Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Scarborough, Peter
and
Rayner, Mike
2014.
When nutrient profiling can (and cannot) be useful.
Public Health Nutrition,
Vol. 17,
Issue. 12,
p.
2637.
Tseng, Marilyn
and
Hodge, Allison
2014.
Profiling foods and diets.
Public Health Nutrition,
Vol. 17,
Issue. 12,
p.
2625.
Newson, R.S.
van der Maas, R.
Beijersbergen, A.
Carlson, L.
and
Rosenbloom, C.
2015.
International consumer insights into the desires and barriers of diners in choosing healthy restaurant meals.
Food Quality and Preference,
Vol. 43,
Issue. ,
p.
63.
Drewnowski, Adam
2015.
Preventive Nutrition.
p.
71.
Rizk, Marianne T.
and
Treat, Teresa A.
2015.
Perceptions of food healthiness among free-living women.
Appetite,
Vol. 95,
Issue. ,
p.
390.
Nemecek, Thomas
Jungbluth, Niels
i Canals, Llorenç Milà
and
Schenck, Rita
2016.
Environmental impacts of food consumption and nutrition: where are we and what is next?.
The International Journal of Life Cycle Assessment,
Vol. 21,
Issue. 5,
p.
607.
Hingle, Melanie D.
Kandiah, Jayanthi
and
Maggi, Annette
2016.
Practice Paper of the Academy of Nutrition and Dietetics: Selecting Nutrient-Dense Foods for Good Health.
Journal of the Academy of Nutrition and Dietetics,
Vol. 116,
Issue. 9,
p.
1473.
Moreira-Ascarrunz, Sergio
Larsson, Hans
Prieto-Linde, Maria
and
Johansson, Eva
2016.
Mineral Nutritional Yield and Nutrient Density of Locally Adapted Wheat Genotypes under Organic Production.
Foods,
Vol. 5,
Issue. 4,
p.
89.
Berendsen, Agnes A.M.
van Lieshout, Lilou E.L.M.
van den Heuvel, Ellen G.H.M.
Matthys, Christophe
Péter, Szabolcs
and
de Groot, Lisette C.P.G.M.
2016.
Conventional foods, followed by dietary supplements and fortified foods, are the key sources of vitamin D, vitamin B6, and selenium intake in Dutch participants of the NU-AGE study.
Nutrition Research,
Vol. 36,
Issue. 10,
p.
1171.
Bucher, Tamara
Hartmann, Christina
Rollo, Megan
and
Collins, Clare
2017.
What Is Nutritious Snack Food? A Comparison of Expert and Layperson Assessments.
Nutrients,
Vol. 9,
Issue. 8,
p.
874.
Fernandez, Melissa A.
Fisberg, Mauro
and
Marette, André
2017.
Yogurt in Health and Disease Prevention.
p.
491.
Kubena, Karen S.
and
McIntosh, W. Alex
2017.
Nutrition and Functional Foods for Healthy Aging.
p.
345.
Hess, Julie M.
and
Slavin, Joanne L.
2017.
Healthy Snacks: Using Nutrient Profiling to Evaluate the Nutrient‐Density of Common Snacks in the United States.
Journal of Food Science,
Vol. 82,
Issue. 9,
p.
2213.
Fernandez, Melissa Anne
and
Marette, André
2017.
Potential Health Benefits of Combining Yogurt and Fruits Based on Their Probiotic and Prebiotic Properties.
Advances in Nutrition,
Vol. 8,
Issue. 1,
p.
155S.
De Vlieger, Nienke M.
Collins, Clare
and
Bucher, Tamara
2017.
What is a nutritious snack? Level of processing and macronutrient content influences young adults' perceptions.
Appetite,
Vol. 114,
Issue. ,
p.
55.
2017.
Yogurt: Roles in Nutrition and Impacts on Health.
p.
23.
Drewnowski, Adam
2017.
Uses of nutrient profiling to address public health needs: from regulation to reformulation.
Proceedings of the Nutrition Society,
Vol. 76,
Issue. 3,
p.
220.
Eriksen, Rebeca
Gibson, Rachel
Lamb, Kathryn
McMeel, Yvonne
Vergnaud, Anne-Claire
Spear, Jeanette
Aresu, Maria
Chan, Queenie
Elliott, Paul
and
Frost, Gary
2018.
Nutrient profiling and adherence to components of the UK national dietary guidelines association with metabolic risk factors for CVD and diabetes: Airwave Health Monitoring Study.
British Journal of Nutrition,
Vol. 119,
Issue. 6,
p.
695.
Drewnowski, Adam
Dwyer, Johanna
King, Janet C
and
Weaver, Connie M
2019.
A proposed nutrient density score that includes food groups and nutrients to better align with dietary guidance.
Nutrition Reviews,
Vol. 77,
Issue. 6,
p.
404.
Sonesson, U.
Davis, J.
Hallström, E.
and
Woodhouse, A.
2019.
Dietary-dependent nutrient quality indexes as a complementary functional unit in LCA: A feasible option?.
Journal of Cleaner Production,
Vol. 211,
Issue. ,
p.
620.
‘Nutrient-dense foods’ are widely recommended; however, there is no consistent definition of what distinguishes a nutrient-dense food. There is also little understanding of how individual nutrient-dense foods fit into healthful dietary patterns. The purpose of this commentary is to discuss how nutrient-dense foods have been defined and address some of the caveats of existing definitions. The commentary also relates the challenges of defining nutrient density to recent efforts at nutrient profiling.
