Acute rheumatic fever is an important cause of mortality and morbidity in developing and underdeveloped countries. It is one of the most common acquired heart diseases in children in developing countries, and a systematic review conducted in 2005 estimated that globally 471,000 cases of acute rheumatic fever are diagnosed annually. Reference Carapetis, Steer, Mulholland and Weber1,Reference Carapetis, Beaton and Cunningham2
The Jones criteria used for the diagnosis of acute rheumatic fever was recently revised in 2015. Reference Gewitz, Baltimore and Tani3 For the first time, separate diagnostic criteria were defined for low-risk and moderate-/high-risk populations to minimise the risk of overdiagnosis and underdiagnosis, respectively. Also, subclinical carditis was defined as a major criterion, and echocardiography was recommended to all patients with suspected or confirmed acute rheumatic fever in high-risk populations. Reference Gewitz, Baltimore and Tani3 The most common presentation of the disease is arthritis and carditis, and carditis is the most important component because of life-long sequelae. Reference Guilherme, Steer, Cunningham, Dougherty, Carapetis, Zühlke and Wilson4
Growth differentiation factor-15 is a member of the transforming growth factor-β cytokine superfamily. Reference Assadi, Zahabi and Hart5 Although growth differentiation factor-15 is not a cardiac-specific biomarker, the cardiovascular system is one of the most studied systems to investigate the role of growth differentiation factor-15. The role of growth differentiation factor-15 in acute and chronic heart failure and acute coronary syndrome has been extensively investigated, and it has been reported that inflammation, oxidative stress, mechanical stress, and myocardial injury may lead to increased expression of growth differentiation factor-15 from the heart. Reference Luo, Duan, Song, Yu and Shi6–Reference Ueland, Gullestad and Kou9
In this single-centre prospective study, we aimed to evaluate the role of growth differentiation factor-15 in children with acute rheumatic fever.
Material and methods
This study was conducted at Health of Ministry Van Training and Research Hospital between the dates of December 2018 and December 2019. Informed consent was obtained from the parents of all participants. The local ethics committee approved the study.
Children diagnosed with acute rheumatic fever in the paediatric cardiology department of our institution constituted the study group and healthy children who admitted to the paediatric cardiology department with symptoms like murmur, palpitation, and non-specific chest pain constituted the control group. Children with a known specific cardiac disease, any chronic disease, any morbidity, and using any specific medication were excluded from the study.
Demographic information and a detailed history were obtained from all the participants. After a detailed physical examination, all the participants were evaluated by electrocardiography and echocardiography. Two experienced paediatric cardiologists performed a detailed echocardiographic evaluation by a Vivid 7 Pro echocardiography device (GE Vingmed Ultrasound AS, Horten, Norway) using 3–6 MHz transducer. Doppler findings and morphological findings defined by the 2015 Revised Jones Criteria were used in the diagnosis of rheumatic valvulitis. Reference Gewitz, Baltimore and Tani3
Carditis was defined as mild in the presence of trivial-to-mild valvular disease, and it was defined as moderate when there was moderate valvular disease with normal left ventricular function and no signs or symptoms of heart failure. Carditis was defined as severe when there was a severe valvular disease or moderate-to-severe valvular disease with signs of heart failure. Reference Cannon, Roberts, Milne and Carapetis10 The study group was further classified into subgroups according to the severity of the carditis on admission as follows: no carditis, mild carditis, and moderate-to-severe carditis. Moderate and severe carditis were grouped together because there were only two cases with severe carditis.
A single dose of intramuscular benzathine penicillin G was administered at the time of diagnosis and continued every 3 weeks for secondary prophylaxis. Reference Lahiri and Sanyahumbi11 The cases with mild carditis were treated with naproxen sodium, whereas oral prednisolone was the drug of choice for cases with moderate and severe carditis. Reference Parnaby and Carapetis12 Anti-congestive therapy was given to the patients in the presence of heart failure. All patients had bed rest and restriction of activity according to the severity of the disease.
