Introduction
Recent archaeological and bioarchaeological research emphasise the intricate relationship between climate change, social organisation and human health (Burke et al. Reference Burke2021; Robbins Schug et al. Reference Robbins Schug2023). Understanding the resilience, adaptation or, ultimately, the collapse of populations in prehistory can help inform sustainability measures and policies related to our current climate crisis. But to recognise the true value of the data, we must first dissect the specific drivers of climate-driven shifts in human society and health. This article aims to assess climate-change-related dietary stress in prehistoric Japan and its potential role in population decline at a time of global climatic cooling, c. 4200 BP.
The Jōmon people (縄文人) were sedentary foragers who inhabited the Japanese islands between 16 500 and 2300 years ago (Koyama Reference Koyama1979; Nakamura et al. Reference Nakamura, Taniguchi, Tsuji and Oda2001; Matsui & Kanehara Reference Matsui and Kanehara2006; Nakashima et al. Reference Nakashima, Ishida, Shigematsu, Goto and Hanihara2010). The Middle Jōmon (c. 5400–4500 BP) marked a peak in population density and cultural complexity at the end of the Holocene Thermal Maximum (HTM), a period of global warming (Koyama Reference Koyama1979; Hoover & Matsumura Reference Hoover and Matsumura2008; Schmidt et al. Reference Schmidt2019). Subsequent climatic cooling from around 4500 BP corresponds with the Late Jōmon period, during which reduced settlement density in eastern and inland Japan is presumed to reflect a decline in population size (Yasuda Reference Yasuda2004; Schmidt et al. Reference Schmidt2019; Taniguchi Reference Taniguchi2019). Conversely, in the west a gradual population increase occurred, albeit not to the degree of the eastern Middle Jōmon (Koyama Reference Koyama1979; Habu Reference Habu2004; Temple Reference Temple2007a). Globally, this cooling event (the 4.2ka event) was arguably instrumental in large social shifts associated with changing health burdens, such as the introduction of agriculture in Southeast Asia and the depopulation of cities in the Indus Valley (Oxenham et al. Reference Oxenham2018; Robbins Schug et al. Reference Robbins Schug2023; Vlok et al. Reference Vlok2023).
Establishing the specific drivers of the Late Jōmon population decline represents a major archaeological challenge. Environmental change with global cooling likely had an impact on human populations but evidence for this is often indirect and ambiguous, with cause and effect open to interpretation. For example, Habu (Reference Habu2008) proposes that the Late Jōmon population decline was the result of an overspecialised reliance on dietary resources that became less available as the climate cooled. This proposal is based on an apparent simplification of technology in the Late Jōmon compared to earlier periods. Similarities in mortuary customs, tooth ablation patterns and artefacts between eastern Middle Jōmon and western Late Jōmon sites also appear to indicate migration to the warmer west as a component of demographic change (Matsumoto et al. Reference Matsumoto, Habu, Matsui, Habu, Lape and Olsen2017). Yet Crema and Kobayashi (Reference Crema and Kobayashi2020) use Bayesian dating to demonstrate that site density declined in eastern Japan centuries prior to climatic cooling (from 5000 BP), casting doubt on a direct relationship between climate and population change, at least in the east. Viewed in another light, the archaeological research could suggest a model of regionally focused adaptation and resilience rather than a blanket vulnerability to a changing climate.
Palynological analyses indicate that a shift from evergreen to coniferous ecological zones in western and central Japan accompanied the cooling period. This ecological shift would have had a substantial impact on available food sources (Koyama Reference Koyama1979). Evergreen woodlands provide an abundance of flora and fauna, including nut-bearing trees, in contrast to the lower bioavailability of coniferous forests (Koyama Reference Koyama1979; Pearson Reference Pearson2006; Hoover & Matsumura Reference Hoover and Matsumura2008). The isotopic evidence, however, shows a marked increase in the use of terrestrial resources from the Late Jōmon period, particularly in western Honshū (Kusaka et al. Reference Kusaka, Uno, Nakano, Nakatsukasa and Cerling2015). The contrasts presented by prior research underscore the social, environmental and biological complexity of the impacts of climate change.