The 2005 Dietary Guidelines Advisory Committee placed a major emphasis on the selection of nutrient-dense foods, which were defined as ‘those foods that provide substantial amounts of vitamins and minerals and relatively few calories’( 1 ). Conversely, non-nutrient-dense foods were foods that were defined as supplying energy but relatively small amounts of micronutrients. Consumption of nutrient-dense foods was endorsed by the 2005( 2 ) and 2010( 3 ) Dietary Guidelines for Americans (DGA). However, neither the 2005( 2 ) nor the 2010 DGA( 3 ) actually defined the terms ‘nutrient dense’ or ‘nutrient density’; rather, both listed food groups which they deemed ‘nutrient dense’: whole grains, fruit, vegetables, fat-free or low-fat milk and milk products, seafood, lean meats and poultry, eggs, beans and peas, and nuts and seeds, prepared without added fats or sugars( 3 ). The implication was that these foods were healthful and others were not. Neither the 2005( 2 ) nor the 2010 DGA( 3 ) made an effort to use a more useful method of categorizing the nutrients that foods contributed at the expense of their SFA, added sugars or Na content.
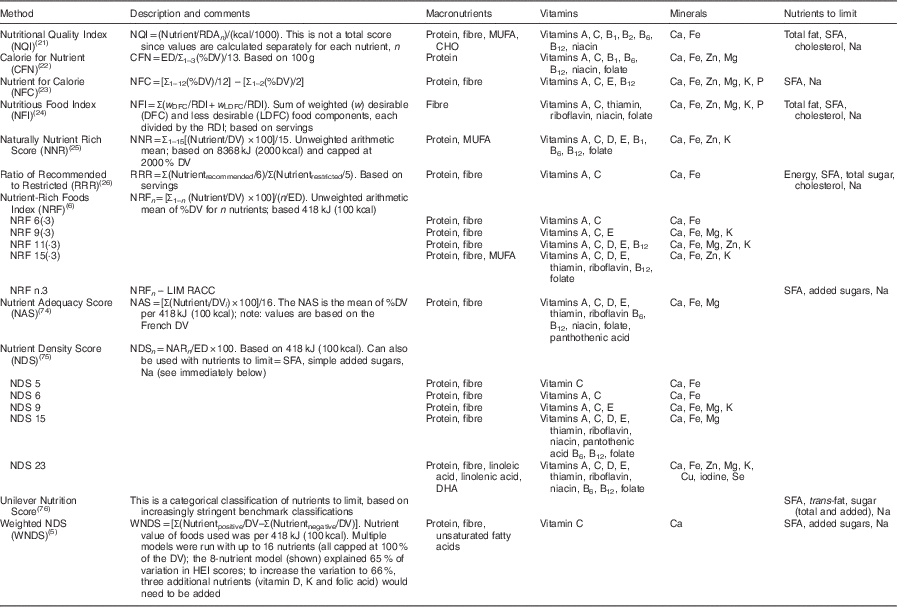
Nutrient profiling of foods is the science of ranking foods based on nutrient composition. The algorithms used to profile foods generate a numerical score which can then be used to help consumers make healthier choices( 3 – Reference Katz, Njike and Faridi 7 ), influence regulatory issues( Reference Drewnowski 8 , Reference Tetens, Oberdörfer and Madsen 9 ), help consumers rate foods using front-of-package labels or labels on store shelves( Reference Katz, Njike and Rhee 10 , Reference Kunkel and McKinley 11 ), define healthy foods that can be advertised to children( Reference Scarborough, Payne and Agu 12 – Reference Drewnowski, Fulgoni and Young 15 ) and motivate industry to develop more healthful foods( Reference Drewnowski, Fulgoni and Young 15 ). Table 1 shows a sample of the variety of nutrient density algorithms that have been used. The continuous systems that generate scores calculate and assign a score to each food item, which can then be used to rank and compare foods. There is no score or scoring system that identifies an individual food as ‘nutrient dense’; foods are only nutrient dense in comparison with other foods. The criteria used in nutrient profiling systems are not currently regulated and not all algorithms have been validated.
Table 1 Summary of selected nutrient profile models
RDA, Recommended Dietary Allowance; DV, Daily Value; ED, energy density; RDI, Reference Daily Intake; LIM, limit; RACC, Reference Amount Customarily Consumed; MRV, maximum recommended value; HEI, Healthy Eating Index; CHO, carbohydrates.
Unanswered questions and challenges in defining nutrient-dense foods
There are many unanswered questions and challenges relating to the development of a scientific definition of nutrient density and the application of this term( Reference Miller, Drewnowski and Fulgoni 16 – Reference Scarborough, Rayner and Stockley 18 ). Many of the questions surrounding the definitions of nutrient density were identified in a Practice Paper of the Academy of Nutrition and Dietetics( Reference Pennington, Kandiah and Nicklas 17 ) and by Scarborough et al. ( Reference Scarborough, Rayner and Stockley 18 ). The DGA’s all-encompassing definition of nutrient-dense foods( 3 ) also adds to these challenges/questions. Using these published references, herein we focus on eight of the important challenges related to defining nutrient density. We expand and explain these important challenges; highlight the situation in the USA; and relate the concept of defining nutrient density and its challenges to wider international efforts in nutrient profiling.