In addition to routine laboratory tests used in the diagnosis and the treatment of acute rheumatic fever, blood samples for growth differentiation factor-15 were withdrawn from all the cases in the study group at the time of diagnosis and the end of the treatment. Blood samples for growth differentiation factor-15 were also withdrawn from the control group. Blood samples for the analysis of growth differentiation factor-15 were assigned a number by a nurse, and neither the paediatric cardiologist nor the biochemist was aware whether the blood samples belonged to the study or control group. Venous blood samples withdrawn for growth differentiation factor-15 analysis were centrifuged for 15 minute at 1000 × g at 2–8°C after clotting at room temperature for 2 hours. The serum was collected and stored at −80°C until the day that all the samples would be assayed. The concentration of growth differentiation factor-15 was analysed by micro enzyme-linked immunosorbent assay using commercial kits (Wuhan Elabscience Biotechnology Co. Ltd, Wuhan, Hubei, China, with LOT number MF4LTGELGA) following the manufacturer’s instructions.
Statistical analyses
Statistical analyses were performed by the SPSS 20.0 statistical package (IBM Corp., Armonk, NY, USA). The mean, standard deviation, and frequency were used for descriptive statistics. Student t-test was used for comparison of the groups, and one-way ANOVA test was used for the comparison of subgroups. Paired sample t-test was used to compare the findings before and after the treatment in the study group. Pearson’s correlation analysis was used for correlation analysis. The confidence interval was given as 95 % and statistical significance was set at p < 0.05.
Results
The study included a total of 50 participants. The study group included 25 children diagnosed with acute rheumatic fever. The mean age of the study group and the control group were 10.98 ± 2.71 and 12.04 ± 2.44 years, respectively (p = 0.154). Eleven (44%) of the cases in the study group and 14 (56%) of the cases in the control group were female, and the gender distribution was similar between the groups (p = 0.572).
The distribution of clinical and laboratory manifestations of the study group according to the diagnostic criteria is shown in Table 1. None of the cases had Sydenham chorea, subcutaneous nodules, or erythema marginatum. At the time of diagnosis, a total of 21 (84%) cases had carditis, and the severity of carditis was as follows: 12 (48%) mild, 7 (28%) moderate, and 2 (8%) severe carditis. Although none of the patients had severe carditis after treatment, 7 cases had moderate and 14 cases had mild carditis.
Table 1. Findings of the study group according to the diagnostic criteria

ESR: erythrocyte sedimentation rate, CRP: C-reactive protein; ASO: anti-streptolysin O; AV: atrioventricular.
The distribution of growth differentiation factor-15 levels of both groups at the time of diagnosis and after the treatment is shown in Figure 1. The mean growth differentiation factor-15 level of the study group at the time of diagnosis (918.40 ± 605.65 pg/ml) was significantly higher than the mean post-treatment level (653.08 ± 330.92 pg/ml) (p = 0.015). Similarly, the mean growth differentiation factor-15 level of the study group at the time of diagnosis was significantly higher than the control group (p = 0.04). However, there was not any difference between groups in means of growth differentiation factor-15 levels after the treatment. The comparison of mean growth differentiation factor-15 levels of study and control group is shown in Table 2. When subgroups were compared with the control group, cases with mild and moderate-to-severe carditis had significantly higher growth differentiation factor-15 levels than the control group at the time of diagnosis; however, cases without carditis had similar growth differentiation factor-15 levels with the control group. The distribution of growth differentiation factor-15 levels according to the severity of carditis is shown in Figure 2.

Figure 1. The distribution of growth differentiation factor-15 levels of groups.
Table 2. Comparison of mean growth differentiation factor-15 levels of the study and control group

t-test.
Growth differentiation factor-15 levels are presented as pg/ml.
SD: standard deviation.
