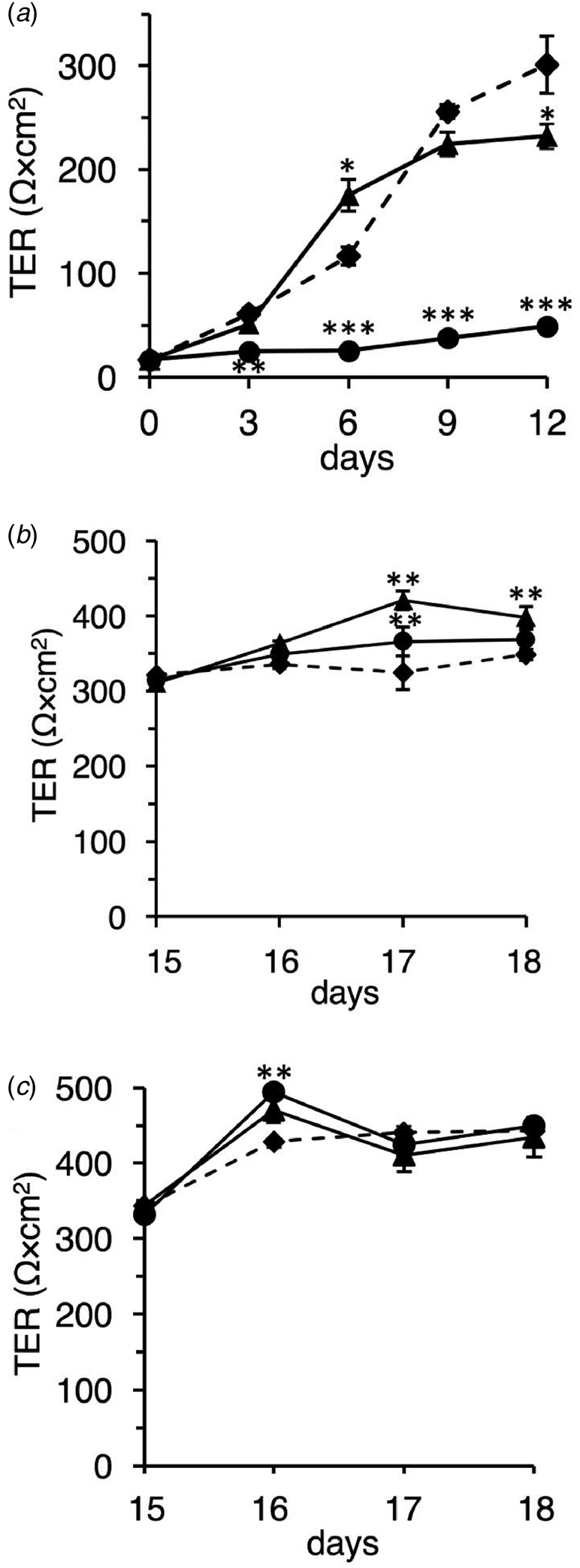
Fig. 2. Effect of the native and TFE-treated bovine whey proteins on differentiating and differentiated Caco-2 cells. (a) Effects of the native and TFE-treated β-LGs on differentiating Caco-2 cells. Caco-2 cells were seeded on permeable membranes and pre-cultured for 5 h. After incubation, the samples were added to the insert, DMEM containing 1% FCS without a sample (control, diamonds and dotted line), or with 10 mg/ml of native β-LG (triangles and solid line), or with 10 mg/ml of the TFE-treated β-LG (circles and solid line). The experimental medium was replaced on alternate days. TER measurements were performed every 3 d. (b and c) Effects of the native and TFE-treated bovine whey proteins on differentiated Caco-2 cells. Caco-2 cells were seeded on permeable membranes, and DMEM medium containing 10% FCS was replaced on alternate days up to 15 d. After that, during the experimental period, cells were exposed from the apical side in DMEM containing 1% FCS without a sample (control; diamonds and dotted line) or with 10 mg/ml of native sample (triangles and solid line) or with 10 mg/ml of the TFE-treated sample (circles and solid line): β-LG (b) and α-LA (c). Replacement of experimental medium and TER measurements were performed every day. Each measurement was performed in quadruplicate, and each value represents the mean ± sd. ***P < 0.001, **P < 0.01, and *P < 0.05 indicate significant difference from the respective control.
