Introduction
Angiostrongylus costaricensis is the causative agent of abdominal angiostrongyliasis (AA), a zoonosis that has been reported over an area extending from North America to subtropical regions of South America (Rodriguez et al., Reference Rodriguez, Dequi, Peruzzo, Mesquita, Garcia and Fornari2008). Its life cycle involves terrestrial molluscs as intermediate hosts (Rodriguez et al., Reference Rodriguez, Sandri and Porto2019) and rodents as definitive hosts (Graeff-Teixeira et al., Reference Graeff-Teixeira, Avila-Pires, Machado, Camillo- Coura and Lenzi1990). Humans are considered to be accidental hosts (Mota & Lenzi, Reference Mota and Lenzi2005).
The clinical signs of this disease are abdominal pain, anorexia, fever, malaise, vomiting, nausea, diarrhoea or constipation (Graeff-Teixeira et al., Reference Graeff-Teixeira, Camillo-Coura and Lenzi1987). In laboratory tests, leucocytosis, marked eosinophilia and anaemia are observed; and, due to hepatomegaly, there may be changes in liver enzymes (Rodriguez et al., Reference Rodriguez, Dequi, Peruzzo, Mesquita, Garcia and Fornari2008). Therefore, as a result of the biological cycle, not only the intestine is injured, but also other organs end up affected by the action of the parasite (Mota & Lenzi, Reference Mota and Lenzi2005).
In humans, the diagnosis of AA is based on histopathological findings, through identifying worms, eggs or larvae in arterial or vascular lumina (Rodriguez et al., Reference Rodriguez, Dequi, Peruzzo, Mesquita, Garcia and Fornari2008). Presence of adult nematodes in the mesenteric arteries and the inflammatory reactions that occur as a result of larvae and eggs give rise to pathogenesis of AA. Haemorrhagic or ischaemic infarctions and granulomatous reactions that can progress to intestinal stenosis are detected. These lesions are seen mainly in the ileocaecal region. The macroscopic findings consist of intestinal wall thickening and occurrences of congestive necrotic lesions. Microscopically, there are three histopathological findings: granulomatous reactions; eosinophil vasculitis; and eosinophil infiltration into the intestinal wall layers (Graeff-Teixeira et al., Reference Graeff-Teixeira, Camillo-Coura and Lenzi1991).
An oxidative stress (OS) process can be established as a result of the occurrence of an imbalance between oxidant and oxidant compounds that leads to the excessive generation of free radicals or to a decrease in the speed of their removal (Amer et al., Reference Amer, Dkhil, Hikal and Al-Quraishy2015). Free radicals are linked to a wide variety of diseases, including liver disease, cancer and aging (Lima & Abdalla, Reference Lima and Abdalla2001). Excess free radicals in the body are countered by antioxidants produced by the body or absorbed from the diet. The human organism suffers constant action of reactive oxygen species (ROS) and reactive nitrogen species generated in inflammatory processes, due to some biological dysfunction or from food. When ROS are produced in excess or for long periods, they can exert toxic effects that damage cells and tissues, resulting in the dysfunction of physiological processes. This process leads to oxidation of biomolecules, with consequent loss of their biological functions and/or homeostatic imbalance. These consequences are manifested as a potential for oxidative damage to cells and tissues, causing OS (Halliwell & Whiteman, Reference Halliwell and Whiteman2004). Thus, during its life cycle, A. costaricensis may be exposed to different ROS generated by the parasite's metabolism, to the host's immune response, as it occurs with infections by Trypanosoma cruzi, in Chagas disease (Docampo, Reference Docampo1990; Turrens, Reference Turrens2004).