1. What units of measure should be used to determine nutrient density?
It seems to be accepted that nutrient density refers to nutrients compared with kilocalories (or kilojoules), but should other measures also be considered or used in place of kilocalories/kilojoules? A number of standard ‘units’ have been used in the calculation of nutrient density scores, including 418 kJ (100 kcal), the Reference Amount Customarily Consumed (RACC) and 100 g. Scarborough et al. ( Reference Scarborough, Rayner and Stockley 18 ) stated that 100 g is commonly used in nutrient profiling; however, those authors pointed out the caveat that if the food was high in a nutrient if 100 g were consumed, what would happen if that food (for example, mustard) was not customarily consumed in that amount? Similarly, 418 kJ (100 kcal) may also be an implausible serving for some foods, especially fresh vegetables with no fat added, and consumers who consume a standard serving size may not understand that they are not getting as many nutrients as they think. Ultimately, the decision on what units to use may need to reflect how a nutrient profiling system will be used; if consumers are introduced to 418 kJ (100 kcal) or the MyPlate serving size( 19 ) it might be the easiest for them to understand. Use of MyPlate serving sizes would also send a consistent message to the US public; however, these servings are often inconsistent with RACC servings and these serving sizes may not apply worldwide.
2. What (and how many) nutrients should be included in algorithms that assess nutrient density?
The specific nutrients and the number of nutrients that should be used in determining the nutrient density of a food have been debated( Reference Fulgoni, Keast and Drewnowski 6 , Reference Drewnowski and Fulgoni 20 ); and although the selection of nutrients is arbitrary( Reference Arsenault, Fulgoni and Hersey 5 ), many models use similar nutrients. In general, however, the nutrients chosen for determination of nutrient density scores have included those that have been identified as shortfall nutrients, such as dietary fibre, Ca and vitamins A, C and E( 2 , 3 ); and as nutrients to limit, including total fat, SFA, trans-fat, cholesterol, total and added sugars, and Na( Reference Arsenault, Fulgoni and Hersey 5 , Reference Fulgoni, Keast and Drewnowski 6 , Reference Hansen, Wyse and Sorenson 21 – Reference Scheidt and Daniel 26 ) and, often, protein. Protein is included in many models, not because it is a shortfall nutrient or a nutrient to limit in the diets of most Americans( Reference Fulgoni 27 ), but because of its importance in the diet. Several models use other nutrients( Reference Arsenault, Fulgoni and Hersey 5 , Reference Fulgoni, Keast and Drewnowski 6 , Reference Hansen, Wyse and Sorenson 21 – Reference Scheidt and Daniel 26 ); however, this overall approach suggests that some nutrients may not be ‘as important’ in contributing to diet quality or to overall health promotion and disease reduction.
A bigger problem is that selection of nutrients for nutrient profiling suggests that nutrients not generally used in scoring may not be ‘as important’ or do not contribute to overall health promotion and disease reduction. An example of this is K, which is seldom included in nutrient profiling, although it has been identified as a nutrient of public health concern because of its inverse relationship with blood pressure( 3 ).
As another example, fruit and vegetables have been defined as nutrient-dense foods( 3 ). The importance of many fruit and vegetables for health promotion and disease prevention is related not only to their dietary fibre and micronutrient content, but also to their phytochemical content( Reference Leiherer, Mündlein and Drexel 28 – Reference Davinelli, Sapere and Zella 31 ). Phytochemicals have been shown to have a wide array of health benefits, including reducing inflammation associated with diabetes( Reference Leiherer, Mündlein and Drexel 28 ) or other chronic diet-related illnesses such as CHD( Reference Boeing, Bechthold and Bub 29 ) and osteoprotective( Reference Shen, von Bergen and Chyu 30 ) or neuroprotective effects( Reference Davinelli, Sapere and Zella 31 ). If the nutrient density score does not consider these components, then fruits and vegetables may be undervalued.
Several algorithms use only nutrients to limit to determine the nutrient density of a food. One of these has traditionally been SFA; however, since new information suggests that some dietary SFA do not play a role in influencing circulating LDL-cholesterol levels, and subsequently the risk of CVD( Reference Huth and Park 32 ), should SFA be included as a nutrient to limit? Further, the role of SFA must be examined in light of macronutrient substitutions made when SFA is reduced in the diet – notably addition of other solid fats or sugars( Reference Siri-Tarino, Sun and Hu 33 ). Trans-fats, while shown to be associated with increased risk of CVD( Reference Ganguly and Pierce 34 ), are not in the US Department of Agriculture Nutrient Database( 35 ), so it is difficult to include them in some of the algorithms. The rapid elimination of trans-fats from processed foods in the USA and many other countries also questions their current public health importance and their role in a nutrient density algorithm. Cholesterol intake has a recommended limit of no more than 300 mg/d, and it is seldom included in nutrient density algorithms; in part, because dietary cholesterol has only a tenuous link to serum cholesterol levels( Reference Kanter, Kris-Etherton and Fernandez 36 ).
The levels of selected nutrients to define nutrient density are also ambiguous. The words ‘substantial’ for amounts of vitamins and minerals and ‘few calories’ (or kJ) used by the DGA are vague, and without definition, not only for scientists but also consumers. The best estimate for ‘substantial amounts of vitamins and minerals’ comes from the US Food and Drug Administration’s criteria for foods that can be considered for a health claim. These foods must provide >10 % of the Daily Value (DV) of protein, dietary fibre, vitamin A, vitamin C, Ca or Fe per RACC, prior to supplementation, and less than 13 g of total fat, 4 g of SFA, 60 mg of cholesterol or 480 mg of Na per RACC( 37 ). The concept of ‘relatively few calories’ has no reference standard at all.