Effect of the native and TFE-treated whey proteins on differentiated Caco-2 cells
Additionally, we investigated the effect of the native and TFE-treated whey proteins on differentiated Caco-2 cells using TER measurement. We measured the TER value for 4 d after differentiation.
As shown in Figure 2b, native β-LG significantly induced a high TER value compared to that in control, thus suggesting the stabilization of barrier function at the tight junction. Unexpectedly, the TFE-treated β-LG also significantly enhanced the TER value on day 17. We also observed that the native and TFE-treated α-LAs produced cell behavior similar to that of the native and TFE-treated β-LGs (Fig. 2c). These observations in differentiated cells were markedly different from those in undifferentiated and differentiating cells.
Safety assessment of the native and TFE-treated major whey proteins using a healthy mouse model
We first investigated whether α-LA and β-LG that are denatured by TFE treatment have adverse effects on healthy suckling mice. Litters of 6 d old BALB/c mice were given denatured (TFE treated) α-LA and β-LG orally by gavage each day for 4 d. No samples caused diarrhea or even soft stool on any day (data not shown), suggesting that the denatured bovine major whey proteins have no adverse effect in vivo. The result also indicated that the effect of possible residual TFE could be excluded from this experimental system.
Safety assessment of the native and TFE-treated major whey proteins using a diarrheal mouse model
Next, we evaluated the safety and efficacy of the TFE-treated whey proteins using diarrheal suckling mice. Litters of 5 d old suckling mice were orally challenged with the HRV MO strain. After the viral challenge, the sample was administered orally at 24, 48, and 72 hpi. The control mice were given PBS instead of the milk sample.
