Implications
Biting behaviour in growing pigs impairs their welfare and leads to economic losses. We categorized this behaviour into aggressive and non-aggressive biting, the latter often being directed towards the tail. The environment in which pigs are expressing biting is of major importance, but predisposing factors acting in early life can also influence its expression. This review points out the detrimental influence of large litters on non-aggressive biting and the positive influence of social interactions between suckling piglets of different litters on aggressive biting later on. No clear conclusion emerged for other factors due to inconsistent results or paucity of information.
Introduction
Group-housed pigs in commercial production systems are susceptible to the performance of a variety of behaviours that contribute to reduced welfare. Most prominent are biting behaviours that directly result in more or less severe skin lesions, or in amputation of part of the tail or ears in post-weaning and fattening pigs. Indirectly, biting behaviour can result in injuries such as lameness due to slipping during fights (e.g. Anil et al., Reference Anil, Anil, Deen, Baidoo and Wheaton2005; Maes et al., Reference Maes, Pluym and Peltoniemi2016) (sometimes lethal), infections due to wounds caused by biting (Schroëder-Petersen and Simonsen, Reference Schroëder-Petersen and Simonsen2001), immunosuppression (de Groot et al., Reference de Groot, Ruis, Scholten, Koolhaas and Boersma2001), reduced growth (e.g. Stookey and Gonyou, Reference Stookey and Gonyou1994) and, in some extreme cases, death (Sinisalo et al., Reference Sinisalo, Niemi, Heinonen and Valros2012). Biting induces a reaction (retreat or attack) by the victim, except in severe cases when the wounded animal gradually gives up its resistance and its effort to flee (Sambraus, Reference Sambraus and Fraser1985) or when limitations imposed by the environment do not allow an effective escape by the recipient pig. Two types of biting can be identified (Simonsen, Reference Simonsen1990) and will be referred to throughout the present text:
aggressive biting,
non-aggressive biting or oral manipulative biting.
Aggressive biting is common in the context of hierarchy formation and occurs mostly in the first hours after creating a new social group (Meese and Ewbank, Reference Meese and Ewbank1973). It can also occur, to a lesser extent, in stable groups when animals compete for limited resources or when some pigs challenge the established hierarchical order (Meese and Ewbank, Reference Meese and Ewbank1972 and Reference Meese and Ewbank1973; Parois et al., Reference Parois, Larzul and Prunier2017; Peden et al., Reference Peden, Turner, Boyle and Camerlink2018). Bites are targeted preferentially at the head and the shoulders (the front third of the body) but can also reach the flanks when delivered in a reverse parallel posture or the rump when delivered to retreating animals (McGlone, Reference McGlone1985; Fraser and Rushen, Reference Fraser and Rushen1987; Turner et al., Reference Turner, Farnworth, White, Brotherstone, Mendl, Knap, Penny and Lawrence2006).
Non-aggressive biting is largely unrelated to hierarchy formation and resource competition. It occurs mainly, though not exclusively, in barren environments where pigs are likely to be thwarted in their need to perform exploration, object play or foraging behaviours (EFSA, 2007). Non-aggressive biting is mainly targeted at the tail, but ears can also be the subject of biting (EFSA, 2007) as well as other parts of the body. These other parts of the body include flank biting (Petersen et al., Reference Petersen, Nielsen, Hassing, Ersboll and Nielsen2008), leg biting (Beattie et al., Reference Beattie, O’Connell and Moss2000), penis biting in entire male pigs (Weiler et al., Reference Weiler, Isernhagen, Stefanski, Ritzmann, Kress, Hein and Zols2016), vulva biting in sows (Ladewig et al., Reference Ladewig, Kloeppel and Kallweit1984) or anus biting in fatteners (Blowey, Reference Blowey2003). Regarding tail or vulva biting, an aggressive motivation may result from competition for food or water in situations of limited access (Hansen et al., Reference Hansen, Hagelso and Madsen1982; Van Putten and Vandeburgwal, Reference Van Putten and Vandeburgwal1990; Rizvi et al., Reference Rizvi, Nicol and Green1998). Tail and ear lesions can also result from necrosis without other pigs’ intervention (Lechner et al., Reference Lechner, Langbein and Reiner2015), although this often leads to biting of the affected parts by other pigs once exudate and blood are present.
The important role of the immediate environment on the two types of biting behaviour is well recognized and has been reviewed (e.g. Schroëder-Petersen and Simonsen, Reference Schroëder-Petersen and Simonsen2001; Van de Weerd et al., Reference Van de Weerd, Docking, Day and Edwards2005; EFSA, 2007). The influence of internal factors related to genotype or health is also recognized (for reviews see Moinard et al., Reference Moinard, Mendl, Nicol and Green2003; Taylor et al., Reference Taylor, Main, Mendl and Edwards2010; D’Eath et al., Reference D’Eath, Arnott, Turner, Jensen, Lahrmann, Busch, Niemi, Lawrence and Sandoe2014; Valros and Heinonen, Reference Valros and Heinonen2015). In addition to these factors, events affecting prenatal life as well as the early postnatal environment may also influence the later predisposition to both types of biting behaviours in pigs. The evidence for the existence of such early predisposing factors is evaluated in the present review, focussing on biting behaviours performed by young pigs after weaning and during the fattening period.
Taking into account that both types of biting involve at least one performer and one recipient, and that the reaction of the recipient is likely to influence the behaviour of the performer, we evaluate, whenever information is available, the effects of potential predisposing factors not only on the propensity of pigs to perform but also to receive such behaviours, as well as on the way pigs react to these behaviours. We consider various factors acting during the prenatal and early postnatal life and their effects during the post-weaning and fattening periods. Early postnatal life is defined as the whole period between birth and weaning. Before analysing the predisposing factors in detail, we firstly describe the main motivations of weaned or growing pigs to perform biting.
Motivations underlying biting behaviours and variability of expression between pigs
Motivations to bite
Aggressive biting and non-aggressive biting are, especially in practice, often discussed as if these are the same behaviours (Bracke et al., Reference Bracke, Lauwere, Wind and Zonerland2013; Benard et al., Reference Benard, Schuitmaker and Buning2014). However, these should be considered differently because of differences in the underlying motivations, the part of the body that is concerned and the reaction of the recipient (Taylor et al., Reference Taylor, Main, Mendl and Edwards2010). Indeed, the recipients of non-aggressive biting, such as tail biting, show no response or little reaction that consists mostly of avoidance (Taylor et al., Reference Taylor, Main, Mendl and Edwards2010), whereas the recipients of aggressive biting often engage in reciprocal fighting (Turner et al., Reference Turner, Farnworth, White, Brotherstone, Mendl, Knap, Penny and Lawrence2006).
Aggressive biting occurs (1) during the formation of dominance relationships that dictate privileged access to potential resources and (2), subsequently, during the maintenance of these relationships when animals are competing for resources with limited access (Figure 1). The formation of dominance relationships occurs when unfamiliar pigs are mixed together to form new social groups, which is a common occurrence in commercial piggeries (Peden et al., Reference Peden, Turner, Boyle and Camerlink2018). The motivation to establish, defend or challenge a high dominance position, or to access resources, results in aggressive behaviour expressed through fighting and biting. In most cases, the target of biting is the front third of the body (Turner et al., Reference Turner, Farnworth, White, Brotherstone, Mendl, Knap, Penny and Lawrence2006), but bites are often delivered to the rump of a retreating animal. In situations when animals try to access a feeder or a drinker, biting can be directed to the tail or the vulva as this is the most accessible part at that moment (Hansen et al., Reference Hansen, Hagelso and Madsen1982; Van Putten and Vandeburgwal, Reference Van Putten and Vandeburgwal1990). Aggressive biting can also occur because of fear-induced and pain-induced aggression, as demonstrated in dogs (Jacobs et al., Reference Jacobs, De Keuster and Simoens2003).

Figure 1 Targets of biting and main motivations of pigs to bite.
Non-aggressive biting largely results from the inability of pigs to express natural behaviour to root, chew and forage, as shown in numerous reviews (e.g. Schroëder-Petersen and Simonsen, Reference Schroëder-Petersen and Simonsen2001; Taylor et al., Reference Taylor, Main, Mendl and Edwards2010; D’Eath et al., Reference D’Eath, Arnott, Turner, Jensen, Lahrmann, Busch, Niemi, Lawrence and Sandoe2014; Valros and Heinonen, Reference Valros and Heinonen2015). When this innate behaviour cannot be appropriately expressed, as is the case in most commercial conditions, this internal drive starts to be expressed in redirected behaviour. This urge to chew and root is redirected towards any available materials in the environment, including penmates. In field situations, aggressive and non-aggressive biting directed to the tail may sometimes be interrelated, since the presence of blood at the tail of one pig may attract other pigs (Fraser, Reference Fraser1987) that will develop non-aggressive tail biting.