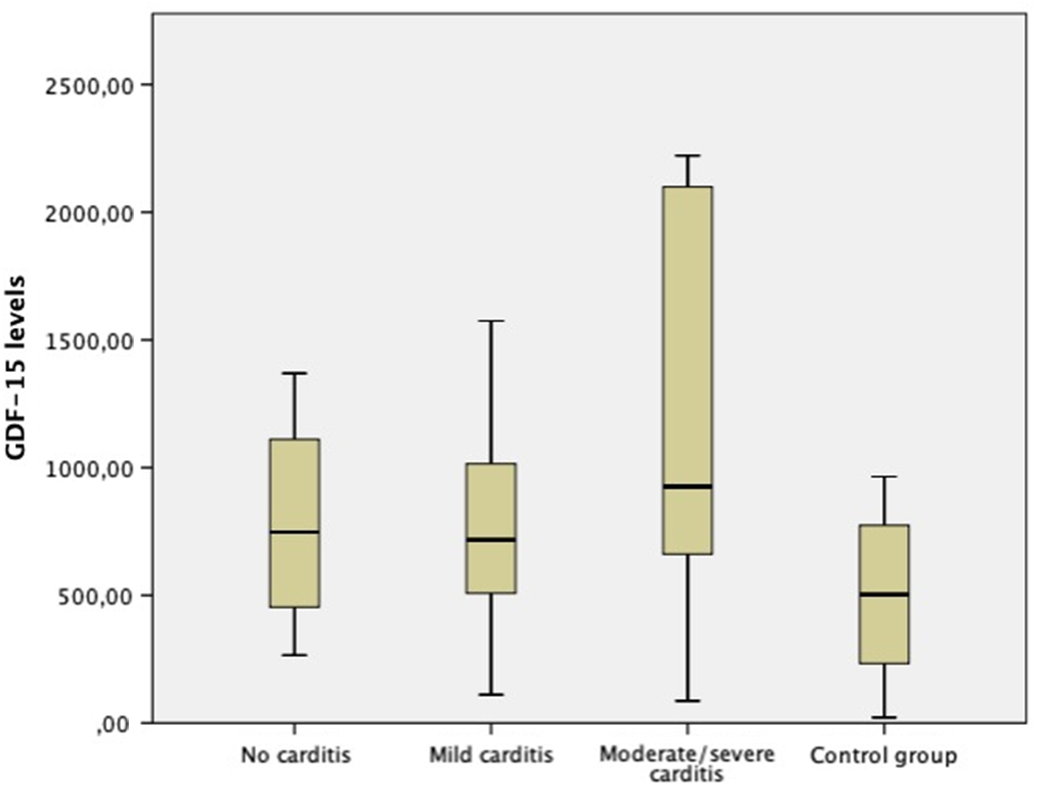
Figure 2. The distribution of growth differentiation factor-15 levels according to the severity of carditis.
The comparison of mean growth differentiation factor-15 levels of the subgroups with each other before and after the treatment is shown in Table 3. There was no significant difference in mean growth differentiation factor-15 levels of subgroups before and after the treatment (one-way ANOVA test, p = 0.309, p = 0.557, respectively).
Table 3. Comparison of mean growth differentiation factor-15 levels of the subgroups

T test.
Although cases with carditis had significantly higher growth differentiation factor-15 levels than the control group, there was no significant correlation between growth differentiation factor-15 levels and the severity of carditis (p = 0.95, correlation coefficient = 239). We think that this result must be interpreted carefully, because the number of cases with moderate and severe carditis were low in our study, and studies with a larger sample size involving higher number of cases with moderate and severe carditis may yield different results.
As expected, the mean erythrocyte sedimentation rate and C-reactive protein levels of the study group were significantly higher at the time of diagnosis than the mean post-treatment level (p < 0.001). The mean erythrocyte sedimentation rate and C-reactive protein levels were similar between the subgroups. (p = 0.325, p = 0.495, respectively). Growth differentiation factor-15 was positively correlated with both C-reactive protein (p < 0.001, correlation coefficient = 888) and erythrocyte sedimentation rate (p = 0.001, correlation coefficient = 633) at the time of diagnosis.