Whether the nutrients considered in defining nutrient density should be country specific is another question. Nutrients that were identified in the (2005) DGA as low in the diets of US populations were Ca, K, dietary fibre, Mg, vitamins A, C and E for adults; Ca, K, dietary fibre, Mg and vitamin E for children and adolescents; vitamin B12, Fe, folic acid and vitamins E and D for specific populations( 2 ). But the 2010 DGA identified dietary fibre, Ca, vitamin D and K as nutrients of public health concern for children and adults, and others as nutrients of concern for sub-populations( 3 ). Are these nutrients shortfall nutrients in other countries? And even within the USA, would updates to DGA mean that new nutrient density algorithms would need to be developed every five years? If fortificants are added to the food supply, would these nutrients remain shortfall nutrients?
The question of how many nutrients are required to make a food nutrient dense also remains unanswered. For example, is a food that contains high amounts of one or two nutrients any less nutrient dense than a food that contains moderate amounts of several vitamins and minerals?
3. Are foods that are high in shortfall nutrients, but also contain nutrients to limit (e.g. SFA and trans-fat, total fat, cholesterol, Na and added sugars), nutrient dense?
The majority of algorithms used to determine nutrient density include nutrients to limit (Table 1); however, there is no standard definition of ‘nutrient to limit’ or the number to include, which is reflected in Table 1. There are also potential problems with adding too many nutrients to the formulas, in that nutrient density can decrease with too many nutrients in the algorithms( Reference Fulgoni, Keast and Drewnowski 6 ).
Here, we give four examples of foods that are high in both shortfall nutrients and nutrients to limit, using the Weighted Nutrient Density Score (WNDS) to illustrate inconsistencies in defining nutrient-dense foods. The WNDS is one algorithm that models nutrient density; it uses eight nutrients (all capped at 100 % of the DV) and explains 65 % of the variance in Healthy Eating Index (HEI)( Reference Guenther, Reedy and Krebs-Smith 38 ) scores( Reference Arsenault, Fulgoni and Hersey 5 ). To increase the variation by only 1 % (to 66 %), three additional nutrients – vitamin D, K and folate – would need to be added. Although this is only one such algorithm to assess nutrient density, it can be used to standardize the examples presented.
Example 1
Poultry without skin has been specifically designated by the DGA as a nutrient-dense food; however, poultry with skin has not been. Why? Poultry skin clearly affects the nutrient density of chicken calculated using the WNDS: chicken leg with skin (WNDS ~18), without skin (WNDS 25·5). However, the fatty acid composition of poultry skin comprises primarily unsaturated fatty acids, not SFA. Chicken skin (per ounce (~28 g)) has 3·4 g of SFA, 5·4 g of MUFA and 2·5 g of PUFA( 35 ). Has poultry with skin been designated as a ‘non-nutrient-dense’ food because of the total fat content, which ignores the beneficial MUFA and PUFA; the relatively low SFA content; or because of the energy (548 kJ (131 kcal)/ounce (~28 g))( 35 ) it adds to the food? There is no reason given in the DGA. By itself, poultry skin would have a near-perfect score of 10 in the fatty acid ratio subcomponent of the HEI-2010. Since the term ‘relatively few calories (kJ)’ required for a nutrient-dense food is undefined, it is unclear if poultry skin fits into this category.
Example 2
The score for whole-wheat bread is higher (WNDS 41·1) than that for white bread (WNDS 9·5), with dietary fibre being the likely nutrient driving the score. So, using this criterion, whole-wheat bread is more nutrient dense than white bread; however, if other nutrients such as Fe, thiamin and folate were used in the calculation of nutrient density, would the nutrient density scores have been different? Whole-wheat bread is recommended by MyPlate( 19 ) and is a ‘Go’ food in the National Heart, Lung, and Blood Institute’s ‘We Can’ list of foods( 39 ). Consumption of whole-grain products has been associated with better diet quality and health biomarkers( Reference O’Neil, Zanovec and Cho 40 , Reference O’Neil, Nicklas and Zanovec 41 ), but because they may not be fortified as refined-grain products are, they may not provide the nutrient profile that refined grains do.
Example 3
The 2005 DGA( 2 ) and the 2010 DGA( 3 ) clearly state that nutrient-dense foods do not contain added fats or sugars: ‘Selecting lower-fat forms of foods in each food group and forms free of added sugars—in other words nutrient dense versions of foods—provides individuals a way to meet their nutrient needs while avoiding the over-consumption of (kilo)calories and of nutrients such as SFA’. Does this definition mean that foods such as pre-sweetened (PS) fortified ready-to-eat cereals (RTEC), low-fat flavoured milk and fruited low-fat yoghurt are not nutrient dense? The effect that nutrients to limit has on the calculated nutrient density is striking; for example, added sugars changes the nutrient density of Cheerios® (WNDS 49·5) to that of Frosted Cheerios® (WNDS 11·3). Both types of cereal are fortified with important nutrients: vitamins A, C, D, B6 and B12, thiamin, riboflavin, niacin, Ca, Fe and Zn. Should both types of cereal be considered nutrient dense? We think so. Consumption of any type of RTEC has also been associated with consumption of milk( Reference Song, Chun and Kerver 42 ), which provides additional nutrients, including three of public health concern (vitamin D, Ca and K)( 3 ), as well as protein, riboflavin, vitamin B12 and P. Both RTEC and PSRTEC have been associated with improved diet quality, dietary adequacy, lower weight and better cardiovascular risk profiles( Reference Albertson, Affenito and Bauserman 43 – Reference Miller, Liska and Fulgoni 48 ), suggesting that the amount of sugar added to PSRTEC is not detrimental to diet quality, dietary adequacy or overall health risk. Further, consumption of PSRTEC by children has not been associated with intakes of added sugars over the Institute of Medicine’s recommendation (CE O’Neil, T Nicklas and VL Fulgoni, unpublished results). Most RTEC, including PSRTEC, are also low in SFA and Na. Thus, should PSRTEC be considered nutrient dense by virtue of their micronutrient fortification, and low fat and Na content, despite their added sugars? The authors believe ‘yes’, despite the results of the algorithm and the recommendation by the 2010 DGA( 3 ). It is also important to note that many individuals add sugar to RTEC, with less control and precision than when PSRTEC are prepared; thus, added sugars in RTEC may actually be higher than those seen in some PSRTEC.