Variability of expression of biting behaviours
During an episode of tail biting, some pigs in a pen perform (performers), receive (recipients), perform and receive (performers/recipients) or are not involved in tail biting (neutral) (Brunberg et al., Reference Brunberg, Wallenbeck and Keeling2011; Zonderland et al., Reference Zonderland, Kemp, Bracke, den Hartog and Spoolder2011a). Regarding performers, there is substantial variation in the amount of tail biting and in other behaviours performed by these pigs. Some performers are considered as ‘fanatical’ biters, being hyperactive and going from one tail to another during an outbreak of biting, whereas other performers bite rather occasionally (Van de Weerd et al., Reference Van de Weerd, Docking, Day and Edwards2005). A great variability also exists for the frequency of receipt of tail biting (Brunberg et al., Reference Brunberg, Wallenbeck and Keeling2011). Concerning aggressive biting, great variability is observed regarding the number of damaging interactions and the number of accumulated skin lesions during the 24 h after mixing unacquainted pigs (Turner et al., Reference Turner, Farnworth, White, Brotherstone, Mendl, Knap, Penny and Lawrence2006). Part of this inter-individual variation within social groups can be explained by genetic factors, the personality or coping style of the animals and, in addition, by the influence of the prenatal and early postnatal environment.
Influence of personality and coping style on biting behaviours
Background
Personality is defined by a correlated set of individual behavioural and physiological traits that are consistent over lifetime and environmental contexts (Finkemeier et al., Reference Finkemeier, Langbein and Puppe2018). In humans, personality is described by five main dimensions. Among these, aggressiveness, exploration and boldness can be easily recognized and tested in farm animals. A coping style is defined by a coherent set of behavioural and physiological responses to an aversive stressor, with the responses being consistent over time (Koolhaas et al., Reference Koolhaas, Korte, De Boer, Van Der Vegt, Van Reenen, Hopster, De Jong, Ruis and Blokhuis1999). Animals are classified as proactive (also referred to as ‘active’ or ‘high resisting’) when they have a strong fight/flight response, and reactive (also referred to as ‘passive’ or ‘low resisting’) when they have a low response. Personality and coping style are closely linked (Korte et al., Reference Korte, Koolhaas, Wingfield and McEwen2005; Finkemeier et al., Reference Finkemeier, Langbein and Puppe2018). Indeed, proactive animals are considered to be more aggressive towards conspecifics, more exploratory, bold and active compared with reactive individuals. In pigs, coping style can be assessed through the backtest performed in suckling piglets (e.g. Bolhuis et al., Reference Bolhuis, Schouten, Schrama and Wiegant2005a). The classification into proactive and reactive is based on the number of escape attempts (i.e. bouts of struggling with at least the hind legs) that piglets display during the course of 60 s when they are gently placed on their back. ‘High resisting’ pigs perform more escape attempts. Backtest responses may change across multiple tests as shown by Zebunke et al. (Reference Zebunke, Repsilber, Nuernberg, Wittenburg and Puppe2015). These authors performed four test repetitions between 1 and 4 weeks of age in 3555 piglets and concluded that the backtest rather indicates a coping disposition, which is modulated by environmental factors such as age and experience.
Aggressive biting behaviour
The potential link between coping style and aggressive biting has been evaluated in several studies in pigs, with inconsistent results. Hessing et al. (Reference Hessing, Hagelso, Vanbeek, Wiepkema, Schouten and Krukow1993) subjected suckling piglets to the backtest five times during the first 3 weeks of life. They also performed a social confrontation test at 1 week of age (mixing three animals from each of two litters together) in order to classify pigs as either aggressive or non-aggressive. Results showed that 75% of proactive pigs were aggressive, whereas 75% of reactive pigs were non-aggressive. Pigs that varied in their behavioural response during consecutive backtests (alternating between proactive and reactive; 21%) were equally distributed between aggressive and non-aggressive pigs. When social behaviour was tested in older animals, Bolhuis et al. (Reference Bolhuis, Schouten, Schrama and Wiegant2005b) observed more aggressive behaviours (including biting) in proactive or ‘high resisting’ pigs, independent of the housing environment (enriched v. barren) applied before and after weaning. In the study of Melotti et al. (Reference Melotti, Oostindjer, Bolhuis, Held and Mendl2011), the relationship between coping style and aggressiveness was more nuanced, with ‘high resisting’ pigs showing the same amount of aggression (head-knocks and/or bites) as ‘low resisting’ pigs, but being more persistent in their aggression. ‘High resisting’ pigs chased (bullied) other pigs more and fought more, independently of relative weight differences. In contrast, other studies found no relationship between the number of struggles in the backtest and aggressiveness indicators (number of attacks, bites, latency to attack) in a resident-intruder test (Forkman et al., Reference Forkman, Furuhaug and Jensen1995; D’Eath and Burn, Reference D’Eath and Burn2002; Janczak et al., Reference Janczak, Pedersen and Bakken2003). Moreover, aggressive traits measured in weaners, growers and gilts in their rearing pens during group mixing were poorly related to the number of escape attempts in the backtest (two repetitions performed at 12 and 19 days of age) with phenotypic correlations varying between −0.05 and 0.02 (Scheffler et al., Reference Scheffler, Stamer, Traulsen and Krieter2016a). The discrepancy between test outcomes may be partly due to the variation in the manner of performing the backtest. Indeed, the findings showing a relationship between backtest response and aggression come from the same research group, where a great similarity in the procedures is expected. It can also be noted that, in all these experiments, the coping style was assessed early in the life of the pigs, and probably before they had time to develop their full personality when being confronted with a wide range of personal experiences. Therefore, increasing the time interval between the evaluation of coping style and aggressiveness may reduce the strength of the relationship between them.
The relationship between aggressiveness and personality traits measured in tests other than the backtest has less often been investigated. The response in the human approach test, commonly used to assess fear tendency (Finkemeier et al., Reference Finkemeier, Langbein and Puppe2018), was poorly correlated (Scheffler et al., Reference Scheffler, Stamer, Traulsen and Krieter2016a) or not correlated (Janczak et al., Reference Janczak, Pedersen and Bakken2003) with aggressiveness observed in rearing pens. Gilts classified as low or high responders after several behavioural tests (including restraint, handling, sudden human approach) did not differ in their number of attacks towards other gilts in a food competition test (Lawrence et al., Reference Lawrence, Terlouw and Illius1991).
Non-aggressive biting behaviour
It has been reported that the total frequency of manipulatory behaviours towards penmates was lower from weaning until the end of the fattening period in ‘high resisting’ than in ‘low resisting’ pigs (in the backtest) that were kept in a barren environment (Bolhuis et al., Reference Bolhuis, Schouten, Schrama and Wiegant2005b; Bolhuis et al., Reference Bolhuis, Schouten, Schrama and Wiegant2006). This effect was not seen in an enriched environment, but it should be noted that the level of manipulatory behaviours was already very low in the ‘low resisting’ pigs. Specific tail manipulation behaviour was observed so rarely that the influence of the coping style could not be reliably tested. ‘Low resisting’ pigs showed more oral manipulation than ‘high resisting’ pigs when they experienced a change in the environment from enriched to barren housing (Melotti et al., Reference Melotti, Oostindjer, Bolhuis, Held and Mendl2011).
Piglets that showed a less fearful response pre-weaning in a novel environment test (‘Novel Box Test’) performed less tail biting later in life when housed in barren pens (Ursinus et al., Reference Ursinus, Reenen, Reimert and Bolhuis2014a). Chewing propensity at an early age has been tested as a personality trait that could predispose pigs for tail or ear biting later in life (Beattie et al., Reference Beattie, Breuer, O’Connell, Sneddon, Mercer, Rance, Sutcliffe and Edwards2005). Indeed, chewing may refer to the exploration dimension of personality. Behaviour during a ‘Tail Chew Test’ (a salty rope and a plain one were presented to a piglet for 10 min) performed a couple of days before weaning (at 4 weeks of age) and 2 weeks later showed some stability over time. The behaviour directed towards these ropes was slightly positively correlated with ear biting observed between 4 and 7 weeks of age in the home pen, whereas tail biting was only positively correlated with results from the test at 6 weeks of age (Beattie et al., Reference Beattie, Breuer, O’Connell, Sneddon, Mercer, Rance, Sutcliffe and Edwards2005).
Conclusion: personality and coping style
Currently, there is only limited evidence that personality and coping style, evaluated through behavioural tests before weaning, can predict aggressive or non-aggressive biting behaviour later in life. The response to the backtest has shown some relationship with both types of behaviours, with ‘low resisting’ pigs being more prone to non-aggressive biting (oral manipulation) and ‘high resisting’ ones more prone to show aggressive biting. This latter relationship was not observed in all studies being probably influenced by various factors. Consequently, the link between coping style, personality and biting behaviour would merit further investigation in different environments and at different ages. The various aspects of personality should also be considered, including boldness and exploration, and not solely the coping style.
Prenatal effects on biting behaviours
Foetal brain development is highly dependent upon adequate nutritional and endocrine support. Therefore, nutritional deficit or stress applied to the pregnant mother may have long-term consequences on cognitive and behavioural abilities of the offspring and hence on behavioural predisposition to bite.
Effects related to undernutrition of the foetus
Background
Undernutrition during prenatal life can be displayed by low birth weight. Low birth weight has a strong influence on growth rate during and after lactation, and hence on the liveweight at weaning or later at a given age. For example, Poore and Fowden (Reference Poore and Fowden2003) found that low-birth-weight piglets had a lower growth rate until 3 months of age. These piglets also had higher adrenal-to-liveweight and adrenal cortex-to-medulla ratios and a greater cortisol response to ACTH stimulation at 3 months of age, even though differences were no longer detectable at 12 months of age.