Discussion
Acute rheumatic fever develops as a result of the autoimmune response of the host to the pharyngeal infection caused by group A Streptococcus. The exact pathogenesis of the disease is not completely understood, but molecular mimicry is the mechanism that is thought to be responsible for multiple tissue damage. Molecular mimicry can be defined as the similarity between human tissues and other exogenous proteins. Reference Carapetis, Beaton and Cunningham2 Brain, joints, heart, skin, and subcutaneous tissues are affected; however, the most important component of the disease is carditis because of life-long sequelae. Reference Guilherme, Steer, Cunningham, Dougherty, Carapetis, Zühlke and Wilson4
The risk of acute rheumatic fever is 1.5–2-fold higher in females, and it is most common between the ages of 5 and 14 years. Reference Parnaby and Carapetis12,Reference Lawrence, Carapetis, Griffiths, Edwards and Condon13 In our study, the age of the study group ranged from 5 to 15.5 years, but there was a male (56%) predominance. Similar to previous reports, the frequency of carditis, fever, and arthritis/polyarthralgia were 84, 72, and 80%, respectively. Reference Gewitz, Baltimore and Tani3,Reference Kumar and Tandon14 None of the cases had Sydenham chorea, erythema marginatum, or subcutaneous nodules. The frequency of mild, moderate, and severe carditis were 48, 28, and 8% respectively. C-reactive protein and erythrocyte sedimentation rate were elevated in all cases at the time of diagnosis.
Growth differentiation factor-15 is a highly attractive biomarker that has been studied in various diseases. It is a member of the transforming growth factor-β cytokine superfamily and has several roles such as a hormone, stress-induced cytokine, or stress-sensitive circulating factor. Reference Assadi, Zahabi and Hart5 Although the physiologic role of growth differentiation factor-15 is not completely understood, it participates in the regulation of inflammatory and reparative responses. It is known to have an antitumorigenic and proapoptotic role and a protective role in the heart, liver, and kidney under pathological conditions. Reference Liu, Wang and Tao15,Reference Richter, Uray and Krychtiuk16 Although the main site of expression of growth differentiation factor-15 is the prostate and placenta, several tissues in the body can express lower levels of growth differentiation factor-15. Cytokines and growth factors, angiotensin 2, and non-steroidal anti-inflammatory drugs may lead to increased expression of growth differentiation factor-15. Reference Assadi, Zahabi and Hart5 Elevated growth differentiation factor-15 levels have been reported in diseases involving immune homoeostasis and surveillance and their regulation. Liver injury, type 1 diabetes mellitus, rheumatoid arthritis, atherosclerosis, endothelial dysfunction, obesity, insulin resistance, chronic kidney diseases in diabetes, colorectal, ovarian, and early-stage lung carcinoma may lead to increased expression of growth differentiation factor-15. Reference Wischhusen, Melero and Fridman17
Although mostly increased expression of growth differentiation factor-15 have been reported, there a few studies about decreased expression of growth differentiation factor-15. It has been reported that deficiency of growth differentiation factor-15 is beneficial against vascular injury and inflammation. Reference Adela and Banerjee18 Reduced levels of growth differentiation factor-15 was reported in women with pre-eclampsia, and also the reduction was more profound in late-onset pre-eclampsia than early-onset pre-eclampsia. Reference Chen, Wang, Zhao, Hyett, da Silva Costa and Nie19 An association between reduced growth differentiation factor-15 levels and atrophic inflammatory lesions of the prostate has also been reported. Reference Lambert, Whitson and Iczkowski20 Age and gender are other demographic factors that may affect the level of growth differentiation factor-15, and females are shown to have higher levels of growth differentiation factor-15 in comparison to males. Reference Wesseling, de Poel and de Jager21
Under physiological conditions, growth differentiation factor-15 is weakly expressed in the heart, but ischemia, inflammation, oxidative stress, tissue hypoxia, and mechanical stretch may lead to increased expression of growth differentiation factor-15. Reference Assadi, Zahabi and Hart5,Reference Luo, Duan, Song, Yu and Shi6,Reference Liu, Wang and Tao15 Growth differentiation factor-15 may play a cardioprotective role by inhibiting cell apoptosis, myocyte hypertrophy, and cardiac remodelling. Reference Desmedt, Desmedt, De Vos, Delanghe, Speeckaert and Speeckaert22 The cardioprotective role of growth differentiation factor-15 has been studied in mice models. It was shown that growth differentiation factor-15 expression was increased