There is increasing scientific evidence showing that OS has important implications for the mechanisms that culminate in the development of future diseases in humans (Furukawa et al., Reference Furukawa, Fujita and Shimabukuro2004). OS plays an important role in intestinal, liver and pancreatic damage. It acts as the activating factor for organ barrier dysfunction, thus triggering immune imbalance and inflammation (Que et al., Reference Que, Cao, Ding, Hu, Mao and Wang2010; Han et al., Reference Han, Yao, Liu, Li, Zhang, Hei and Xia2016; Yang et al., Reference Yang, Zhang, Jiang, Lei, Yu, Xie and Chen2019). In this context, we tested the hypothesis that infection by A. costaricensis causes severe intestinal, liver and pancreatic damage, and interferes with the homeostasis of the organism. Thus, the objective of the present study was to evaluate the relationship between histopathological lesions and markers for OS.
Materials and methods
Animals
Twenty-eight male Swiss mice, from the Animal Laboratory of the Institute of Biological Sciences of the University of Passo Fundo, located in the city of Passo Fundo, in the state of Rio Grande do Sul, Brazil, were used in this study. These animals were kept in plastic cages (polypropylene) with stainless steel grids. These do not have ‘living corners’ inside them, as the animals tend to gnaw at any ledge to try to escape; they are rectangular and closed at the top to allow the accommodation of the pelleted feed (Nuvilab®) and the bottle containing water; they are safe, not allowing the animals to escape; easily allow observation and feeding; have proper ventilation; they are hygienic and easy to clean; comfortable, allowing animals ample freedom in their movements; and allow easy access to food and water. Animals are kept under ideal temperature conditions (23 ± 2°C), ad libitum and lighting conditions (12/12 hour light/dark cycle) (Andrade et al., Reference Andrade, Pinto and Oliveira2002).
The ‘three Rs principle’, that is, reduction, replacement and refinement, was followed in this experiment. This principle envisages that, whenever possible, data obtained through previous animal experimental studies should be used, and it encourages reduction of animal use when there is no real need for this (Benvegnú et al., Reference Benvegnú, Hermes, Guizzo, Soares, Costa, Rodriguez, Frandoloso and Vieira2021).
Formation of experimental groups
These 28 animals were selected in two groups containing 14 animals each, for this training. The animals were selected based on their characteristics, such as same weight, size and age. In each of the two groups (G1 and G2) eight animals were infected and six served as uninfected controls. The experimental infection was performed using the gavage method with a dose of 10 infective larvae (L3) per animal (0.1 to 0.3 ml). The G1 animals were euthanized at 14 days post-infection (dpi) and the G2 animals, at 24 dpi. Blood samples were collected at 07:30 am through intracardiac puncture, with previous anaesthesia of the animals with Isoflurane (Isoforine®). The blood was stored in tubes with ethylenediaminetetraacetic acid and the serum was separated by centrifugation at 2500 rotations per minute for five minutes and was frozen at −70°C until use (Benvegnú et al., Reference Benvegnú, Hermes, Guizzo, Soares, Costa, Rodriguez, Frandoloso and Vieira2021).
Sample preparation
Serum samples were obtained on the same day that blood was collected and homogenized in phosphate-buffered saline (pH 7.5), using an Ultra-Turrax homogenizer, and centrifuged (3000 × g for 10 min) (Llesuy et al., Reference Llesuy, Milei, Molina, Boveris and Milei1985). The supernatant was separated and aliquoted to assess the levels of proteins, non-protein thiols (NPTs) and nitric oxide (NO).
Histopathology
The preparations and histopathological analyses were performed according to the methodology previously described by our team (Sandri et al., Reference Sandri, Rodriguez, Costa, Porto, Schwingel and Vieira2018). Macroscopic evaluation to identify anatomical alterations, such as the presence of ischaemic lesions and formation of pseudotumours, was performed in the animals during necropsies. Specimens (pancreas, liver and intestine) with visible changes were fixed in 10% formalin for 24 h and embedded in paraffin to obtain histological sections (5 μm thick). The slides were prepared for histological analysis from three sections and stained with haematoxylin and eosin, for later analysis under an optical microscope. The following aspects were investigated: (a) presence of infarction; (b) identification of eggs, larvae and adult worms; (c) presence of eosinophilic infiltrate (focal, multifocal and diffuse); (d) granuloma formation (focal, multifocal and diffuse); and (e) characterization of vasculitis, thrombosis and pancreatitis (Hermes et al., Reference Hermes, Benvegnú, Costa, Rodriguez and Vieira2020; Benvegnú et al., Reference Benvegnú, Hermes, Guizzo, Soares, Costa, Rodriguez, Frandoloso and Vieira2021) (figs 1–3).