There are many potential causes of undernutrition during prenatal life. Reduced nutrient supply can occur because of intra-uterine crowding and reduced placental area (Foxcroft and Town, Reference Foxcroft and Town2004), undernutrition of the dam (Worobec et al., Reference Worobec, Duncan and Widowski1999; Bell and Ehrhardt, Reference Bell and Ehrhardt2002) or maternal diseases that limit nutrient exchange to the foetuses (Gaccioli and Lager, Reference Gaccioli and Lager2016). In pigs, litter size is of particular interest, mainly because of the relationship with intra-uterine crowding (Foxcroft et al., Reference Foxcroft, Dixon, Novak, Putman, Town and Vinsky2006) and decreased average birth weight (Rutherford et al., Reference Rutherford, Baxter, D’Eath, Turner, Arnott, Roehe, Ask, Sandoe, Moustsen, Thorup, Edwards, Berg and Lawrence2013).
Aggressive biting behaviour
Taking into account the importance of liveweight at birth for growth rate (Douglas et al., Reference Douglas, Edwards, Sutcliffe, Knap and Kyriazakis2013), an influence of nutrition during foetal life on biting behaviour is expected at least via the influence of liveweight on aggressiveness of pigs (cf. Postnatal undernutrition). However, in a competitive feeding test performed in gilts, the proportion of aggressive interactions initiated and the dominance ratio (ratio of the number of gilts she dominated to the number that dominated her) were not significantly predicted by size of litter at birth, body mass at birth or crown-rump length (which might be indicative of intra-uterine growth retardation) (Drickamer et al., Reference Drickamer, Arthur and Rosenthal1999). Ruis et al. (Reference Ruis, Brake, Burgwal, Jong, Blokhuis and Koolhaas2000) also found that pigs showing higher resistance in a backtest, which was moderately correlated to aggressiveness during a group-feeding competition test, were not heavier at birth.
Non-aggressive biting behaviour
In the study by Beattie et al. (Reference Beattie, Breuer, O’Connell, Sneddon, Mercer, Rance, Sutcliffe and Edwards2005), there was no significant difference in birth weight between pigs that expressed high or low levels of tail-chewing behaviour after weaning. Similarly, in the study by Ursinus et al. (Reference Ursinus, Wijnen, Bartels, Dijvesteijn, van Reenen and Bolhuis2014b), birth weight did not differ between non-tail or ear biters, ‘medium’ tail or ear biters, and ‘high’ tail or ear biters, regardless of the environment (provided with a jute sack or not) and the stage of growth (post-weaning: 6 to 8 weeks of age; rearing: 11 weeks of age).
Conclusion: foetal undernutrition
The current balance of evidence fails to provide support for a role of prenatal nutrient deficiency in the ontogeny of later damaging behaviour.
Influence of non-nutritional sources of prenatal stress on biting behaviours
Background
Prenatal stress, that is, stress experienced while in the foetal environment, can result in long-term behavioural and biological changes of the offspring. This has been studied in various farm species, including pigs (reviewed by Kranendonk et al., Reference Kranendonk, Mulder, Parvizi and Taverne2008; Rutherford et al., Reference Rutherford, Donald, Arnottt, Rooke, Dixon, Mehers, Turnbull and Lawrence2012; Merlot et al., Reference Merlot, Quesnel and Prunier2013). Prenatal stress can occur from a single or repeated stressor during gestation, such as malnutrition (discussed above), or from disease and social stress of the dam. In pigs, prenatal stress has been elicited through the dam by the administration of stress hormones (e.g. ACTH: Haussmann et al., Reference Haussmann, Carroll, Weesner, Daniels, Matteri and Lay2000; hydrocortisone: Kranendonk et al., Reference Kranendonk, Hopster, van Eerdenburg, van Reenen, Fillerup, de Groot, Korte and Taverne2005), pain (e.g. Otten et al., Reference Otten, Kanitz, Tuchscherer and Nurnberg2001), rough handling (e.g. Lay et al., Reference Lay, Kattesh, Cunnick, Daniels, Kranendonk, McMunn, Toscano and Roberts2011) or social stress through group mixing during gestation (e.g. Couret et al., Reference Couret, Otten, Puppe, Prunier and Merlot2008; Rutherford et al., Reference Rutherford, Robson, Donald, Jarvis, Sandercock, Scott, Nolan and Lawrence2009). According to the stage of maturation of the foetus, the consequences of prenatal stress or of hormonal treatment may be different (reviewed by Kranendonk et al., Reference Kranendonk, Mulder, Parvizi and Taverne2008; Rutherford et al., Reference Rutherford, Donald, Arnottt, Rooke, Dixon, Mehers, Turnbull and Lawrence2012; Merlot et al., Reference Merlot, Quesnel and Prunier2013).
When natural stressors are applied, they stimulate the hypothalamic–pituitary–adrenal axis of the dam (Brunton, Reference Brunton2013; Merlot et al., Reference Merlot, Quesnel and Prunier2013). As a consequence, the nutrient supply to the foetuses can be modified and the transfer of cortisol to them can be increased. Both phenomena may alter the maturation of their neuroendocrine systems, with possible consequences after birth (Brunton, Reference Brunton2013; Merlot et al., Reference Merlot, Quesnel and Prunier2013). In addition, undernutrition of the foetuses might influence biting behaviour through reduced birth weight per se (cf. Undernutrition of the foetus). Furthermore, the sympathetic nervous system (SNS) of the dam, and hence catecholamine release, is very likely to be stimulated with, again, possible consequences on the nutrient supply to the foetuses and long-term effects. When using hormonal treatment to mimic prenatal stress, the SNS component is not included.
Aggressive biting behaviour
During a social test (i.e. mixing with an unfamiliar pig for 60 min) performed at about 1.5 months of age, piglets born from sows treated with hydrocortisone during early (days 21 to 50), mid (days 51 to 80) or late (days 81 to 110) pregnancy performed the same number of aggressive encounters during the first 30 min as piglets born from control sows (Kranendonk et al., Reference Kranendonk, Hopster, Fillerup, Ekkel, Mulder and Taveme2006). However, piglets born from sows treated during mid-pregnancy showed more aggressive encounters during the second 30 min of the test compared to piglets born from control sows or from sows treated during early or late gestation, suggesting a greater persistence of aggressive behaviour. In contrast, Lay et al. (Reference Lay, Kattesh, Cunnick, Daniels, Kranendonk, McMunn, Toscano and Roberts2011) found no effect of sow stress treatment (ACTH administration or rough handling at days 42 to 77 of gestation) on the amount of offspring aggression during mixing. The influence of catecholamines was not specifically evaluated.
Non-aggressive biting behaviour
The hypothesis that prenatally stressed piglets will be better prepared for receiving stress (in the form of pain) and thus respond differently from control pigs has been challenged (Rutherford et al., Reference Rutherford, Robson, Donald, Jarvis, Sandercock, Scott, Nolan and Lawrence2009; Sandercock et al., Reference Sandercock, Gibson, Rutherford, Donald, Lawrence, Brash, Scott and Nolan2011). Data show that prenatal stress due to social stress applied to the dam during mid-gestation increases the offsprings’ response to pain (Rutherford et al., Reference Rutherford, Robson, Donald, Jarvis, Sandercock, Scott, Nolan and Lawrence2009). Therefore, it can be hypothesized that prenatally stressed piglets are less susceptible to be recipients of biting due to an increased reaction to being bitten.
Offspring of sows that had received an ACTH challenge had significantly higher concentrations of plasma cortisol and healed slower after biopsy damage compared to control pigs (Haussmann et al., Reference Haussmann, Carroll, Weesner, Daniels, Matteri and Lay2000). Therefore, they might be more prone to being bitten by other pigs due to the presence of persisting lesions.
Conclusion: prenatal stress
There are too few reports to reliably determine the influence of prenatal stress on aggressive behaviour. Prenatal stress might, however, have a favourable influence (pigs more responsive and hence probably more reactive to pain) on non-aggressive biting counterbalanced by a detrimental one (slower healing).
Postnatal effects on biting behaviours
An important part of the pig brain development takes place after birth and depends on nutritional and environmental inputs. Therefore, nutritional deficit or scarcity of sensory stimuli during that period may have long-term consequences on cognitive and behavioural abilities of pigs and hence on behavioural predisposition to bite.
Effects related to undernutrition
Background
Undernutrition of piglets during lactation can arise because of excessive competition at the udder in large litters (cf. Postnatal social stress), poor health and agalactia of the dam (Sauber et al., Reference Sauber, Stahly and Nonnecke1999; Pend et al., Reference Pend, Jenny, Torgerson, Spring, Kuemmerlen and Sidler2017) or poor health of the individual piglet itself. In the latter case, infection-induced cytokine production can reduce appetite and growth (Williams et al., Reference Williams, Stahly and Zimmerman1997). Such undernutrition can be considered as a stressor with possible long-term consequences on the maturation of the neuroendocrine systems. In addition, it clearly influences the growth of pigs. Indeed, liveweight of growing pigs is greatly influenced by their liveweight at weaning, and hence milk intake and growth during lactation (Quiniou et al., Reference Quiniou, Dagorn and Gaudré2002; Douglas et al., Reference Douglas, Edwards, Sutcliffe, Knap and Kyriazakis2013).