in mice with dilated and hypertrophic cardiomyopathy. Reference Xu, Kimball and Lorenz23,Reference Harding, Yang, Yang, Shesely, He and LaPointe24 The growth differentiation factor-15-deficient mice were compared with transgenic mice with induced growth differentiation factor-15 expression. Transverse aortic constriction procedure was performed to all mice to produce pressure overload-induced hypertrophy. It was shown that growth differentiation factor-15-deficient mice had greater cardiac hypertrophy, whereas transgenic mice with induced growth differentiation factor-15 expression showed a reduction in hypertrophy after transverse aortic constriction procedure. Reference Xu, Kimball and Lorenz23 The cardioprotective role of growth differentiation factor-15 in myocardial infarction is thought to be due to the downregulation of the recruitment of polymorphic nuclear cells. Reference Assadi, Zahabi and Hart5 Another role of growth differentiation factor-15 in the heart under pathological conditions is claimed to be maintaining a high number of viable and functional cardiomyocytes to maintain cardiac output. Reference Wesseling, de Poel and de Jager21 Although growth differentiation factor-15 upregulation in pathological states may seem contradictory with its protective role in various tissues, this contradiction might be explained with the fact that growth differentiation factor-15 plays its physiological regulatory role in response to the tissue damage as a part of compensatory mechanism. Reference Assadi, Zahabi and Hart5
The increased levels of growth differentiation factor-15 is reported to be associated with various cardiovascular diseases. Reference Luo, Duan, Song, Yu and Shi6–Reference Ueland, Gullestad and Kou9,Reference Liu, Wang and Tao15,Reference Desmedt, Desmedt, De Vos, Delanghe, Speeckaert and Speeckaert22 It may provide additional information and act as a predictor in chronic heart failure. Reference Liu, Wang and Tao15 It was shown to be a prognostic factor independent of other biomarkers in heart failure with both preserved and reduced ejection fraction, but it is not discriminative or diagnostic for heart failure. Reference Wesseling, de Poel and de Jager21 It was also shown to be a predictor of survival after cardiac arrest and serious arrhythmic events in patients with non-ischemic dilated cardiomyopathy. Reference Richter, Uray and Krychtiuk16,Reference May, Kochi and Magalhaes25 It has been suggested as a biomarker of heart failure in patients with unrepaired CHD with a left to right shunt. Reference Kagiyama, Yatsuga and Kinoshita26
In light of the data about the role of growth differentiation factor-15 in cardiovascular diseases, with this study, we aimed to evaluate the role of growth differentiation factor-15 in children with acute rheumatic fever. To the best of our knowledge, this is the first study to evaluate the role of growth differentiation factor-15 in children with acute rheumatic fever. Our study showed that the mean growth differentiation factor-15 level of children with acute rheumatic fever was significantly higher at the time of diagnosis compared to post-treatment level. Also, the mean growth differentiation factor-15 level of the children with acute rheumatic fever was significantly higher than the healthy children at the time of diagnosis (p = 0.04), and this significant difference disappeared after the treatment. Growth differentiation factor-15 was positively correlated with both C-reactive protein and erythrocyte sedimentation rate at the time of diagnosis. It is known that age, gender, and other diseases may affect growth differentiation factor-15 levels. In our study, the mean age and gender distribution were similar between the groups, and none of the patients had any other known disease or were using any medication.
The significantly increased levels of growth differentiation factor-15 in children with acute rheumatic fever may be explained by the increased expression of growth differentiation factor-15 in various cardiac diseases. In our study, the majority (21/25) of children had carditis at the time of admission, but only two children had severe carditis and heart failure. These two children recovered from heart failure and severe carditis after treatment. They were also the cases with the highest growth differentiation factor-15 levels (2221.8 pg/ml and 2098.7 pg/ml) on admission and after treatment (1536.5 pg/ml and 1223 pg/ml), even though there was a significant decline in their growth differentiation factor-15 levels with treatment. Although this result supports the acknowledged fact that growth differentiation factor-15 is increased in patients with heart failure and seems superior to C-reactive protein and erythrocyte sedimentation rate predicting this, it must be kept in mind that there were only two patients with heart failure.