Example 4
Similarly, low-fat chocolate milk and fruited low-fat yoghurt have added sugars, but should these foods be considered nutrient dense by virtue of their nutrient contribution to the total diet? The nutrient density score of chocolate milk varies with the amount of SFA in the product. Whole milk-based chocolate milk has a WNDS score of −3·1, whereas a skimmed milk-based chocolate milk has a WNDS score of 31·3 (compared with 54·73 for skimmed white milk). Skimmed milk-based chocolate milk has virtually no SFA; however, an 8-ounce (~237 ml) serving has 15·5 g of added sugars and 165 mg of Na (6·9 % DV)( 35 ). Does the presence of added sugars and the high Na content negate the fact that chocolate milk also provides >10 % of the DV for protein, Ca, P, K, riboflavin, pantothenic acid and vitamins A, D and B12 (35) ? High levels of three nutrients of public health concern, identified above, are present in chocolate milk and contribute to the nutrient intake of children( Reference Nicklas, O’Neil and Fulgoni 49 , Reference Murphy, Douglass and Johnson 50 ). Further, consumption of chocolate milk has not been negatively associated with weight in children( Reference Murphy, Douglass and Johnson 50 ). If children will not drink recommended amounts of plain milk, but will drink chocolate milk, should the added sugars content deter health professionals from recommending it because it is not ‘nutrient dense’? A similar argument could be made for non-fat fruited yoghurt with added sugar or vegetables served with Ranch Dressing®. By the DGA definition, foods like low-fat chocolate milk, fruit yoghurt or vegetables with dressing that contain added fats or sugars are not nutrient dense. The DGA definition of nutrient-dense foods needs to be revisited.
4. Should all foods within a specific food group designated by the Dietary Guidelines for Americans as nutrient dense actually be considered as nutrient dense?
The DGA has ‘awarded’ nutrient dense status to all fruit, vegetables, lean meats, poultry without skin, beans, non-fat/low-fat milk products and whole/enriched grain products by fiat( 3 ). But foods within a group can show considerable heterogeneity in nutrient content. Many questions revolve around the nutrient density of fruit and vegetables; and these questions result from the lack of definition of nutrient density. Some vegetables (e.g. iceberg lettuce, celery, mushrooms and courgettes) have a high water content and contain few vitamins and minerals, but they are low in energy and contain some fibre( 35 ). This leads to another question: should there be distinctions in nutrient density within the fruit and vegetable groups to be consistent with the distinctions in the other food groups, i.e. refined v. whole grains?
Fruit and vegetables vary widely in their nutrient density scores. For fruit, the WNDS( Reference Arsenault, Fulgoni and Hersey 5 ) ranges from 3·5 for dried cranberries to 204 for raw blackberries. Does this imply that dried fruits are not healthful? Dried fruit is a rich source of nutrients( 35 ) and its consumption has been associated with better diet quality and reduced levels of obesity in the USA( Reference Keast, O’Neil and Jones 51 ). Although data are limited, there also appears to be a clear effect that processing or preparation has on the nutrient density of a given food; for example, the WNDS of an apple is 66, of unsweetened apple sauce is 36 and of 100 % apple juice is 21.
The WNDS( Reference Arsenault, Fulgoni and Hersey 5 ) for vegetables ranges from approximately 35 for cooked corn to 287 for raw endive, chicory, escarole or romaine lettuce. The WNDS for lettuce, celery and mushrooms is 148, 146 and 103, respectively. By comparison, raw spinach has a WNDS of 216. Although across-method comparisons cannot be made, within-method comparison of scores suggests that while lettuce, celery and mushrooms are not as nutrient dense as raw spinach, they have reasonably high nutrient density scores, when compared with some non-plant foods. Scores may have been higher if phytochemicals had been included; for example, iceberg lettuce and celery have some lutein + zeaxanthin, but raw spinach has very high levels of phytochemicals( 35 ). Many mushrooms have β-glucans and phenolic compounds that provide health benefits; those mushrooms that have been irradiated are high in vitamin D, a nutrient of public health concern( Reference Guillamón, García-Lafuente and Lozano 52 ). How can these important components of these foods be captured in the currently available algorithms to determine nutrient density? The gradation in nutrient density among vegetables suggests that it is important to follow MyPlate’s recommendation for variety in vegetable selection( 19 ); although it is not clear the extent to which both inter- and intra-variety is needed.
