Article contents
Hoganite and paceite, two new acetate minerals from the Potosi mine, Broken Hill, Australia
Published online by Cambridge University Press: 05 July 2018
Abstract
Hoganite, copper(II) acetate monohydrate, and paceite (pronounced ‘pace-ite’), calcium(II) copper(II) tetraacetate hexahydrate, occur as isolated crystals embedded in ferruginous gossan from the Potosi Pit, Broken Hill, New South Wales, Australia. They are associated with goethite, hematite, quartz, linarite, malachite, azurite, cerussite and cuprian smithsonite. Hoganite is bluish green with a pale blue streak and a Mohs hardness of 1½; it possesses perfect {001} and distinct {110} cleavages and has a conchoidal fracture. Chemical analysis of hoganite gave (wt.%) C 23.85; H 3.95; Cu 31.6; Fe 0.4; O (by difference) 40.2, yielding an empirical formula of C4H7.89O5.07Cu1.00Fe0.01. The simplified formula is C4H8O5Cu or Cu(CH3COO)2.H2O, the mineral being identical to the synthetic compound of the same formula. Single-crystal X-ray data for hoganite are: monoclinic, space group C2/c, a = 13.162(3), b = 8.555(2), c = 13.850(3)Å, β = 117.08(3)°, Z = 8. The density, calculated from single-crystal data, is 1.910 g cm−3. The strongest lines in the X-ray powder pattern are [dobs (Iobs) (hkl)] 6.921 (100) (011); 3.532 (28) (202); 6.176 (14) (200); 3.592 (11) (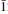 22); 5.382 (10) (
22); 5.382 (10) (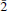 11); 2.278 (10) (204); 5.872 (9) (002). Hoganite (orientation presently unknown) is biaxial positive with α = 1.533(2), β = 1.541(3), γ = 1.554(2), 2V(meas.) = 85(5)°, 2V(calc.) = 76.8°, dispersion is r < v, medium (white light); it is strongly pleochroic with X = blue, Y = pale bluish, Z = pale bluish green and absorption X > Y > Z. The mineral is named after Graham P. Hogan of Broken Hill, New South Wales, Australia, a miner and well-known collector of Broken Hill minerals.
11); 2.278 (10) (204); 5.872 (9) (002). Hoganite (orientation presently unknown) is biaxial positive with α = 1.533(2), β = 1.541(3), γ = 1.554(2), 2V(meas.) = 85(5)°, 2V(calc.) = 76.8°, dispersion is r < v, medium (white light); it is strongly pleochroic with X = blue, Y = pale bluish, Z = pale bluish green and absorption X > Y > Z. The mineral is named after Graham P. Hogan of Broken Hill, New South Wales, Australia, a miner and well-known collector of Broken Hill minerals.
Paceite is dark blue with a pale blue streak and a Mohs hardness of 1½; it possesses perfect {100} and {110} cleavages and has an uneven fracture. Chemical analysis of paceite gave (wt.%) C 21.25; H 5.3; Ca 9.0; Cu 14.1; O (by difference) 50.35, yielding an empirical formula of C8H23.77O14.23Ca1.02-Cu1.00. The simplified formula is C8H24O14CaCu or CaCu(CH3COO)4.6H2O, the mineral being identical to the synthetic compound of the same formula. Unit-cell data (refined from X-ray powder diffraction data) for paceite are: tetragonal, space group I4/m, a = 11.155(4), c = 16.236(17)Å, Z = 4. The density, calculated from refined cell data, is 1.472 g cm−3. The strongest lines in the X-ray powder pattern are [dobs (Iobs) (hkl)] 7.896 (100) (110); 3.530 (20) (310); 5.586 (15) (200); 8.132 (8) (002); 9.297 (6) (101); 2.497 (4) (420); 3.042 (3) (321). Paceite is uniaxial positive with ω = 1.439(2) and ɛ = 1.482(3) (white light); pleochroism is bluish with a greenish tint (O), pale bluish with a greyish tint (E), and absorption O ⩾ E. The mineral is named after Frank L. Pace of Broken Hill, New South Wales, Australia, an ex-miner and well-known collector of Broken Hill minerals.
- Type
- Research Article
- Information
- Copyright
- Copyright © The Mineralogical Society of Great Britain and Ireland 2002
References
- 20
- Cited by