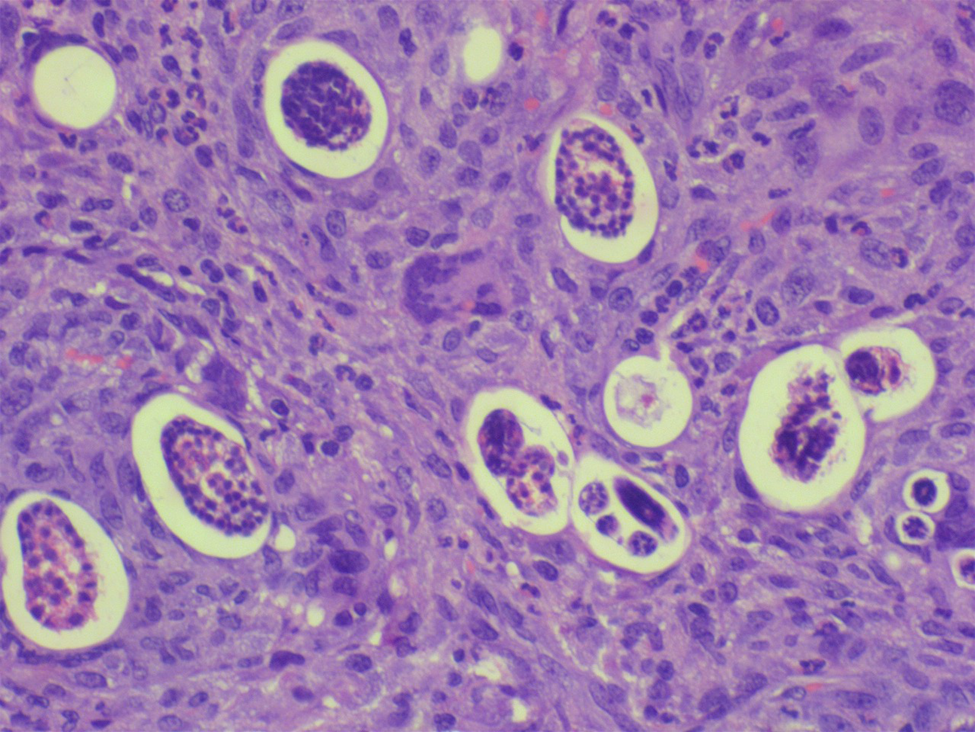
Fig. 1. Submucosa of small intestine with granuloma enveloping the eggs and larvae (haematoxylin and eosin-200×).

Fig. 2. Acute pancreatitis at 24 days post-infection – necrosis area with eosinophilic infiltrate (haematoxylin and eosin-100×).