Aggressive biting behaviour
Liveweight at the time of mixing pigs into new groups is a major determinant of their aggressive behaviour, especially biting behaviour (e.g. Andersen et al., Reference Andersen, Andenæs, Bøe, Jensen and Bakken2000; Desire et al., Reference Desire, Turner, D’Eath, Doeschl-Wilson, Lewis and Roehe2015; Scheffler et al., Reference Scheffler, Stamer, Traulsen and Krieter2016b). Lighter animals demonstrate fewer aggressive behaviours. Even though lower liveweight at mixing may be related to a lower nutrient supply during lactation, it is far from being sufficient to demonstrate the role of early nutrition. To the best of our knowledge, only one study has reported the long-term influence of growth during lactation on aggressive biting (Drickamer et al., Reference Drickamer, Arthur and Rosenthal1999). The results indicated that, in newly formed groups of 6- to 7-month-old gilts, the proportion of aggressive behaviours that each gilt initiated around feeding was positively correlated with her liveweight at 21 days of age. It was, however, not influenced by her liveweight at birth nor by her daily gain between birth and 21 days of age. This suggests that early-life nutrient supply, including both prenatal and lactational supply, may be important in competitive aggression.
Non-aggressive biting behaviour
It has anecdotally been reported that the pigs which perform injurious tail-biting behaviour are the smallest individuals within the group, or the so-called ‘runt’ pigs (Sambraus, Reference Sambraus and Fraser1985). When this has been investigated under experimental conditions, conflicting results have been obtained. Van de Weerd et al. (Reference Van de Weerd, Docking, Day and Edwards2005) reported that, while there was no difference in weight between pigs showing occasional tail biting and non-biting penmate controls, pigs showing persistent tail-biting behaviour were indeed significantly smaller individuals. These persistent biters, so-called ‘fanatical biters’, were described as being hyperactive pigs going from one tail to the other during a biting outbreak (Van de Weerd et al., Reference Van de Weerd, Docking, Day and Edwards2005). In other studies where persistent tail-biting pigs have been identified, they have also tended to be lighter in weight compared with penmates (Zupan et al., Reference Zupan, Janczak, Framstad and Zanella2012).
While smaller body size has therefore often been associated with tail-biting predisposition, it is less often documented when exactly – in pre or postnatal life – this reduced growth rate has occurred. In the study by Van de Weerd et al. (Reference Van de Weerd, Docking, Day and Edwards2005), ‘fanatical’ tail-biting pigs were lighter at the time of biting outbreaks but did not differ from other pigs (non-biters or sporadic biters) in their weight at birth or at weaning. This suggests a growth effect shortly prior to the appearance of injurious behaviour rather than an early-life effect. However, in the study by Beattie et al. (Reference Beattie, Breuer, O’Connell, Sneddon, Mercer, Rance, Sutcliffe and Edwards2005), although there was no significant difference in birth weight between pigs that expressed high or low tail-chewing behaviour after weaning, pigs that chewed most frequently showed significantly lower growth rates between birth and weaning (260 v. 285 g/day). This suggests an increased predisposition arising from nutrient deficiency during lactation. Zonderland et al. (Reference Zonderland, Schepers, Bracke, den Hartog, Kemp and Spoolder2011b) also found tail-biting pigs in the post-weaning stage to have a significantly lower weaning weight compared with victims and a numerically lower weight (0.5 kg less) compared with control contemporaries. Further circumstantial evidence of a link between impaired early growth and tail biting comes from the observation of van Staaveren et al. (Reference van Staaveren, Teixeira, Hanlon and Boyle2017) of a negative correlation between average tail lesion score and weight at sale/transfer of a batch of weaners, and between the percentage of pigs with severe tail lesions in a herd and average daily gain in weaners. Several reasons may explain lower weight in biters. They may use up more energy due to their increased activity (e.g. Van de Weerd et al., Reference Van de Weerd, Docking, Day and Edwards2005). They may have a reduced growth due to internal causes (e.g. health disorder as suggested by Valros and Heinonen, Reference Valros and Heinonen2015) or may use tail biting as a strategy to displace heavier pigs from the feeder or the drinker when access is difficult (D’Eath et al., Reference D’Eath, Arnott, Turner, Jensen, Lahrmann, Busch, Niemi, Lawrence and Sandoe2014).
Contrary to a negative relationship between growth and tail biting, Ursinus et al. (Reference Ursinus, Wijnen, Bartels, Dijvesteijn, van Reenen and Bolhuis2014b), showed that liveweight at weaning and growth rate during lactation were higher in gilts classified as high tail biters compared with medium and non-tail biters during the first 4 weeks after weaning. However, these results were dependent on the rearing environment, since they were observed only when jute sacks were provided and hence when biting directed to congeners was mitigated. In addition, they were not consistent across ages, since the existing difference was not observed when animals were classified according to their behaviour 3 weeks later.
Conclusion: postnatal undernutrition
The influence of growth during lactation and weight at weaning on aggressive biting has been scarcely investigated. Available data suggest that reduced early nutrition decreases the occurrence of this behaviour. The current balance of evidence provides clear, though not unambiguous, evidence of a predisposing effect of undernutrition during lactation on subsequent manipulatory behaviour of weaned piglets. The tail-biting behaviour of growing or finishing pigs may be more related to subsequent growth rate immediately preceding onset of the problem.
Effects related to social stress due to competition for teats or other resources
Background
Colostrum and milk are essential to piglet survival as they provide nutrients necessary for thermoregulation and growth, as well as immunoglobulins and other cellular and humoral factors necessary for protection against diseases (Edwards, Reference Edwards2002; Salmon et al., Reference Salmon, Berri, Gerdts and Meurens2009). During parturition and shortly after, colostrum is continuously available to the piglets, but thereafter milk can be consumed only during discrete ejections (De Passillé and Rushen, Reference De Passillé and Rushen1989; Fraser and Rushen, Reference Fraser and Rushen1992). Disputes at the teats appear very early, in the first hours after birth of the first piglets (De Passillé and Rushen, Reference De Passillé and Rushen1989). These disputes enable winning piglets to gain access to a better functional teat during the brief period of time when milk is ejected (Fraser and Rushen, Reference Fraser and Rushen1992). As a consequence, a stable ‘teat order’ emerges whereby piglets occupy the same teat at each suckling bout. A larger litter size is generally believed to increase disputes at the udder (Rutherford et al., Reference Rutherford, Baxter, D’Eath, Turner, Arnott, Roehe, Ask, Sandoe, Moustsen, Thorup, Edwards, Berg and Lawrence2013), but data from De Passillé and Rushen (Reference De Passillé and Rushen1989) do not support this hypothesis for the first day of life when the teat order is being established. However, in fully established lactation, the occurrence of skin lesions in suckling piglets increases with litter size, suggesting a positive relationship between fighting and litter size (Norring et al., Reference Norring, Valros, Munksgaard, Puumala, Kaustell and Saloniemi2006). Increased competition in large litters is also associated with a more variable and lower growth rate on average (Ocepek et al., Reference Ocepek, Newberry and Andersen2017). The current genetic selection for increasing litter size is likely to increase this competition (Ocepek et al., Reference Ocepek, Newberry and Andersen2017). Another source of variation in the intensity of the competition to which piglets are subjected is their position at suckling. Piglets that use teats in the middle of the udder have potentially more competitors for the teats than those that use the anterior or posterior teats.
Aggressive biting behaviour
There is evidence that piglets that need to compete strongly for milk retain a heightened aggressiveness after weaning. Using a resident-intruder test at 18 to 19 days post-weaning, D’Eath and Lawrence (Reference D’Eath and Lawrence2004) found that piglets from larger litters were more aggressive after weaning. In contrast, Chaloupková et al. (Reference Chaloupková, Illmann, Bartos and Spinka2007) did not observe any influence of litter size on the frequency of agonistic behaviours in newly weaned and mixed piglets. Litter size in this study, however, was relatively small (10.8 in average compared to 12.5 in D’Eath and Lawrence, Reference D’Eath and Lawrence2004) and may therefore have not resulted in much competition. Subsequently, Skok et al. (Reference Skok, Prevolnik, Urek, Mesarec and Skorjanc2014) showed that piglets that had sucked from middle teats were involved in more aggressive interactions with unfamiliar pigs post-weaning than those that had sucked from other parts of the udder. This effect did not seem to be related to liveweight at weaning, which was similar in piglets sucking anterior and middle teats.
Sibling competition is likely to occur in other contexts. As an example, piglets are born with little insulation and therefore face a major thermoregulatory challenge (Herpin et al., Reference Herpin, Damon and Le Dividich2002). Securing access to a warm resting area is essential for survival and, as for other resources that affect fitness, competition should be expected where a warm area is too small. While there has been little work to quantify how much biting occurs to access a nest or creep area of fixed size, it most likely increases with litter size, and this early-life competition probably has similar effects on later behavioural development to that resulting from competition for access to teats.