In the control group (Fig. 3a), 5 of the 11 mice presented with diarrhea at 24 hpi (DI = 1.73 ± 0.91). Thereafter, most of the mice developed liquid diarrhea from 48 to 96 hpi. At 72 hpi, the DI of the control group was 2.71 ± 0.88. In the native β-LG group (Fig. 3c), although we observed that 5 of the 11 mice also presented with diarrhea at 24 hpi (DI = 1.73 ± 0.91, P > 0.05), the administration of native β-LG relieved the symptoms from 48 hpi onward. At 72 hpi, the observation on the native β-LG group suggest a modest and non-significant relieving effect (DI = 1.56 ± 0.88, P = 0.07). In the TFE-treated β-LG group (Fig. 3e), 4 of the 9 mice developed severe diarrhea at 24 hpi (DI = 1.89 ± 1.05, P > 0.05), followed by symptomatic levels at 72 hpi (DI = 1.88 ± 1.04, P > 0.05).
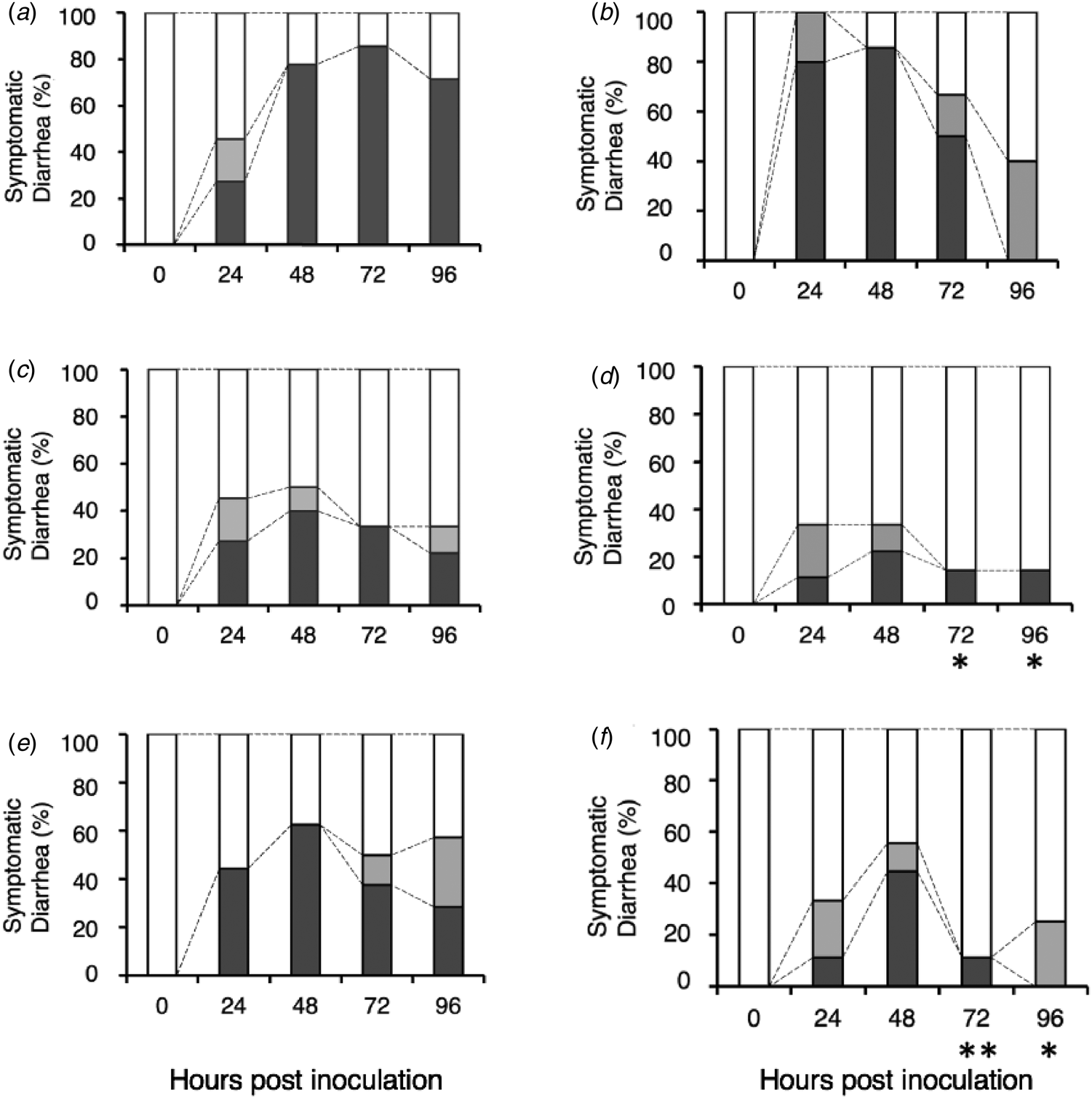
Fig. 3. Safety assessment of the native and TFE-treated bovine whey proteins against HRV-infected diarrhea in suckling mice. Litters of 5-d-old mice were orally inoculated with HRV MO strain. After viral inoculation, a sample was orally administered by gavage at 24, 48, and 72 h post virus inoculation: PBS (a), bovine lactoferrin (b), native β-LG (c), native α-LA (d), TFE-treated β-LG (e), and TFE-treated α-LA (f). Symptomatic diarrhea is expressed as the percentage of mice presenting with diarrhea compared with the total number of mice (100%): Normal brown formed stool or no stool (open box), soft brown stool (gray box), and liquid yellow stool (black box). **P < 0.01 and *P < 0.05 indicate significant difference from the respective control.
Furthermore, the native and TFE-treated α-LA groups showed that two and four of the nine mice respectively presented with diarrhea at 48 hpi (native α-LA group: DI = 1.56 ± 0.88, P > 0.05 (Fig. 3d); TFE-treated α-LA group: DI = 2.00 ± 1.00, P > 0.05 (Fig. 3f)). All mice significantly recovered from their diarrheal symptoms at 72 hpi (native α-LA group: DI = 1.29 ± 0.76, P = 0.01; TFE-treated α-LA group: DI = 1.25 ± 0.71, P < 0.01) and 96 hpi (native α-LA group: DI = 1.29 ± 0.76, P < 0.05; TFE-treated α-LA group: DI = 1.25 ± 0.46, P < 0.05). We performed the same test about bovine lactoferrin and observed no remarkable effect (Fig. 3b). These results suggest that TFE-treated β-LG and α-LA have no adverse effects, even in mice with diarrheal symptoms.