Nuts are another example. Nuts are considered by the DGA to be nutrient dense, but one serving (1 ounce (~28 g)) of cashew nuts has an SFA content of 14 %, which exceeds the ‘recommendation’ for SFA of <10 % of energy recommended for the total diet( 35 ). Macadamia nuts, Brazil nuts and some varieties of pine nuts are also relatively high in SFA( 35 ). Does this mean that these nuts are not nutrient dense and cannot be included in an overall healthy diet? Do all foods need to contribute less than 10 % of energy from SFA or can the whole diet be balanced? No it doesn’t; consumers of tree nuts have consistently been shown to have better nutrient intake profiles, diet quality, lower weight and better health risk markers than non-consumers( Reference O’Neil, Keast and Nicklas 53 , Reference O’Neil, Keast and Nicklas 54 ).
5. Should definitions for nutrient density consider the bioavailability of nutrients by food source?
Bioavailability of nutrients and its effect on nutrient density are nearly impossible to determine. For example, 418 kJ (100 kcal) of raw spinach (432 g) has 431 mg Ca, more than the 359 mg Ca in 418 kJ (100 kcal) of non-fat milk (293·8 g)( 35 ). But while the 418 kJ (100 kcal) of non-fat milk is a reasonable serving size (1·2 cups; although not that promulgated by MyPlate), 418 kJ (100 kcal) of raw spinach is not (61 cups). Also, some plant foods, like spinach, contain high concentrations of indigestible salts, such as oxalic acid, which is a potent inhibitor of Ca. Thus, mean Ca absorption from spinach is only 5 % compared with a mean of 27 % from milk ingested at a ‘similar load’( Reference Heaney, Weaver and Recker 55 ). However, the oxalate content of spinach varies widely (400–900 mg/100 g fresh weight) among samples( Reference Koh, Charoenprasert and Mitchell 56 ), which would alter interference with absorption in the spinach in different samples.
The same argument can be made for Fe; the non-haem Fe found in spinach (11·79 g/418 kJ (100 kcal) – an implausible intake) is not as bioavailable as the haem Fe found in meat. Moreover, the overall efficiency of Fe absorption is linked to the Fe status of the individual; if someone is Fe deficient, he/she will absorb more Fe. Absorption of non-haem Fe is also influenced by other foods consumed; for example, vitamin C increases absorption of non-haem Fe, as does concomitant consumption of foods with haem Fe. These physiological variations, both in the animal and plant foods and in the absorption potential by consumers, make it virtually impossible to include the bioavailability of nutrients in foods in any consideration of a nutrient density algorithm, although they are extremely important.
6. Should nutrient density be defined differently for naturally nutrient dense foods v. fortified nutrient dense foods?
With few exceptions, such as supplemental crystalline vitamin B12 for those over 50 years of age, the DGA( 3 ) recommends that nutrients come from foods. So, should nutrient density be defined differently for fortified foods? Many foods in the USA are micronutrient fortified, for example those made with enriched flour, as mandated by law. Other foods are not fortified by law but as a public health measure, for example iodized salt; and yet other foods, such as RTEC, are nutrient fortified voluntarily by the manufacturer. Finally, the potential exists that some forms of nutrients used in food fortification are better absorbed than those occurring in some natural forms; for example, the form of vitamin B12 used in RTEC is better absorbed than those forms in meat or eggs( Reference Tucker, Rich and Rosenberg 57 ). This underscores the effect that the bioavailability of nutrients has on nutrient density.
7. Should the nutrient density of individual foods be considered, or is it more important to consider the nutrient density of the entire diet?
People do not eat individual foods alone; they either prepare mixed dishes, such as pastas, stews or casseroles, or serve a variety of foods at a single meal or snack – some of which may be nutrient dense, whereas others may not be. For example, with few exceptions, a fruit salad or vegetable with no sugar or oils added would have a high nutrient density score; however, if an oil- or sugar-based dressing were added, would the food still be nutrient dense? Mixed dishes tend to have low nutrient density scores, since they are made from both nutrient-dense and non-nutrient-dense foods; during preparation, they often have SFA, sugars or Na added. Lasagne with meat, for example, has a WNDS of 2·9. Nutrient-dense foods in the lasagne are the carrots, onions and celery; non-nutrient-dense foods, as defined by the DGA, include whole milk, commercial tomato paste (high in Na), refined-grain pasta, pancetta, wine, Parmesan cheese, ground meat and butter. Oils, especially heart-healthy olive oils, are also used; although these are not defined by the 2010 DGA as a nutrient-dense food, they do have a recommendation in the US Department of Agriculture meal patterns (27 g (5·4 tsp) in a 8368 kJ (2000 kcal) diet). Can the nutrient density score be improved without compromising the dish? To a degree: lean meat, low-Na tomato paste, fat-free milk and whole-grain pasta can be used; also the amount of cheese can be reduced. Vegetables, including aubergine and courgette, can also be added to the lasagne or can replace the pasta altogether. Will this produce a similar menu item? Maybe not. Will this mixed dish be a truly nutrient-dense food? Probably not. Can it provide substantial amounts of nutrients? Yes. Can the food fit into an overall healthy diet? Yes. Can the menu item be acceptable to consumers? It’s unclear, but it may be acceptable to some consumers. Do consumers know how to prepare a more nutrient-dense version of this food and other mixed foods? It’s unclear, and consumer education is likely needed.
8. What organization, institution or government agency will take up the task of defining nutrient density and nutrient-dense foods?