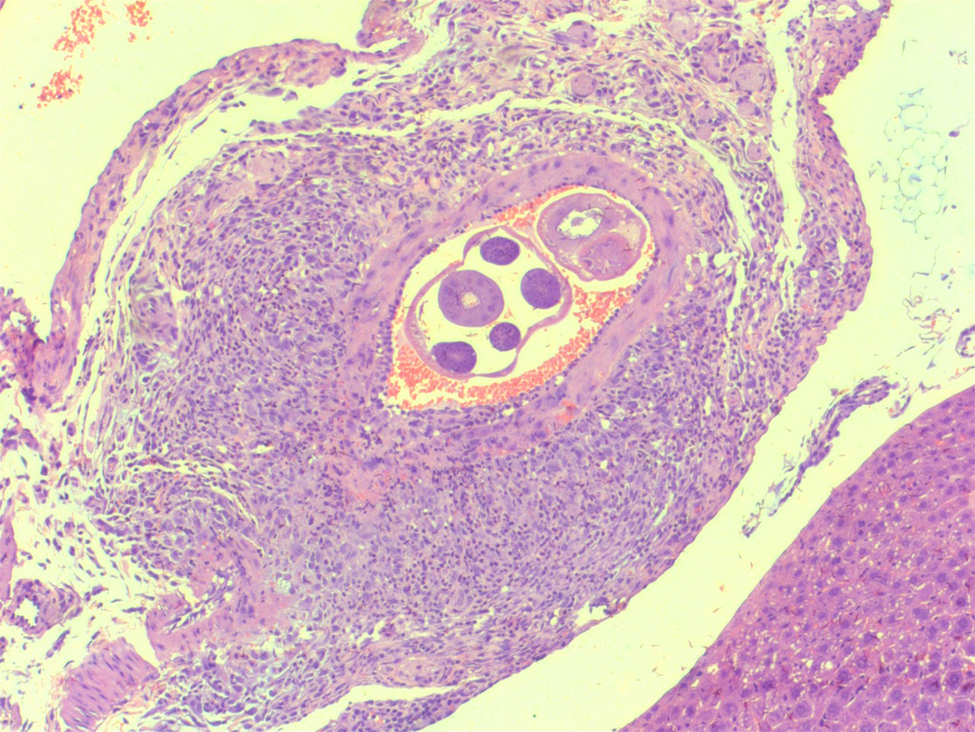
Fig. 3. Artery in the liver with thrombi and worm of Angiostrongylus costaricensis (haematoxylin and eosin-50×).
Oxidative damage parameters
NO
The NO concentration was indirectly evaluated using the Griess reagent (Bracht & Ishii-Iwamoto, Reference Bracht and Ishii- Iwamoto2003). NO end products (nitrite/nitrate) were collorimetrically quantified by means of spectrophotometry at 540 nm. The results were expressed as μgmol/g of protein.
NPTs
The levels of NPTs were measured through indirect intracellular measurement of glutathione levels (Ellman, Reference Ellman1959). The product from this reaction was quantified by means of spectrophotometry at 412 nm. The results were expressed as μgmol/g of protein.
Protein quantification
Protein concentrations were determined using Coomassie blue, in the Bradford method (Bradford, Reference Bradford1976) and bovine serum albumin was used as a standard. Absorbance was measured at 595 nm and the results were expressed in g/l.
Statistical analysis
For comparisons between the groups (G1: control vs. infected; G2: control versus infected), the normality and homoscedasticity of the variables of NO and NPTs levels were tested using the Shapiro–Wilk and Levene tests, respectively. The variables that met the above prerequisites, were compared using the t-test for independent samples, with the exception of the variable of NO in G2, which showed heteroscedasticity of variances, and thus, required Welch correction in the t-test for independent samples.
For comparison between histopathology lesions of G1 and G2, a cross table was used for analysing the observed frequencies, and differences in proportions were analysed by Yates’ correction Chi-square.
To describe the effects of lesions on the levels of NO and NPTs, multiple linear regression models were used for the different periods of time analysed (G1 and G2). Given the large number of predictor variables in each model, they were selected through their t-values (values that presented P < 0.05), and in terms of whether the model with any given variable was significantly better than the model without it. To select the best models, they were subjected to simplification using the methodology of the Akaike information criterion, with correction for small sample size (corrected Akaike information criterion (AICc)) (Hurvich & Tsai, Reference Hurvich and Tsai1989; Burnham et al., Reference Burnham, Anderson and Huyvaert2011; Symonds & Moussalli, Reference Symonds and Moussalli2011). The adjusted coefficient of determination (R²) was calculated to determine the variability of the best model. Data were considered significantly different with a probability (P) of less than 5%. The R statistical package was used for the statistical analysis and the GraphPad Prism software, version 7.01, was used to generate graphs.