Non-aggressive biting behavior
In the study by Ursinus et al. (Reference Ursinus, Wijnen, Bartels, Dijvesteijn, van Reenen and Bolhuis2014b), females expressing a relatively high level of tail chewing and biting (both behaviours were registered in a single category) originated from larger litters (number of live-born piglets) compared with females with a relatively low level of tail chewing and biting. However, this result was dependent on the rearing environment, since it occurred only when pigs were housed in an environment enriched with jute sacks during lactation and after weaning. In a poor environment, high litter size was associated with a higher level of chewing directed to parts of the body other than the tail and ears.
Conclusion: postnatal competition
Taken together, these studies suggest that social competition experienced by piglets during lactation increases aggressive biting behaviour after weaning. In addition, non-aggressive biting behaviour may be increased in piglets originating from large litters. Taking into account the low number of studies, more data are needed to consolidate this conclusion.
Effects related to socialization of piglets by contact with piglets from other litters
Background
Under commercial conditions, pigs usually first encounter unfamiliar pigs at weaning at around 4 weeks of age, which is often accompanied by intense fighting and injuries from biting. Under natural conditions, young wild boar interact with piglets from other litters from the first week of life, without a high level of aggression or injurious bites (Gundlach, Reference Gundlach1968). Piglets of domestic sows reared in a free-range environment also start interacting with non-familiar pigs within the first 12 days of life (Jensen and Redbo, Reference Jensen and Redbo1987). As such, early-life socialization with unfamiliar animals is the norm in the wild ancestors of domestic pigs and, given the opportunity, domestic pigs revert to this practice.
In the wild, social groups usually comprise pigs that are related (Gabor et al., Reference Gabor, Hellgren, Van den Bussche and Silvy1999). An early-life window of greater tolerance to unfamiliar conspecifics may be an adaptive response to the need of litters of wild pigs to integrate into this larger and related social group. Avoidance of damaging biting may promote individual fitness by reducing the energetic costs of fighting and the risk of attracting predators.
Domestic piglets in indoor housing seem to retain this willingness to engage in minimal aggression with unfamiliar litters pre-weaning. Indeed, even if the number of fights and skin lesions is increased by mixing litters (Wattanakul et al., Reference Wattanakul, Stewart, Edwards and English1997a and Reference Wattanakul, Sinclair, Stewart, Edwards and English1997b; Pedersen et al., Reference Pedersen, Studnitz, Jensen and Giersing1998), pre-weaning socialization also stimulates play, resting together (Weary et al., Reference Weary, Pajor, Bonenfant, Ross, Fraser and Kramer1999) and sharing of home pen areas (Weary et al., Reference Weary, Pajor, Bonenfant, Fraser and Kramer2002). Therefore, a window of greater sociality is present in domestic pigs, and the indoor environment can be modified to allow voluntary integration of litters at a similar time as in the wild.
Aggressive biting behavior
Pre-weaning socialization reduces fighting when piglets are later mixed at weaning, although the mechanism of how it does so is not fully understood. Indeed, studies unanimously show evidence of a reduction in the frequency and/or duration of biting behaviour at post-weaning regrouping in pigs that have had the opportunity to socialize in early life (Weary et al., Reference Weary, Pajor, Bonenfant, Ross, Fraser and Kramer1999; D’Eath, Reference D’Eath2005; Kanaan et al., Reference Kanaan, Pajor, Lay, Richert and Garner2008; Kutzer et al., Reference Kutzer, Buenger, Kjaer and Schrader2009; Salazar et al., Reference Salazar, Ko, Yang, Llonch, Manteca, Camerlink and Llonch2018). While D’Eath (Reference D’Eath2005) reported that socialized pigs were quicker to attack a small, unfamiliar intruder introduced into the home pen, Wattanakul et al. (Reference Wattanakul, Stewart, Edwards and English1997a) and Kanaan et al. (Reference Kanaan, Pajor, Lay, Richert and Garner2008) found that socialized pigs took longer to attack a new pig. This discrepancy in attack latency is likely to result from the different social contexts in which aggressiveness was tested. The studies of Wattanakul et al. (Reference Wattanakul, Stewart, Edwards and English1997a and Reference Wattanakul, Sinclair, Stewart, Edwards and English1997b) and Kanaan et al. (Reference Kanaan, Pajor, Lay, Richert and Garner2008) involved mixing pigs in a novel environment with others of similar competitive ability, in contrast to that of D’Eath (Reference D’Eath2005) in which one pig had a clear competitive and residency advantage. Taken together, the evidence would suggest that socialized pigs take longer to enter into a fight, unless they have a home pen advantage and are faced with an inferior opponent, and are thus better able to efficiently establish dominance relationships. In addition, during a social encounter test performed a couple of days before or after weaning, piglets reared in a group farrowing system approached an unfamiliar piglet more quickly, stayed closer to it and were more active compared with piglets reared in individual farrowing pens (Hillmann et al., Reference Hillmann, von Hollen, Bunger, Todt and Schrader2003). This was interpreted by the authors as a lesser but better adapted reaction to an unfamiliar pig. Finally, a recent work showed that socialized pigs solve dominance relationships sooner in a dyadic contest setting (Camerlink et al., Reference Camerlink, Turner, Farish and Arnott2019). It is assumed, but has never been tested, that the opportunity to engage in play fighting and other forms of social contact with unfamiliar animals pre-weaning allows a more rapid acquisition of mature social skills or cognitive ability.
Pre-weaning socialization can be achieved by allowing piglets, but not the sows, from adjacent farrowing pens to mix, or by using a multi-suckling system in which multiple sows and litters are allowed to integrate. It is possible that the benefit of socialization derives both from early-life contact with unfamiliar piglets but also from a more complex and larger physical environment. For example, Weary et al. (Reference Weary, Pajor, Bonenfant, Ross, Fraser and Kramer1999) allowed piglets to socialize between 11 days of age and weaning at 28 days, but the socialized piglets also had access to a communal area in which different enrichment objects were available. Similarly, Hillmann et al. (Reference Hillmann, von Hollen, Bunger, Todt and Schrader2003) offered more space per piglet and a more complex environment to socialized litters compared with un-socialized control ones. However, the work of Wattanakul et al. (Reference Wattanakul, Stewart, Edwards and English1997a) and Kutzer et al. (Reference Kutzer, Buenger, Kjaer and Schrader2009) showed that removing the division between adjacent farrowing pens reduced post-weaning aggression and skin injuries, even though the floor space per piglet and level of enrichment of the environment remained the same. This indicates that socialization itself can reduce subsequent aggression independently of, even though potentially in addition to, environmental enrichment.
Pigs are often regrouped several times after weaning and, at present, it is unknown whether the benefits of socialization are evident only during mixing at weaning or persist into later regrouping episodes. In pigs maintained in stable groups after weaning, a recent work has shown that socialized pigs had 19% fewer skin lesions from aggression compared with controls 4 weeks after weaning (Camerlink et al., Reference Camerlink, Farish, D’Eath, Arnott and Turner2018).
Non-aggressive biting behavior
In the study by Klein et al. (Reference Klein, Patzkewitschl, Reese and Erhard2016), piglets were allowed to socialize with piglets from three other litters starting at 10 days after parturition. Although tail biting occurred in all groups, a higher percentage of pigs from the early socialized groups had intact tails at day 100 of the fattening period, and their tails were significantly longer.
Conclusion: socialization
Altogether, these studies indicate that socializing piglets during lactation, by allowing them to interact with piglets from other litters, reduces aggressive biting at weaning and probably until some weeks after weaning. Even though more research is needed to substantiate the effect of socialization on non-aggressive biting, the first results are also in favour of a reduction of tail biting.
Effects related to cross-fostering
Background
Litter size has increased to such an extent that the number of live-born piglets often exceeds the number of functional teats. With the trend for more piglets than teats, management solutions such as cross-fostering and fostering to a nurse sow or supplementing with milk replacer have become standard practice in commercial pig husbandry (Baxter et al., Reference Baxter, Rutherford, D’Eath, Arnott, Turner, Sandoe, Moustsen, Thorup, Edwards and Lawrence2013). If performed correctly, cross-fostering enhances survival prospects of piglets and can reduce the need for further management interventions. It is recommended to take place within the first 24 h after birth. As piglets get older, aggression after fostering is more intense and is associated with higher pre-weaning mortality (Straw et al., Reference Straw, Dewey and Burgi1998). Piglets that are fostered may suffer from hunger and chilling during the process of acceptance, while all the piglets in the litter may suffer from social stress. Indeed, Heim et al. (Reference Heim, Mellagi, Bierhals, Souza, Fries, Piuco, Seidel, Bernardi, Wentz and Bortolozzo2012) observed more fighting just after milk ejection in litters where half of the piglets were adopted, as well as in litters where all piglets were adopted, compared with litters with no adoption. Similarly, Robert and Martineau (Reference Robert and Martineau2001) found more fighting in fostered litters compared with control litters, both during and between nursings. There are reports of long-term detrimental impacts of cross-fostering on survival, growth, behaviour, reproductive success and immunity (Baxter et al., Reference Baxter, Rutherford, D’Eath, Arnott, Turner, Sandoe, Moustsen, Thorup, Edwards and Lawrence2013). Therefore, long-term effects of cross-fostering on aggressive and non-aggressive behaviours are expected.