Bioarchaeological research and the Jōmon population decline
Reduction in resource returns, as the climate cooled, is proposed to have driven nutritional stress and thus population decline among the Jōmon (Kawahata et al. Reference Kawahata, Yamamoto, Ohkushi, Yokoyama, Kimoto, Ohshima and Matsuzaki2009; Kawahata Reference Kawahata2019), but this hypothesis is not supported by physical evidence of physiological stress in skeletal assemblages. Temple (Reference Temple2007a) found little difference in markers of non-specific stress (linear enamel hypoplasia (LEH) and cribra orbitalia) in Jōmon through time and concluded there was no evidence for increased dietary stress following the HTM. A decrease in long bone growth at Late to Final Jōmon sites is apparent (Temple Reference Temple2008; Temple et al. Reference Temple, Nakatsukasa and McGroarty2012) but a lack of change in femoral robusticity, and an increase in the prevalence of carious lesions, across the Middle to Late/Final Jōmon periods seems to suggest adaptations to subsistence within the pre-existing sociocultural system indicative of resilience to climate change (Temple Reference Temple, Temple and Stojanowski2018).
No significant difference in any marker of stress across time or space was identified in a study of 294 Jōmon skeletons from Honshū (Suzuki Reference Suzuki1998). Suzuki uses this lack of difference, and the low overall levels of bone pathology among the Jōmon, to argue that individuals succumbed rapidly to any infection, prior to the development of advanced bone infection, due to nutritional inadequacy. Research on Jōmon palaeopathology has almost exclusively investigated non-specific stress markers in aggregated assemblages (Yamamoto Reference Yamamoto1988; Koga Reference Koga2002; Hoover & Matsumura Reference Hoover and Matsumura2008; Sawada et al. Reference Sawada, Suzuki, Yoneda, Sato, Hirata and Dodo2008; Fujita & Nishizawa Reference Fujita and Nishizawa2022). These markers are multi-aetiological, their formation being influenced by many factors including psychological, traumatic, infectious, nutritional and developmental stress; hence the bone markers are considered ‘non-specific’. As a result, the presence of markers of non-specific stress is not direct evidence of dietary deficiency—a state in which the body becomes ‘diseased’ from a primary nutritional external source (Meyer Reference Meyer2016)—and in isolation, these markers may not be sensitive enough to investigate a relationship between nutrition and population decline. They are, however, valuable for consideration of the overall health burden of populations and do suggest clear regional differences in the health profiles of northern, western, central and eastern Jōmon, indicating great variety in the biosocial contexts of these populations and their consequences on health (Temple Reference Temple2007a & Reference Templeb; Oxenham & Matsumura Reference Oxenham and Matsumura2008; Fujita & Nishizawa Reference Fujita and Nishizawa2022).
We propose that skeletal evidence of a specific micronutrient deficiency is a more useful proxy for assessing resource shortage, specifically. Micronutrient deficiencies are a consequence of insufficient intake of vitamins and/or minerals. Thus, they are suitable for identifying the restriction of resource variety and availability that can occur with ecological shifts, which in turn may be associated with changing climate. Scurvy is a disease caused by extreme vitamin C deficiency. Macroscopic skeletal evidence of scurvy is readily observable and if untreated the disease is often fatal, making it a useful proxy for evidence of nutritional disease in the archaeological record. Lack of vitamin C weakens the walls of blood vessels which leads to repeated haemorrhaging throughout the body. Haemorrhage close to the bone surface can cause inflammation which promotes an osteoblastic response that is visible as discrete lesions of new bone formation. Increased capillary formation to drain haematomas increase cortical porosity on the bone surface. Disruption in bone growth in subadults with vitamin C deficiency may be observed radiographically as radiodense white lines of Fraenkel and radiolucent Trümmerfeld zones, providing corroboration for macroscopic scurvy diagnoses (Jaffe Reference Jaffe1972).
An association has been suggested between scurvy and agricultural transitions with an over-reliance on a reduced range of micronutrient-poor staple crops (Snoddy et al. Reference Snoddy, Halcrow, Buckley, Standen and Arriaza2017). Yet low vitamin C levels are not just experienced by agriculturalists and have been recorded among the Masai from Kenya and the San Bushman from southern Africa (Gatenby Davies & Newson Reference Gatenby Davies and Newson1974; Kreike Reference Kreike2010). Given the marked seasonal climate of temperate Japan, available dietary resources fluctuated significantly throughout the year. During the winter months, reliance on boar and deer increased (see online supplementary material (OSM) Table S1). Vitamin C rich roots, tubers, fruits and nuts that were readily available during the summer and springtime declined (Habu Reference Habu2004). Nuts in the west were stored in water for weeks which will have leached out a significant component of the water-soluble vitamin C (Sakaguchi Reference Sakaguchi2009). Nuts that naturally contain little vitamin C also appear to predominate in western Jōmon sites (Takahashi & Hosoya Reference Takahashi, Hosoya, Mason and Hather2016). These factors may have predisposed the Jōmon to the threat of scurvy if resource returns were also lean due to environmental pressures on food sources. Other cooking and storing methods, including exposure to oxygen, frying, boiling, cutting and stirring, also considerably reduce the original vitamin C content in food (Brickley et al. Reference Brickley, Ives and Mays2020). There is, however, no evidence for significant changes in food preparation strategies among the Jōmon over time, so food preparation is unlikely to be a major factor in any diachronic changes to scurvy prevalence.