This depends on the ultimate use(s) of the nutrient profiling and the country involved. If nutrient profiling is to be used for regulatory purposes, appropriate agencies will need to be involved; for example, if the terms are to be used for food labels in the USA, the Food and Drug Administration should be the agency to establish criteria. Consumer education uses could potentially involve consumer groups in conjunction with regulatory agencies and industry. For advertising to children, the Institute of Medicine might set the criteria( 58 ), in collaboration with industry. If nutrient profiling is to be used for other purposes, such as television advertising for children, other agencies might need to be involved. In Europe, the consensus of the European Union (EU) would be important.
The perspective of the European Union
The issues about nutrient profiling of foods and the nutrient density of foods confronting Europeans actually seem very similar to those we face in the USA. Conditions for the EU-wide use of nutrition and health claims were set by the European Parliament and Council Regulation 1924/2006( 59 ). In order to carry a claim, all foods and beverages had to have a favourable nutrient profile( 59 ). Nutrient profiles were to be based primarily on excessive content of disqualifying nutrients, salt, sugar and fat. Researchers and industry were encouraged to develop nutrient profile models to provide a scientific basis for the regulation of health claims( 59 ). This regulation gave the EU commission the ‘task to establish specific nutrient profiles, including exemptions, which foods or certain categories of foods must comply with in order to bear nutrition of health claims and the conditions for the use of nutrition of health claims’. The goal was to restrict products considered to be unhealthy from making nutrition and health claims. However, the development of nutrient profiling systems, originally scheduled for 2009, has not been finalized due to a lack of consensus and both internal and external opposition( 60 ).
One concern was that nutrient profiling would be used as reference by regulators to tax or restrict the marketing and sale of ‘bad foods’. In 2010, members of the European Parliament voted to delete the nutrient profiling provision from the Nutrition and Health Claims Regulation for the EU. With the European Parliament evenly split, nutrient profiling became a political issue. At this time, there is still uncertainty about developing a pan-European nutrient profiling system, since any Commission initiative in this area would need the approval of all twenty-seven EU member states( 61 ).
In the meantime, individual country jurisdictions, not to mention private industry, have been developing their own nutrient profiling systems. Among these are the SAIN,LIM system adopted by the French regulatory agency ANSES( Reference Masset 62 ) and the FSA–Ofcom system developed for the corresponding regulatory agency in the UK( Reference Masset 62 ). A recent study published in a peer-reviewed journal provided validations of nutrient profiling systems with respect to independently obtained measures of a healthy diet( Reference Darmon, Vieux and Maillot 63 ).
The technical concerns about the methodology of nutrient profiling are comparable in the EU and in the USA. In 2006, representatives from nearly fifteen different countries, attending an International Life Sciences Institute Europe workshop, concluded that a ‘food category’ approach was better than an ‘across the board’ system for nutrient profiling and that further studies were needed to identify the final list of nutrients to be considered in nutrient profiling( Reference Tetens, Oberdörfer and Madsen 9 ).
The member states of the EU have dietary recommendations for nutrients, foods or dietary patterns that are consistent with health promotion and reduction of diet-related chronic disease( 64 , 65 ). However, as seen in the USA, in Europe too there is confusion as to what constitutes a healthy diet and what recommendations should be for diets that support health. In particular, nutrient profiling systems need to balance scientific rigour and policy goals. Even in the absence of a nutrient profiling system, the European Commission approved only 222 health claims (listed in the EU online register) but rejected more than 1600. The work of the European Commission on nutrient profiling has resumed, although no target date has been specified as yet.
Apparent contradictions and inconsistencies in nutrient profiling systems
There are some contradictions and inconsistencies when defining nutrient density. It is also apparent that the nutrient density of foods varies depending on the standard ‘unit’ used in the definition. The challenge is to identify a standard unit that reflects a reasonable serving for some foods and is a concept that consumers can understand. The implications of the 2010 DGA are that all fruits and vegetables with no added fat or added sugar are nutrient dense. However, as seen above, this may not be the case. From this commentary, fruits and vegetables vary in terms of their nutrient density. Moreover, fruits and vegetables would attain higher nutrient density scores if phytochemicals were considered in the nutrient density definition.
Lean meats have been considered a nutrient-dense food because of the nutrient profile unique to this food group. However, lean meats still have relatively high levels of SFA compared with other food groups. Moreover, some of the nutrients rich in lean meats, including vitamin B12, Fe and Zn, have not been typically considered in nutrient profiling systems. Thus, lean meats may be excluded as a nutrient-dense food group by virtue of the nutrient density definition. Ignoring or excluding a food group may have important nutritional implications in the diets of Americans. Further, although there is an ‘official’ US Department of Agriculture definition of lean meat (the portion of total beef containing 9·28 g of total fat or less per 100 g (after cooking))( Reference Bowman, Friday and Moshfegh 66 ), it is unlikely that consumers are aware of it.
Another issue is that total energy content is not considered in most nutrient density definitions. Thus, foods that are both nutrient dense and energy dense may pose some challenges when translating the nutrient density concept to consumers. It is unclear if this nutrient- and energy-dense concept will be taken to another level of complexity in that they should be consumed in ‘moderation’. Finally, mixed dishes may be identified as nutrient dense yet one or more of the ingredients may be categorized as non-nutrient dense when consumed alone.