Aggressive biting behavior
Compared with piglets originating from litters with no cross-fostering, piglets from litters with fostering at 6 days of age fought less immediately following weaning and social mixing performed around 18 days of age, as well as at 1 and 20 days later (Giroux et al., Reference Giroux, Robert and Martineau2000). Being a resident or an intruder piglet at fostering did not change this effect. The occurrence of less fighting was accompanied by a tendency for fewer body lesions in the first week post-weaning, but not later on. The authors attributed the lower fighting frequency in litters with fostered piglets to prior experience of encountering unfamiliar piglets. It may have similar effects to the socialization performed later on during lactation (cf. Socialization of piglets). Similarly, Scheffler et al. (Reference Scheffler, Stamer, Traulsen and Krieter2016b) observed that pigs which had not been raised by their own dam showed fewer agonistic interactions and were less aggressive compared with non-cross-fostered animals when observed shortly after mixing at weaning or at transfer to the growing pens. More recently, Diaz et al. (Reference Diaz, Manzanilla, Diana and Boyle2018) compared piglets originating from litters with no cross-fostering or from litters subjected to early (first week of life) or late (second and third weeks of life) cross-fostering. Pigs were inspected individually for the presence of body lesions during the post-weaning and fattening periods. Results did not show any difference in the presence of body lesions between treatments. This lack of difference may be due to the fact that lesions were determined several weeks after regrouping, whereas skin lesions commonly disappear in a couple of days, and to a binary scoring method unable to pick up differences in the severity and frequency of lesions.
Non-aggressive biting behavior
Moinard et al. (Reference Moinard, Mendl, Nicol and Green2003) found a higher incidence of tail biting in farms where cross-fostering was practised compared with farms with no cross-fostering. Since this was an epidemiological study, it cannot be elucidated whether fostering contributed directly to a later likelihood of tail-biting occurrence or whether this association was related to a common causal factor (e.g. herd size or litter size increasing the likelihood of fostering). In the study by Diaz et al. (Reference Diaz, Manzanilla, Diana and Boyle2018), the presence of ear and tail lesions was not influenced by the occurrence of cross-fostering. However, pigs from fostered litters were more at risk of death and euthanasia, with severe tail lesions being one of the reasons for euthanasia. It suggests that cross-fostering promotes severe tail biting.
Conclusion: cross-fostering
Few studies have examined the influence of cross-fostering on aggressive and non-aggressive biting. Current evidence suggests that this practice may reduce aggressive behaviour and the amount of body lesions, particularly at regrouping, but with a decreasing influence over time. In contrast, it may increase non-aggressive biting after weaning. Taking into account the very low number of studies, more data are needed to consolidate this conclusion, especially regarding non-aggressive biting.
Effects related to age at weaning or artificial rearing
Background
In current intensive pig farms, weaning is abrupt and occurs usually between 3 and 5 weeks of age. This is much earlier than would be the case in natural conditions, where weaning is a very progressive process lasting for several weeks and ending at about 17 weeks of lactation (Jensen and Recen, Reference Jensen and Recen1989). Abrupt early weaning is highly stressful for the animals, as shown by the activation of the adrenal axis and changes in behaviour (Colson et al., Reference Colson, Orgeur, Foury and Mormede2006 and Reference Colson, Martin, Orgeur and Prunier2012). Alteration in behaviour is more profound when pigs are younger at weaning. Therefore, the behaviour of pigs during the post-weaning and fattening periods could differ according to the age at weaning. An extreme situation arises with ‘artificial rearing’ of piglets shortly after birth. This is performed when highly prolific sows have more piglets than teats and cross-fostering cannot be applied (Baxter et al., Reference Baxter, Rutherford, D’Eath, Arnott, Turner, Sandoe, Moustsen, Thorup, Edwards and Lawrence2013). In this situation, piglets are usually allowed to suck colostrum from the dam and then transferred to a rearing pen, where they are provided with milk from a cup. This gives no opportunity to suckle, even though motivation to do so remains high (Noyes, Reference Noyes1976; Frei et al., Reference Frei, Wurbel, Wechsler, Gygax, Burla and Weber2018).
Aggressive biting behavior
Comparison of pigs weaned at about 10 or 30 days of age showed no difference between treatments in the occurrence of fighting behaviour, evaluated between 40 and 150 days of age (Hohenshell et al., Reference Hohenshell, Cunnick, Ford, Kattesh, Zimmerman, Wilson, Matteri, Carroll and Lay2000). Similarly, the frequency of aggressive behaviours measured at 42 days of age did not differ between pigs weaned at 7, 14 or 28 days of age (Worobec et al., Reference Worobec, Duncan and Widowski1999).
Non-aggressive biting behavior
Artificial rearing of piglets, separated from the sow between 3 and 6 days of age, resulted in high levels of belly nosing that lasted until at least 50 days of age (Hosp et al., Reference Hosp, Rzezniczek, Weber, Hillmann, Erhard, Pollmann, Reiter and Waiblinger2014; Rzezniczek et al., Reference Rzezniczek, Gygax, Wechsler and Weber2015). Whether this very early separation from the dam results in a higher propensity for tail biting has not been evaluated. However, it is highly probable since significant correlations between tail-biting and belly-nosing behaviours have been described (Edwards, Reference Edwards2003; Brunberg et al., Reference Brunberg, Wallenbeck and Keeling2011).
Pigs weaned at 7 or 14 days of age showed a higher frequency of massaging penmates at 42 days of age than did pigs weaned at 28 days of age, but there was no effect on the occurrence of nosing-chewing penmates (Worobec et al., Reference Worobec, Duncan and Widowski1999). Comparing pigs weaned at around 10 and 30 days of age, Hohenshell et al. (Reference Hohenshell, Cunnick, Ford, Kattesh, Zimmerman, Wilson, Matteri, Carroll and Lay2000) found a transient increase in manipulatory behaviours (nosing + biting + pushing + suckling part of another pig’s body) at 40 days of age, but no difference at 65, 102, 137 and 165 days. Furthermore, Algers (Reference Algers1984) found no difference between pigs weaned at 3 and 6 weeks of age in injuries caused by manipulation. Comparing pigs weaned at 4 and 6 weeks, Boe (Reference Boe1993) found a higher frequency of massaging and sucking penmates at the beginning of the fattening period in pigs weaned at the youngest age, but no increase in tail biting and tail lesions. Results indicated that the effect of the post-weaning environment had more influence than the age at weaning (Algers, Reference Algers1984; Boe, Reference Boe1993).
Conclusion: age at weaning or artificial rearing
Early weaning stimulates, at least transiently, massaging and/or chewing of penmates, with the risk of provoking damage if it is persistent. However, available data suggest that age at weaning has no clear influence on aggressive and non-aggressive biting in growing pigs.
Effects related to acute stress due to handling and routine practices
Piglets usually undergo a series of routine management practices within their first days of life, such as castration, tail docking, teeth clipping, ear tagging and medical treatments. These interventions certainly cause acute stress due to handling and/or pain (Prunier et al., Reference Prunier, Mounier and Hay2005; Marchant-Forde et al., Reference Marchant-Forde, Lay, McMunn, Cheng, Pajor and Marchant-Forde2014), but it is not clear if such stressors may have a long-term effect on aggressive or non-aggressive biting. To the best of our knowledge, there are no data in the literature to support or refute such a hypothesis without difficulties of interpretation. For example, the influence of acute stress due to the surgery of tail docking is impossible to evaluate since it is confounded with the influence of shortening the tail, which itself reduces the likelihood of tail biting even if it does not fully eliminate it (EFSA, 2007). Similarly, the influence of acute stress due to surgical castration is confounded with the effect of the removal of testicular steroids that are known to have a great influence on behaviour.
Effects related to the housing environment
Background
The environment provided to piglets in most conventional farms is restrictive and does not fulfil their exploratory needs. This may result in behavioural and physiological disturbances, with potential long-term consequences on the ability of pigs to cope with their rearing conditions, as well as on their social skills and abilities to resolve social conflicts (de Jonge et al., Reference de Jonge, Bokkers, Schouten and Helmond1996). The pre-weaning environment involves a number of aspects that act simultaneously on piglets, and so individual effects are usually difficult to isolate. Among these, restricted space and lack of enrichment material can be considered most important. Construction features such as crates may hinder vision and movement and thus proper communication between pigs, leading to increased agonistic behaviours (Lammers and Schouten, Reference Lammers and Schouten1985). Other environmental aspects, such as continuous fan noise over certain thresholds (>85 dB), may also be important (Algers and Jensen, Reference Algers and Jensen1991).
Experimental data have shown that enriching the environment of growing pigs from birth until slaughter reduces the occurrence of penmate-directed manipulatory (nibbling, sucking or chewing ears, legs, feet or tails) and aggressive behaviours, as well as the occurrence of tail lesions at various ages (Beattie et al., Reference Beattie, O’Connell and Moss2000; Ursinus et al., Reference Ursinus, Wijnen, Bartels, Dijvesteijn, van Reenen and Bolhuis2014b). However, in these experiments, the effects of the early and current environments are confounded. Several experiments have been set up to evaluate the influence of the early environment per se on the behaviour of pigs observed during the subsequent post-weaning or fattening periods (Table 1).