Here, we ask whether there is any evidence for a shift in the morbidity of scurvy over time (i.e. the chance of developing scurvy) and, if so, whether scurvy is associated with a change in age at death that might point to nutritional stress as a proximate cause for population decline. We further aim to contextualise our findings within the wider debate over the role of environmental change as an ultimate cause in the Middle Jōmon population decline. To achieve this, we examine the prevalence of scurvy and its associated mortality (i.e. the risk of scurvy-related death) in two western Honshū sites dating from the Middle (5400–4500 BP) to Late/Final Jōmon (4500–2300 BP).
Materials and methods
We studied 32 skeletons from the Middle Jōmon site of Ōta (太田; 5400–4500 BP based on relative dates) and 30 skeletons from the Late to Final Jōmon site of Tsukumo (津雲; 4500–2300 BP based on relative dates and a single human bone radiocarbon date of 2370–2010 cal BP (White et al. Reference White2021)). Excavated in the 1920s, the sites are both large burial and shell midden complexes located on the Inland Sea in western Honshū (Figure 1; see OSM section 1). No dwelling structures are apparent (Tomioka Reference Tomioka2020). While the sample sizes are small, the proximity of the sites to each other and the unique temporal position, spanning this climate cooling period, means they have the potential to contribute considerably to understanding of health in Japanese prehistory.

Figure 1. Location of Ōta and Tsukumo (and modern-day Osaka for reference) (figure by authors).
Previous reconstruction of the Ōta and Tsukumo diet through carbon and nitrogen stable isotope analysis indicates that food resources were predominantly terrestrial, with reliance on marine resources lower at Tsukumo (Kusaka et al. Reference Kusaka, Hyodo, Yumoto and Nakatsukasa2010). Higher prevalence of carious lesions at Tsukumo also supports the consumption of more starchy foods such as tubers that are relatively low in vitamin C (Temple Reference Temple2007b; US Department of Agriculture 2019).
Skeletal analysis and diagnosis
Detailed methods for the analysis of skeletal material and the differential diagnosis of lesions are provided in OSM section 2. Standards following recommendations by Buikstra and Ubelaker (Reference Buikstra and Ubelaker1994) and Schaefer and colleagues (Reference Schaefer, Black and Scheuer2009) were applied to assess age at death. All lesions were recorded macroscopically and skeletal elements from subadults were also radiographed. Following differential diagnosis, the threshold criteria of Snoddy and colleagues (Reference Snoddy, Buckley, Elliott, Standen, Arriaza and Halcrow2018) for the identification of scurvy were applied (after Vlok et al. Reference Vlok2023). These criteria consider the bone changes due to subperiosteal haemorrhage: new bone formation, porosity and vascular impressions (Klaus Reference Klaus2017; Snoddy et al. Reference Snoddy, Buckley, Elliott, Standen, Arriaza and Halcrow2018). These criteria also identify whether a potential case of scurvy meets a threshold consistent with a probable case compared to a possible case. While possible cases are those with a greater degree of diagnostic ambiguity, they still need to be reported to consider the full extent of the condition on the entire population. Probable cases are those with a high degree of diagnostic certainty and require multiple lesions valuable for diagnosis to be present (Snoddy et al. Reference Snoddy, Buckley, Elliott, Standen, Arriaza and Halcrow2018). The Snoddy criteria were chosen for this analysis to provide consistency and maintain a high degree of scientific rigour for the inclusion of cases within the statistical analysis.