Results
Histopathology findings
Liver nodules were identified in both infected groups (28% at 14 dpi and 62.5% at 24 dpi) and the infected group at 24 dpi had splenic infarction (37.5%). Changes in the pancreas, such as pancreatitis, were observed in both infected groups (37.5% at 14 dpi and 75% at 24 dpi). At 24 dpi, 62.5% of the mice had eosinophilic infiltrate, in addition to vasculitis, granuloma necrosis and thrombi. The animals in G2 (24 dpi) presented, in their entirety, worms, eggs and larvae in different organs (table 1 and figs 1–3) indicating patent infection.
Table 1. Microscopic findings of mice infected with Angiostrongylus costaricensis at days 14 (G1) and 24 (G2) post infection.

* Probability less than 5% (P < 0.05) indicates a significant difference (Chi-square test with Yates correction).
Changes to OS parameters in mice experimentally infected with A. costaricensis
Among the OS parameters, the infected animals of both groups presented NO release, in comparison with the control group, as shown in fig. 4.

Fig. 4. Serum nitric oxide in mice experimentally infected with Angiostrongylus costaricensis (**P < 0.001, n = 13, t-test for independent samples; #P < 0.001, n = 12, t-test for independent samples with Welch correction).
In relation to NPTs, G1 showed a significant decrease, as can be seen in fig. 5.

Fig. 5. Serum non-protein thiols in mice experimentally infected with Angiostrongylus costaricensis (**P < 0.001, n = 13, t-test for independent samples; #P < 0.001, n = 12, t-test for independent samples).
Effects of lesions on levels of NO and NPTs
The multiple linear regression model that was proposed to describe the variability of NO in G1 (AICc = 178.1; difference in corrected Akaike information criterion values of the best model (dAICc) = 0.00; model weight = 0.96; df = 5) included the degree of eosinophil infiltration in the liver and intestine, and any presence of pancreatitis. The model was able to explain 97.72% (R²) of this variability. Thus, the presence of a mild or moderate infiltrate of eosinophils in the liver promoted on average increases in serum NO of 1520.19 and 40.99 μgmol/g, respectively. Moreover, the presence of moderate eosinophil infiltrate in the bowel and pancreatitis added, on average, 1666.46 and 132.29 μgmol/g of serum NO (table 2).
Table 2. Multiple linear regression model (MRL) proposed to describe the variability of nitric oxide (NO) in mice infected by Angiostrongylus costaricensis, over different periods of infection (G1 and G2).

β, coefficient; SE, standard error.
The multiple linear regression model proposed for the levels of NPTs in G1 (AICc = 182.3; dAICc = 0.00; model weight = 0.49; df = 5) included the degree of infiltration of eosinophils in the liver and intestine. This model was able to explain 92.16% (R²) of the variability of this parameter. Thus, the presence of mild or moderate eosinophil infiltrate in the liver promoted reductions in serum NPTs of, on average, 747.94 and 8.83 μmol/g, respectively. Lastly, the presence of moderate eosinophil infiltrate in the intestine promoted a reduction in serum NPTs of, on average, 1081.92 μmol/g (table 3).
Table 3. Multiple linear regression (MLR) model proposed to describe the variability of non-protein thiols (NPTs) in mice infected with Angiostrongylus costaricensis, at different periods of infection (G1 and G2).

β, coefficient; SE, standard error.