Table 1 Influence of the pre-weaning (preW) environment on the behaviour of pigs during the post-weaning (postW) or fattening periods. Positive effects are highlighted in light grey, negative effects in dark grey and lack of effects are not highlighted
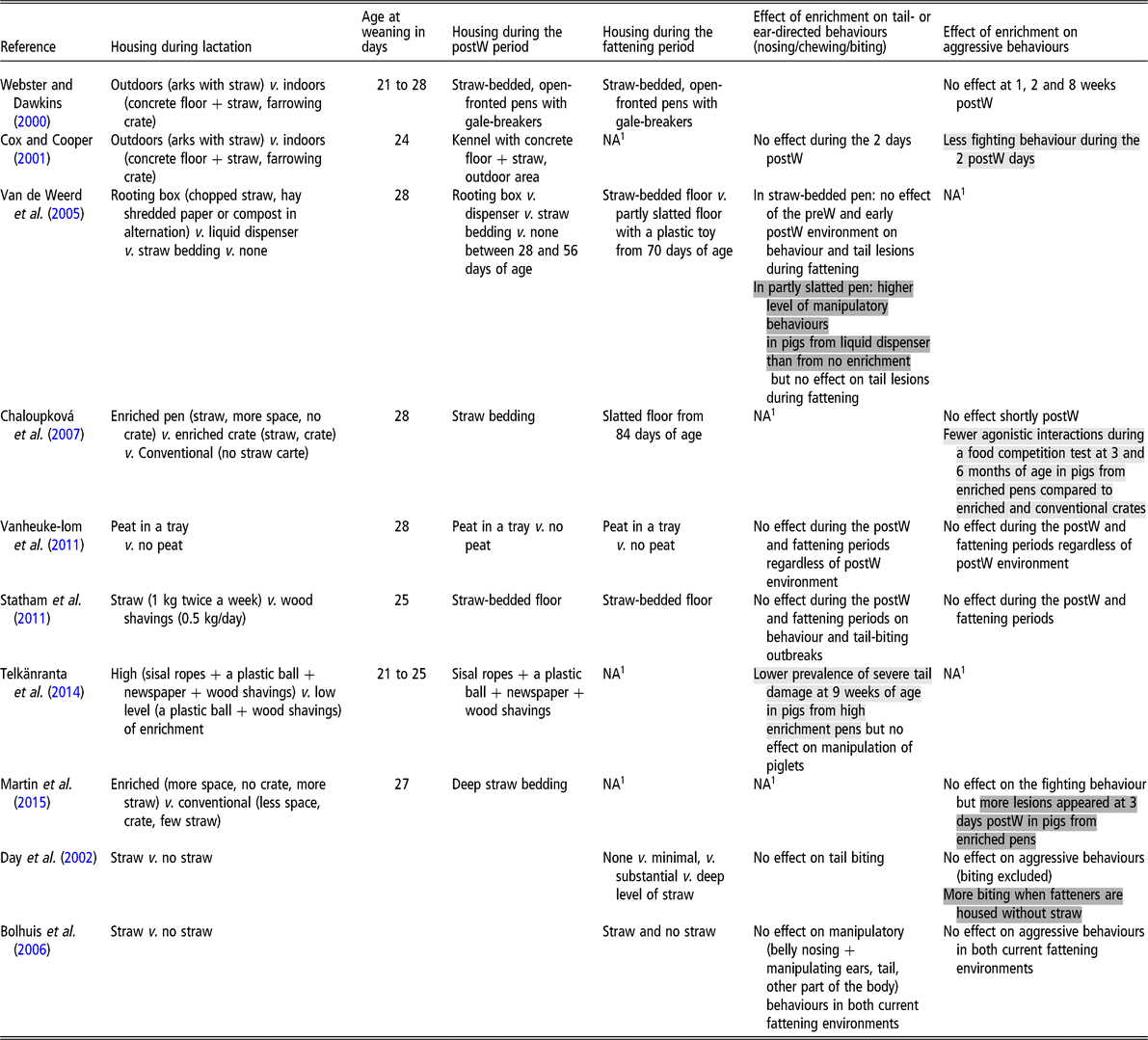
1 NA = no data available.
Aggressive biting behavior
Webster and Dawkins (Reference Webster and Dawkins2000) studied how piglets raised indoors or outdoors before weaning differed in their behaviour after weaning into a pen with concrete floor covered by straw. Compared to the indoor environment, the outdoor environment offered piglets more space, more rooting material and opportunities for social interactions with other litters, and so many factors were confounded. The authors did not observe any difference between indoor and outdoor pigs concerning the fighting behaviour observed just after weaning, as well as at 1, 2 and 8 weeks after weaning. Using the same experimental model, Cox and Cooper (Reference Cox and Cooper2001) focussed more on the period after weaning with a more detailed ethogram. Indoor piglets showed more fighting behaviours during the first 2 days after weaning. Working only with indoor pigs, Chaloupková et al. (Reference Chaloupková, Illmann, Bartos and Spinka2007) tested the influence of the pre-weaning environment on agonistic behaviours on the day after weaning at 4 weeks of age, and on the behaviour during resource competition tests at 3 and 6 months of age. Treatments were conventional crates (slatted floor, no straw), enriched farrowing crates (straw-bedded pen, 10% additional area) and enriched farrowing pens (straw-bedded pen, 60% additional area, no crate). Pigs were housed in straw-bedded pens after weaning and in slatted pens thereafter, when food competition tests were performed. No effect of the pre-weaning environment was detected on agonistic behaviours immediately after weaning. However, pigs from the enriched farrowing pens showed fewer agonistic interactions during feed competition tests at 3 and 6 months of age compared with pigs from the two other environments. In a factorial design, Vanheukelom et al. (Reference Vanheukelom, Driessen, Maenhout and Geers2011) evaluated the influence of providing peat during lactation to pigs which either subsequently did or did not have access to peat later on in life. They found no influence of the pre-weaning environment on fighting behaviour during the post-weaning and fattening periods, whereas the presence of peat in the current environment reduced fighting during the post-weaning period. Similarly, Statham et al. (Reference Statham, Green and Mendl2011) did not find an effect of adding straw on the floor of farrowing pens (1 kg twice a week) on agonistic behaviour of pigs during the post-weaning and finishing periods. These pigs were housed after weaning on a solid concrete floor with straw added at regular intervals. Martin et al. (Reference Martin, Ison and Baxter2015) found that housing piglets during lactation in an enriched environment (280% more space plus fresh long-stemmed straw), in comparison to a conventional one, increased the appearance of skin lesions between weaning and 3 days later. However, this did not influence the latency to first fight after weaning nor the occurrence of fighting behaviours during the post-weaning period (28 to 56 days of age).
Prolonging the same level of enrichment before and after weaning (experience with straw v. no straw), Day et al. (Reference Day, Burfoot, Docking, Whittaker, Spoolder and Edwards2002) compared the influence of the early environment in fattening pigs housed with four levels of straw provision (none, minimal, substantial and deep). The early environment had no influence on aggressive behaviours excluding biting. However, there was a significant interaction between the early and current environments for biting any part of another pig except the tail, presumably reflecting aggressive biting. Indeed, biting behaviour was influenced by the early environment only when pigs had no access to straw during the fattening period: an increase was shown in pigs having had an early experience with straw. Similarly, Bolhuis et al. (Reference Bolhuis, Schouten, Schrama and Wiegant2006) prolonged the enrichment with straw during lactation to the post-weaning period. From 70 days of age, pigs were exposed either to straw or not in a two factorial design. These did not find any significant effect of the early environment on aggressive behaviour.
Whether the influence of a poor pre-weaning environment on piglet behaviour is age-dependent remains to be evaluated. It is known that piglets stay close to the sow during the first 4 days of life (Kirkden et al., Reference Kirkden, Broom and Andersen2013). Therefore, it may be expected that they are relatively unaware of their environment beyond the maternal presence and that the consequences of a barren environment are minor. From day 4 of age until weaning, piglets’ environmental needs may change as they grow. According to Lewis et al. (Reference Lewis, Boyle, O’Doherty, Lynch and Brophy2006), piglets would not interact with shredded paper or natural fibre ropes before 10 days of age, although this may not apply to all types of enrichment materials. The same authors found that, when piglets were offered shredded paper or natural fibre ropes in the farrowing crate, enrichment characteristics were already relevant between 14 and 26 days of age, since piglets offered shredded paper and ropes spent substantial time interacting with the enrichment but were much more interested in paper than ropes.
Other forms of enrichment related to olfactory, taste and auditory senses are also possible but have not been investigated for their influence on aggressive biting, except with the aim of familiarizing pigs with their environment between phases of rearing. For example, a pleasant odorant molecule (isoamyl acetate = banana scent) was topically applied on the skin of the sows’ mammary glands during lactation and on the feeders after weaning (Fuentes et al., Reference Fuentes, Otal, Hevia, Quiles and Fuentes2012). Piglets in contact with the scent during lactation had fewer agonistic interactions, including biting after weaning. Since the banana scent is unlikely to inhibit aggressive behaviours, the effect is probably linked to a decrease in stress due to novelty of the environment at weaning. In another attempt, feed supplemented with an anisic flavour using transanethol was given to sows during gestation and/or lactation, and piglets received a feed with the same flavour in addition to a standard feed during the 2 weeks after weaning (Oostindjer et al., Reference Oostindjer, Bolhuis, van den Brand, Roura and Kemp2010). Exposure to the flavour during gestation and/or lactation had no effect on the amount of aggressive behaviour in the hours after weaning. However, latency to fight after weaning increased in pigs exposed to the flavour during gestation but not during lactation.