Statistical analyses of morbidity
StatsDirect 3.0 was used to undertake risk and odds ratios, t-tests, logistic regression and associated residuals and descriptive statistics. Risk ratios are an intuitive way of analysing the risks between two groups, based on exposure/non-exposure and outcome/absence of outcome, while odds ratios differ slightly as they examine the odds of an outcome occurring in exposed and unexposed groups. T-tests compare the means of two samples and address the null hypothesis that there is no difference between the means of the two groups. Logistic regression analyses the relationship between one or more predictor variables and a dichotomous outcome. Multiple tests were performed spanning parametric and non-parametric tests. Parametric tests provided greater power, but were supplemented by non-parametric tests where the data were not normally distributed. As multiple tests were performed, a Bonferroni correction was applied (equal to significance of p < 0.05 divided by the number of tests (n = 4), resulting in a significance threshold of p < 0.0125).
Prevalence is reported for Ōta adults and for Tsukumo subadults (under 15 years of age, n = 8) and adults. We applied relative risk ratio analyses to assess the difference in risks of scurvy across sites (Ōta versus. Tsukumo). Risk ratios were undertaken to assess the impact of probable cases of scurvy. Odds ratios were also calculated and are provided in OSM section 3 and Table S3 to complement the risk ratios provided.
Unpaired t-tests were used to evaluate differences in the ages at death of each population and the distribution of probable scurvy between the two assemblages. We used sample size estimation analysis to determine if the sample size was sufficient for logistic regression. The minimum sample size required was estimated at 39 individuals. Therefore, multiple logistic regression was applied to assess the influences of population differences and age at death on probable scurvy prevalence in the Ōta and Tsukumo assemblages. Logistic regression accommodates outcome and predictor dichotomous variables (in this case probable scurvy presence or absence as the outcome variable, and the influence of belonging to Ōta or Tsukumo samples as a predictor variable) alongside predictor continuous variables (age at death). The parameters for the logistic regression and sample size analysis can be found in OSM section 3. Outputs of the logistic regression included adjusted odds ratios for each variable (population and age at death) as predictors of the presence of probable scurvy (adjusted for the other predictor). For the ‘population’ variable (Ōta versus Tsukumo), the adjusted odds ratio provides the odds of the outcome of probable scurvy based on exposure to the particular population. For age at death, a continuous variable—the adjusted odds ratio—provides the increase in odds of the outcome of probable scurvy with every one-year increase in age. Additionally, pseudo R2 was calculated for the overall model. McFadden's R2 was used as it is a suitable test of the variability explained by a model and is considered a more conservative measure in contrast to other pseudo R2 calculations (Smith & McKenna Reference Smith and McKenna2013).
Results
Diagnosis of scurvy
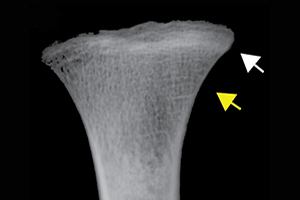
Differential diagnosis indicated scurvy as the cause of the discrete lesions of new bone and abnormal cortical porosity (see OSM section 2 and Table S2). Macroscopically observable diagnostic lesions are present throughout the crania and postcrania of both adults and subadults and, for those associated with cases diagnosed with scurvy, were overwhelmingly symmetrical (Figures 2 & 3; Table 1). Radiographic evidence of disruption in metaphyseal growth in some of the subadults supports the diagnoses (Figure 2a).

Figure 2. Scurvy features in Tsukumo subadults: a) white lines of Fraenkel (white arrow) and Trümmerfeld zones (yellow arrow) of the tibial metaphyseal plates (Tsukumo 71, age 2 years at death (all ages are approximate)), which appear macroscopically (d) as diffuse endochondral porosity (Tsukumo 21, 3 years). New bone and/or porosity on b) a lateral right sphenoid bone with vascular impressions (black arrow; Tsukumo 31, 2 years); c) a posterior left maxilla/zygoma with vascular impressions (black arrow; Tsukumo 56, 3 years); e) a left orbit with vascular impressions (black arrows; Tsukumo 21, 3 years); f) the posterior maxilla (Tsukumo 26, 1.5 years); g) the palatal surface (Tsukumo 21, 3 years); h & j) two medial left coronoid processes of the mandible (h, Tsukumo 56, 3 years; j, Tsukumo 21, 3 years); and i) the anterior maxilla (Tsukumo 31, 2 years) (figure by authors).