For G2, it was included in the descriptive model for NO (AICc = 169.5; dAICc = 0.00; model weight = 0.33; df = 5). There was presence of intestinal granuloma that, as the degree of this lesion increased (mild to moderate to severe), promoted increases in serum NO levels of, on average, 1194.64, 1320.36 and 621.20 μmol/g, respectively (table 2). This model explained 94.60% (R²) of the variability of this molecule. The model proposed for the levels of NPTs (AICc = 173.1; dAICc = 0.00; model weight = 0.50; df = 5) also included the presence of intestinal granuloma, which promoted reductions in the serum levels of NPTs of, on average, 462.41, 529.70 and 779.86 μmol/g, as the degree of granuloma increased from mild to moderate, and from moderate to severe, respectively (table 3).
Discussion
Studies that help to better elucidate AA are important for understanding the parasite–host relationship and, especially, its pathophysiology. Hermes et al. (Reference Hermes, Benvegnú, Costa, Rodriguez and Vieira2018) found that higher infectious doses (with 30 L3) of A. costaricensis L3 increase the amount of faecal shedding of L1 in Swiss mice with no effect on disease development (Hermes et al., Reference Hermes, Benvegnú, Costa, Rodriguez and Vieira2020). Higher doses resulted in abundant presence of eggs and larvae, hepatic infarction, splenitis and intestinal thrombosis, but did not alter the survival of these rodents; this is due to the co-evolutionary relationship between the parasite and the host, in which the parasite benefits from the survival of the parasite. host. enough time to allow the parasite to reproduce and propagate (Gandon et al., Reference Gandon, Buckling, Decaestecker and Day2008).
The mechanisms of immune response in helminth infections are multiple, due to the metabolic diversity of the parasites, which are antigenically complex. An additional problem is that the parasites can survive for many years in the host, by being able to evade the host immune system (Neva & Brown, Reference Neva and Brown1994). Another characteristic of parasitic infections, in situations in which the parasite has not yet adapted well to its definitive host, is that its persistence can induce an inadequate immune response, in addition to inducing morbidity and severe pathogenesis, as has already been observed in infections with A. costaricensis, in BALB/c mice (Geiger et al., Reference Geiger, Abrahams-Sandi, Soboslay, Hoffmann, Pfaff, Graeff-Teixeira and Schulz-Key2001).
At 14 dpi, we observed the presence of moderate eosinophil infiltration in the liver and intestine, along with pancreatitis, which contributed to the increase in NO levels. During this same period, there was the presence of a mild or moderate eosinophil infiltrate in the liver and intestine, and this may have led to the reduction in the serum levels of NPTs. In an experiment carried out by Benvegnú et al. (Reference Benvegnú, Hermes, Guizzo, Soares, Costa, Rodriguez, Frandoloso and Vieira2021), 28% of the infected animals in their G1 (14 dpi) presented liver nodules and 37.5% of the infected animals showed pancreatitis.
However, at 24 dpi, the presence of an intestinal granuloma was observed and, as the size of the granuloma increased, it led to an increase in the serum levels of NO. During this same period, there was a reduction in the levels of NPTs as the severity of the granuloma increased. Benvegnú et al. (Reference Benvegnú, Hermes, Guizzo, Soares, Costa, Rodriguez, Frandoloso and Vieira2021) observed the following among their animals at 24 dpi: splenic infarction (37.5%); liver nodules (62.5%); pancreatic changes (75%); and eosinophil infiltration (62.5%), besides granulomas, thrombi, vasculitis and necrosis.
In our study, the groups infected by A. costaricensis showed greater NO levels of systemic release. This may have been an attempt by the animals to wrestle the infection caused by the parasite, since NO is an important neurotransmitter and acts as a muscle relaxant, thereby increasing blood flow and showing a possible protective effect (Boeckxstaens et al., Reference Boeckxstaens, Pelckmans, Bult, De Man, Herman and Van Maercke1991; Whittle et al., Reference Whittle, Boughton-Smith and Moncada1992; Di Lorenzo et al., Reference Di Lorenzo, Bass and Krantis1995). Moreover, NO expression can be the result of an inflammatory response to the infection that is regulated by pro-inflammatory cytokines such as interferon gamma and tumour necrosis factor α (Body et al., Reference Body, Hartigan, Shernan, Formanek and Hurford1995; Creagh & O'Neill, Reference Creagh and O'Neill2006; Vieira, Reference Vieira2007; Benvegnú et al., Reference Benvegnú, Hermes, Guizzo, Soares, Costa, Rodriguez, Frandoloso and Vieira2021).