Discussion
The primary objective of this study was to investigate the safety of the denatured whey proteins in vivo because the high concentrations of α-LA isolated from market milk exhibited cytotoxicity in cultured intestinal IEC-6 cells (Inagaki et al., Reference Inagaki, Kawai, Xijier, Fukuoka, Yabe, Iwamoto and Kanamaru2017). However, the heat-denatured major whey proteins in market milk were presumed to be insufficient in the amount for in vivo study. Hence, we used the TFE-treated whey proteins that had been shown to strongly induce cell death in IEC-6 cells (Xu et al., Reference Xu, Sugiura, Nagaoka and Kanamaru2005b; Xijier et al., Reference Xijier, Mori, Fukuoka, Cairangzhuoma, Inagaki, Iwamoto, Yabe and Kanamaru2012). Suckling mice were fed 2.5 mg/d of each of the milk proteins, and this dosage was appropriate for the safety test, according to a previous report by Wolber et al. (Reference Wolber, Broomfield, Fray, Cross and Dey2005). Since it is commonly presumed that suckling mice do not have the capability to secrete digestive enzymes fully, it seems that intact (undigested) samples reach their gastrointestinal tract. The results demonstrated that both the native and TFE-treated α-LAs and β-LGs never caused diarrhea or even soft stool during the administration period (data not shown). Based on this finding, the experiments using an HRV-infected diarrheal mouse model were performed. there were no adverse effects, rather we found that the TFE-treated α-LA and β-LG had mild relieving effects against HRV-infected diarrhea in suckling mice (Figs 3e and f).
It is known that HRV impairs the normal formation of tight junctions in polarized Caco-2 cells (Obert et al., Reference Obert, Peiffer and Servin2000). The nonstructural protein 4 in HRV causes a drastic remodeling of the cells’ microtubule network (Yang & McCrae, Reference Yang and McCrae2012) and increases paracellular permeability (Tafazoli et al., Reference Tafazoli, Zeng, Estes, Magnusson and Svensson2001), which induces diarrhea. The tight junctions play an important role in regulating permeability to substances such as ions, solutes, and water (Ballard et al., Reference Ballard, Hunter and Taylor1995). Indeed, both of the denatured whey proteins used in this study maintained or enhanced the differentiated Caco-2 cells’ integrity, resulting in higher electrical resistance measurements, as the native whey proteins did (Figs 2b and c). These results agree with the experimental data of Hashimoto et al. (Reference Hashimoto, Takeda, Nakayama and Shimizu1995, Reference Hashimoto, Nakayama and Shimizu1998). Their TER experiments demonstrated that bovine β-LG improves the tight junction's integrity and modulates cytoskeletal structure using Caco-2 cells treated with inhibitors.
In conclusion, we observed opposite effects of native (stimulatory) and denatured (inhibitory) whey proteins on cell proliferation in undifferentiated Caco-2 intestinal cells. By contrast, in mature (differentiated) cells, cell barrier function was enhanced by both native and denatured whey proteins. The mechanisms underlying these different properties are still unclear. However, our novel finding might illustrate the functional importance of major bovine whey proteins α-LA and β-LG in regulating intestinal cell growth. Although we cannot assess the effect of long-term ingestion of denatured whey proteins, these results suggest that they have no adverse effects on differentiated intestinal cells and digestive tract, at least in short-term ingestion.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022029921000376
Acknowledgements
We thank Drs Osamu Nakagomi and Toyoko Nakagomi (Nagasaki University Graduate School of Biomedical Sciences) for kindly supporting this study. This research was supported by the Grant-in-Aid for Scientific Research (B) (Grant No. 13460117 to Y. K.) and in part by the Grant-in-Aid for Scientific Research (C) (Grant No. 18K05504 to M. I.).