Non-aggressive biting behavior
Comparing commercial farms with a history of tail biting during the previous 6 months with farms with no tail biting, Moinard et al. (Reference Moinard, Mendl, Nicol and Green2003) suggested a link between the degree of enrichment in farrowing accommodation and tail biting. They found that renewing straw daily in the farrowing pen was more frequent in farms with no tail biting. However, this effect may have been confounded with a more frequent use of straw during the later stages of pig production. Cox and Cooper (Reference Cox and Cooper2001) did not find differences between indoor and outdoor pre-weaning environments on tail-biting levels performed by piglets during the first 2 days after weaning. Van de Weerd et al. (Reference Van de Weerd, Docking, Day and Edwards2005) compared different enrichment materials (rooting box with chopped straw, hay, shredded paper or compost in alternation, liquid dispenser, straw bedding or none) provided for 4 weeks either during lactation or during the immediate post-weaning period. From 10 weeks of age until slaughter at around 90 kg liveweight, pigs were reared on partly slatted floors with a minimum legal amount of enrichment or on straw bedding. The early environment had no influence on tail biting observed during fattening, in contrast to effects of the current environment. Some effects of the post-weaning environment were observed on behaviour during fattening, but only in the poor environment, with pigs having the liquid dispenser showing more manipulatory behaviours than those with no enrichment. Similarly, Statham et al. (Reference Statham, Green and Mendl2011) did not find an effect of adding straw on the floor of farrowing pens in pigs subsequently reared on solid concrete floors with straw. Outbreaks of tail biting occurred at the same level in both experimental groups, and frequencies of tail biting and chewing behaviours observed at 7, 11, 15 and 19 weeks of age were also similar. Similarly, Vanheukelom et al. (Reference Vanheukelom, Driessen, Maenhout and Geers2011) did not show an influence of enriching the pre-weaning environment with peat on manipulatory behaviours (chewing and non-violent biting any part of a congener) during the post-weaning and fattening periods, whereas peat during the post-weaning period reduced manipulatory behaviours. Telkänranta et al. (Reference Telkänranta, Swan, Hirvonen and Valros2014) compared two levels of enrichment during lactation (sisal ropes + a plastic ball + newspaper + wood shavings v. a plastic ball + wood shavings) in pigs reared in an identically enriched environment after weaning (sisal ropes + a plastic chewing toy + wood shavings). They observed a lower prevalence of severe tail damage at 9 weeks of age in pigs from the richer lactational environment (10% v. 32%), even though the frequency of penmate manipulation, defined as touching any part of the body, was not influenced by the lactational environment.
Confounding the influence of enrichment before and after weaning (experience with straw v. no straw), Day et al. (Reference Day, Burfoot, Docking, Whittaker, Spoolder and Edwards2002) did not show any significant influence of the early environment on tail biting expressed by fattening pigs, regardless of the level of enrichment in the current environment, whereas the presence of straw in the current fattening environment reduced tail biting. Similarly, Bolhuis et al. (Reference Bolhuis, Schouten, Schrama and Wiegant2006) did not find any influence of early experience with straw on manipulatory behaviours (belly nosing + manipulating ears, tail, other part of the body) in fatteners, regardless of the current environment, whereas the presence of straw in the current environment reduced these behaviours.
The benefit of familiarity with the environment between phases of rearing the pigs was also investigated using a special flavour as a continuous stimulus in the environment (Oostindjer et al., Reference Oostindjer, Bolhuis, Simon, van den Brand and Kemp2011). Piglets were exposed to an anisic flavour for 2 weeks following weaning, after being exposed to a control or to the same anisic flavour during prenatal life and the lactational period via their mother’s feed. Manipulatory behaviour (nibbling, sucking or chewing body parts of penmates) was reduced in piglets exposed to the anisic in their early life. Fewer vocalizations and shorter latency to eat were also observed, suggesting that these pigs were less stressed by weaning.
Conclusion: environmental enrichment
Enriching the environment during lactation has shown diverse effects on both aggressive and non-aggressive biting depending on the study. Some studies do not show any significant effect, whereas others indicate a promising positive reduction in biting or, on the contrary, an increase. This suggests that the nature of the enrichment during the pre-weaning period, as well as the housing after weaning are of great importance to determine the effects. For example, it is likely that pre-weaning-enriched conditions may be detrimental for piglets, at least regarding aggressive biting, if pigs are deprived of enrichment after weaning. Another promising way to reduce harmful behaviours after weaning would be to ensure some familiarity with the environment when animals are moved between houses using, for example, continuous exposure to a familiar pleasant scent.
Overall conclusion
Literature on the early-life factors predisposing to biting is variable according to the type of factor, and results are not always consistent. Regarding non-aggressive biting, its relatively low frequency and unpredictable nature make it difficult to analyse and may explain, at least in part, a lack of the influence of some treatments and/or inconstancy between some studies.
The influence of personality has been poorly examined. Most studies used the response to a backtest to assess coping style, which only reflects one part of personality. Moreover, this assessment is often performed at a very young age, probably well before personality is fully established. Therefore, there is a large scope for new investigations evaluating how personality and its development influence biting. For most of the environmental factors having a potential influence on future biting behaviours, the number of scientific papers is low (less than five) and sometimes there are no data (Figure 2). Only the influence of early socialization on aggressive biting, poor nutrition on non-aggressive biting, and poor environment on both types of biting have been more fully investigated. Sometimes the conclusions differ between studies, suggesting that the influence of one factor may depend on other factors or on the age when effects were observed. This is, for example, the case for the influence of poor housing on both types of biting. Overall, the conditions of the current environment during the post-weaning or fattening periods are probably of greater importance, and may mask or interact with those existing before weaning.
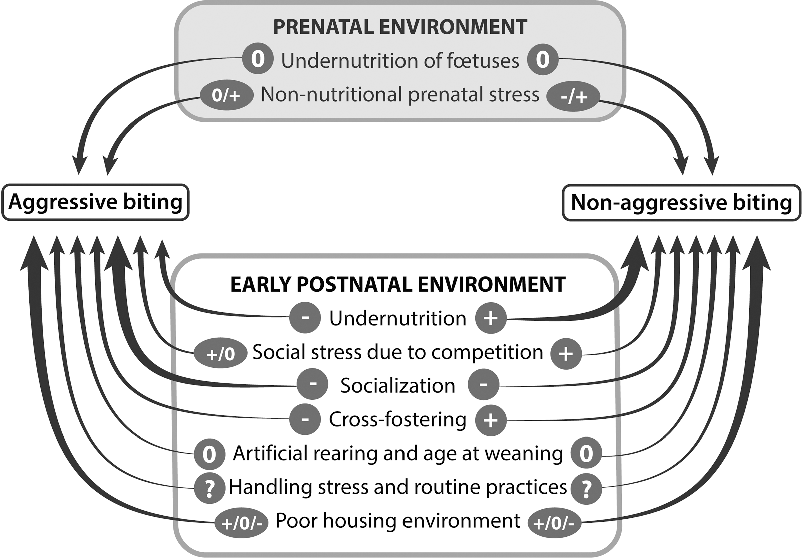
Figure 2 Summary of the effects of prenatal and pre-weaning environments of pigs on the occurrence of their biting behaviours later in life. When at least five studies are available, the arrows are drawn with a thick line. Signs above the arrows indicate that there is at least one study showing that the considered factor increases (+), has no effect (0) or decreases (−) the occurrence of biting. A question mark indicates that there is no information due to a lack of published studies.
Regarding aggressive biting, undernutrition, cross-fostering and socialization early in life reduces its later occurrence. The practical consequence is that any means to allow piglets from different litters to interact from the second week of age should be encouraged. Regarding non-aggressive biting, undernutrition, social stress due to competition and cross-fostering stimulate its occurrence later in life. These three factors are highly dependent on litter size at birth. Therefore, the full consequences of large litters at birth should be evaluated in terms of health, welfare and performance over the whole life of pigs in order to make a more comprehensive assessment of the advantages and drawbacks of a high litter size. Regarding both types of biting, the use of familiar odours may contribute to their reduction when pigs are moved from one stage of production to another, by alleviating the level of stress associated with novelty of the environment. Therefore, this is a promising method of improvement that needs more research for validation and implementation. Finally, it should be remembered that these early environmental factors are likely to interact with genetic predisposing factors.
Acknowledgements
This review paper is based upon work from COST Action CA15134 – Synergy for preventing damaging behaviour in group-housed pigs and chickens (GroupHouseNet), supported by COST (European Cooperation in Science and Technology; www.cost.eu). The text represents the authors’ views and does not necessarily represent a position of the Commission, which will not be liable for the use made of such information.
A. Prunier 0000-0003-3070-6613
Declaration of interest
There is no conflict of interest involved with this paper.
Ethics statement
This paper is a review of published information. No new ethical approval was required.
Software and data repository resources
No new data were generated in this paper.