Another point is that the significantly increased levels of growth differentiation factor-15 may be due to its role in the regulation of inflammatory and reparative responses. Reference Liu, Wang and Tao15,Reference Richter, Uray and Krychtiuk16 It is known that growth differentiation factor-15 may function as an autocrine regulator of macrophage activation and have an anti-inflammatory effect mediated by reducing the migration of leucocytes to areas of inflammation. Reference Sariyildiz, Yazmalar and Batmaz27 Elevated levels of growth differentiation factor-15 have been reported in patients with myocardial involvement in idiopathic inflammatory myopathy. Reference Qiu, Sun and Qi7 The elevated levels of growth differentiation factor-15 and correlation of growth differentiation factor-15 levels with erythrocyte sedimentation rate and morning stiffness have been shown in patients with rheumatoid arthritis. Reference Wischhusen, Melero and Fridman17 Elevated levels of growth differentiation factor-15 have also been reported in patients with myocardial involvement in idiopathic inflammatory myopathy. Reference Qiu, Sun and Qi7 Li et al. Reference Li, Wang and Li28 reported that children with Kawasaki disease had significantly higher growth differentiation factor-15 levels compared to healthy children, and children with coronary artery involvement had also significantly higher growth differentiation factor-15 levels compared to children with Kawasaki disease without coronary involvement. They also reported a positive correlation between growth differentiation factor-15 and white blood cell count, C-reactive protein, and erythrocyte sedimentation rate. They concluded that growth differentiation factor-15 could be a predictor of coronary artery involvement and unresponsive disease in Kawasaki disease, and growth differentiation factor-15 was superior to erythrocyte sedimentation rate in predicting this. Reference Li, Wang and Li28 Similar to these inflammatory diseases, it has been shown that levels of antioxidant enzymes, free radicals, cytokines (tumour necrosis factor-α, interleukin-6, and interleukin-8), homocysteine, and nitric oxide metabolites are significantly increased during the acute phase of rheumatic fever. Reference Kurban, Mehmetoglu, Oran and Kiyici29–Reference Narin, Narin, Pasaoglu, Halici and Aslan34 Besides cardiac involvement, the autoinflammatory nature of this disease with increased levels of free radicals, cytokines, and oxidative stress may be the cause of the increased expression of growth differentiation factor-15. Reference Assadi, Zahabi and Hart5,Reference Luo, Duan, Song, Yu and Shi6,Reference Liu, Wang and Tao15 As Conte et al. Reference Conte, Martucci and Mosconi35 stated, we think that growth differentiation factor-15 levels may be elevated to blunt the destructive effects of inflammation considering the responsiveness of growth differentiation factor-15 to inflammation and its anti-inflammatory effects. Similar to previous studies, growth differentiation factor-15 levels were also positively correlated with C-reactive protein and erythrocyte sedimentation rate. Another interesting point is that although non-steroidal anti-inflammatory drugs are known to increase growth differentiation factor-15 levels and a great proportion of children in the study group were treated with non-steroidal anti-inflammatory drugs, the elevated growth differentiation factor-15 levels of the study group at the time of diagnosis declined to similar levels with the control group after treatment.
Study limitations
The sample size is relatively small taking into consideration the incidence of acute rheumatic fever in underdeveloped and developing countries. Serum concentrations of growth differentiation factor-15 were only studied before and just after the treatment rather than serial multiple measurements. The study itself with its current form is not able to highlight the mechanisms underlying the increased levels of growth differentiation factor-15 in children with acute rheumatic fever.
Conclusion
Growth differentiation factor-15 levels are significantly increased in children with acute rheumatic fever, and growth differentiation factor-15 levels return to similar levels with healthy children after treatment. Growth differentiation factor-15 levels are positively correlated with erythrocyte sedimentation rate and C-reactive protein levels in acute rheumatic fever. Multi-centre studies with larger sample size and with a larger number of subgroups would provide more reliable information about the role of growth differentiation factor-15 and the mechanisms underlying increased levels of growth differentiation factor-15 in acute rheumatic fever.
Acknowledgements
The authors would like to thank C.A. and F.Ç. for their kind helps in collecting, coding, and studying blood samples.
Financial support
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Conflicts of interest
None.
Ethical standards
This study was performed in line with the principles of the Declaration of Helsinki, and the Ethics Committee of Van Training and Research Hospital approved the study (Date: 04.10.2018 No:2018/14).