Potential unintended consequences in using a nutrient density approach
Nutrient-dense foods can also be energy dense( Reference Nicklas, O’Neil and Mendoza 67 , Reference Huth, Fulgoni and Keast 68 ). As Huth et al.( Reference Huth, Fulgoni and Keast 68 ) pointed out: ‘three of the top 10 sources of (kilo) calories (or kJ) and SFA (beef, milk and cheese) contribute 46·3 % of the calcium, 49·5 % of the vitamin D, 42·3 % of the vitamin B12 as well as other essential nutrients to the American diet’. Despite that these foods provide nearly one-half of Ca and vitamin D, identified by the 2010 DGA as nutrients of public health concern( 3 ), should these foods be eschewed because they are high in energy and SFA? Nuts and avocados are other examples of nutrient-dense, energy-dense foods that have been associated with diet and health benefits( Reference O’Neil, Keast and Nicklas 53 , Reference O’Neil, Keast and Nicklas 54 , Reference Fulgoni, Dreher and Davenport 69 ). Further, as stated previously, some food sources that are high in energy, added sugars and SFA make major contributions to dietary fibre and micronutrients. Any dietary modifications that include reduction in energy, added sugars or SFA should consider such modifications so as not to induce the unintended consequence of lowering overall diet quality( Reference Huth, Fulgoni and Keast 68 ).
Is it reasonable to assume that Americans will consume vegetables without added fats or Na? A study has shown that children will consume more bitter flavoured vegetables, like broccoli, if they are served with a Ranch Dressing® flavoured dip( Reference Fisher, Mennella and Hughes 70 ). If the recommendation is to choose a lower-fat version of the food, a low-fat dip may then add sugars or Na to the diet. With the dip, would the broccoli remain nutrient dense? Is it more important for children to consume nutrient-rich foods like broccoli, even at the expense of adding sugars and Na to the diet?
Labelling foods as either nutrient dense or not may seem to consumers to be a ‘good food–bad food’ approach. Promoting nutrient-dense foods as ‘good foods’, without regard to energy content, could mislead the consumer into thinking they can eat as many or as much of nutrient-dense foods as they want, which could result in energy intakes that exceed recommendations. Caution needs to be taken in applying nutrient density measures to individual foods rather than to total diets. Labelling foods as ‘good foods’ and ‘bad foods’ is inconsistent with a total diet approach and could cause people to abandon efforts to make dietary improvements, leading to unhealthy eating habits( Reference Freeland-Graves and Nitzke 71 ). The value of a food should be determined within the context of the total diet( Reference Freeland-Graves and Nitzke 71 ). A focus on moderation and proportionality in the context of the total diet, rather than on specific nutrients and foods, can help reduce consumer confusion. It is the position of the Academy of Nutrition and Dietetics that ‘the total diet and overall pattern of food eaten is the most important focus of a healthful eating style. All foods can fit within this pattern’( Reference Freeland-Graves and Nitzke 71 ). It is important that consumer messaging regarding nutrient density emphasizes a balance of foods, rather than any one food, within the context of the total diet. Programmes such as the National Institutes of Health’s National Heart, Blood, and Lung Institute’s ‘Go, Slow, Whoa’ classification( 39 ) and NuVal( Reference NuVal 72 ) come very close to ‘good food–bad food’ systems. Although these may be easy for consumers to understand, it can also lead to omitting important foods from the diet.
Practical implications and conclusions
The 2005 report of the Dietary Guidelines Advisory Committee( 1 ) and the EU have called for the development of a scientifically valid definition for nutrient density and recommended that it would also be useful on the food label so consumers can use it to make educated decisions about buying and consuming healthier foods. Developing a valid measure for assessing the nutrient density of foods has the potential to assist consumers in making more nutritious food choices. Understandably, a consumer education programme will be needed to demonstrate how to use the nutrient density measure within the concept of the total diet. Katz et al.( Reference Katz, Njike and Faridi 7 , Reference Katz, Njike and Rhee 10 ) and Glanz et al. ( Reference Glanz, Hersey and Cates 73 ) showed that using the Nutrient Rich Foods approach was effective in improving the diet of consumers. Larger and longer studies are needed to confirm the findings once a consensus has been reached on whether a nutrient dense score should be developed and validated for single foods or more broadly for assessing nutrient density of the total diet( Reference Freeland-Graves and Nitzke 71 ).
Due to the difficulties in defining the concept of nutrient density, it seems ultimately that a real science-based definition may be unlikely due to the inherent complexities and lack of answers to the unresolved questions. As a result, in addition to the outstanding scientifically relevant questions that need to be addressed in developing such a definition, it is imperative to address whether consumers will understand and apply the concept of the nutrient density approach. Essentially, will the application of a nutrient density system be feasible, economical and culturally relevant to consumers, and will it help consumers change behaviour that translates into making wise food choices and consuming a healthier diet? Both the development of a standard nutrient density definition and the relevance to the consumer will need to be considered equally for such an approach to translate into appropriate and effective dietary changes that will result in a healthier and sustainable eating pattern.
Acknowledgements
Acknowledgements: The authors extend a special thanks to Lori Briones for help in preparing the manuscript and to Bee Wong for obtaining research articles. Weighted Nutrient Density Scores (WNDS) given throughout this manuscript are courtesy of Victor L. Fulgoni III, PhD. Financial support: This research project was supported by the US Department of Agriculture (USDA)/Agricultural Research Service (ARS) through specific cooperative agreement 58-6250-6-003 and from the Hatch project LAB 93951. This work is a publication of the USDA/ARS Children’s Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, Texas. The USDA/ARS had no role in the design, analysis or writing of this article. The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products or organizations imply endorsement from the US government. Conflict of interest: None. Authorship: T.A.N. conceptualized the study and wrote the first draft of the manuscript. A.D. contributed to sections of the manuscript. C.E.O’N. substantially revised the manuscript with critical feedback. Ethics of human subject participation: Ethical approval was not required.