In addition, we observed that oxidative damage occurred in the G1 animals, as shown by the decrease in the levels of NPTs. Glutathione (GSH) is an antioxidant that has been recognized as the most important NPT in living systems. It exhibits a state of balance with oxidized glutathione (GSSG). The ratio between GSSG and GSH is a reliable indicator of OS, as it reflects the balance between an antioxidant state and the pro-oxidant reactions in cells (Viña, Reference Viña1990; Jones, Reference Jones2006). Changes to GSH metabolism have been linked to the pathogenicity of several diseases, such as acute pancreatitis, in which reduction of GSH in pancreatic tissue is a hallmark during the early stage of this disease (Neuschwander-Tetri et al., Reference Neuschwander-Tetri, Ferrell, Sukhabote and Grendell1992; Schoenberg et al., Reference Schoenberg, Birk and Beger1995). In this way, a change from cellular GSH to GSSG can lead to changes in the redox balance that favour continual presence of ROS, as occurred in our study.
The results obtained in the present study complement what was done by our team, by Hermes et al. (Reference Hermes, Benvegnú, Costa, Rodriguez and Vieira2020) and Benvegnú et al. (Reference Benvegnú, Hermes, Guizzo, Soares, Costa, Rodriguez, Frandoloso and Vieira2021). This indicated that the degree of inflammatory lesions is a result of parasite migration during the development of the biological cycle and is not related to the dose of infection. OS plays an important role in intestinal, liver and pancreatic damage, acting as an activating factor for organic barrier dysfunction, thus triggering immune and inflammatory imbalance (Que et al., Reference Que, Cao, Ding, Hu, Mao and Wang2010; Han et al., Reference Han, Yao, Liu, Li, Zhang, Hei and Xia2016; Yang et al., Reference Yang, Zhang, Jiang, Lei, Yu, Xie and Chen2019). The lesions caused by AA damage one of the main antioxidant defences, in addition to interfering with the body's homeostasis; therefore, infected animals become susceptible to greater tissue damage.
Conclusion
From the histopathological lesions found in the intestine, liver and pancreas, in mice experimentally infected with A. costaricensis, we concluded that they contributed to the increase in NO levels and decrease in NPTs, thus favouring the emergence of OS, causing damage to one of the body's main antioxidant defences.
Acknowledgements
To the University of Passo Fundo and to the Postgraduate Program on Bioexperimentation.
Financial support
The first author N.R. Zorzi was funded with a PROSUC/CAPES scholarship, mode II. The funding agency did not contribute to the study design, data collection, analysis or interpretation of data, in writing the manuscript. This was done independently by the investigators.
Conflicts of interest
None.
Ethical standards
This experiment was approved by the Ethics Committee on Animal Use of the University of Passo Fundo (CEUA-UPF - protocol no. 034/2016).
Authors’ contributions
All authors contributed to the conception and design of the study. Natalie Renata Zorzi and Maria Isabel Botelho Vieira conceived the ideas. Natalie Renata Zorzi, Elise Benvegnú, Caroline Hermes, Rubens Rodriguez, Natália Freddo, Bruno Webber, Francieli Ubirajara do Amaral, Cláudia Almeida Scariot, Márcio Costa, Luciana Grazziotin Rossato-Grando and Maria Isabel Botelho Vieira designed the methodology and collected the data. Márcio Costa, Luciana Grazziotin Rossato-Grando, Rubens Rodriguez and Maria Isabel Botelho Vieira analysed the data. Natalie Renata Zorzi wrote the manuscript. All authors read and approved the final manuscript.