Figure 3. Scurvy features in Ōta and Tsukumo adults. New bone and/or porosity on a) the posterior maxilla (black arrow; Tsukumo 3, young-adult male); b) the medial right coronoid process of the mandible (Tsukumo 23, old-adult male); c) the left lateral sphenoid and temporal bones (Tsukumo 3, young-adult male); d) the superior orbital roof (note how the porous lesions extend from the supraorbital foramen (black arrows) and are cortically restricted; Ōta 710, young-adult female); e) the supraspinous fossa of the right scapula (Tsukumo 23, old-adult male); f) the medial aspect of the left coronoid process of the mandible (Ōta 718, middle-aged-adult male); and g) the posterior left zygoma (Tsukumo 60, young-adult female) (figure by authors).
Table 1. Summary of scurvy at Ōta and Tsukumo, showing possible and probable proportions (%) of individuals affected (aff) out of the total observed (obs) for each sex and age category.

Thirty-five per cent (11/32) of the Ōta adults have lesions consistent with a diagnosis of a possible or probable case of scurvy (Table 1). Seventy-seven per cent (17/22) of Tsukumo adults meet the criteria for probable scurvy and 23 per cent (5/22) meet the criteria for possible diagnosis (Table 1). Seventy-five per cent (6/8) of Tskumo subadults exhibit lesions consistent with probable scurvy (Figure 2; Table 1).
Statistical analysis
While sample sizes for males and females are low in both assemblages, and thus statistical analysis is not appropriate, the prevalence of diagnostic lesions in males and females is similar across both sites, indicating no clear evidence for change in sex-related nutritional stress over time. The relative risk of probable scurvy is significantly higher in the Tsukumo compared to Ōta adults. The confidence interval spanned exclusively detrimental effects (>1) assuring that the Tsukumo adults experienced a greater risk of probable scurvy than the Ōta (Table 2). The unpaired t-test reveals statistically significant differences in the distribution of both probable scurvy and age at death between the two populations (Table 3).
Table 2. Relative risk of scurvy across sites (individuals ≥15 years). Tsukumo was selected as the exposed group due to the higher prevalence of scurvy and the exposure to climate change.

Table 3. Results of unpaired t-test comparing Ōta and Tsukumo (using only individuals ≥15 years).

Power analysis found the sample size to be sufficient to proceed with logistic regression. Tsukumo adults have much greater odds (11.88) of probable scurvy even when age at death is accounted for (Table 4). Age at death has an adjusted odds ratio of just 1.01 and the result is not statistically significant (Table 4). The logistic regression model explains approximately 29 per cent (R2: 0.29) of the observed variance in probable scurvy distribution, which appears to be entirely driven by population differences based on the adjusted odds ratio (Table 4). Descriptive statistics and residuals for the data are provided in OSM section 3.
Table 4. Logistic regression of adults from Ōta and Tsukumo with the presence or absence of probable scurvy. aOR: adjusted odds ratio.

Discussion
The statistical outputs unanimously indicate a significant increase in the prevalence of scurvy over time, suggesting a greater reach of the effects of nutritional deficiency across the Tsukumo adults. The relative risk ratios showed a significantly higher risk of scurvy among Tsukumo compared to Ōta adults. The logistic regression estimated that ‘population’ explained 29 per cent of the variance in probable scurvy. While this leaves 61 per cent of variance unaccounted for, we believe that this explanation of variance is significant explanatory power for a single variable, in the context of a multi-faceted and complex condition such as scurvy that is subject to diverse direct and indirect causes. Other contributing factors may have included social status, individual heterogeneity in the ability to absorb or lose vitamin C and differential environmental exposures, many of which are unobservable in the archaeological record. No significant relationship with age at death was detected by the regression analysis. This result was corroborated by the adjusted odds ratio for age at death of individuals with probable scurvy (near to 1) indicating very little if any effect of probable scurvy prevalence on the mortality of Jōmon populations over time. As only adults could be statistically assessed, this result reflects only those individuals who survived to adulthood and our research could not investigate trends in maternal and subadult health. Our results are consistent with the differential morbidity but not mortality expressed through decreased stature and body size in Tsukumo compared to Ōta (Temple Reference Temple, Temple and Stojanowski2018).
We recognise that differences in the preservation of the two assemblages may contribute to the difference in scurvy prevalence between the sites. Preservation of the cranio-facial region, where scurvy lesions are commonly observed, was better at Tsukumo (see Table S2) but individuals at Tsukumo also had a significantly higher prevalence of possible and probable scurvy, including individuals who had poor preservation. This strongly suggests that the difference in scurvy prevalence between the two populations is not predominantly due to preservation issues of the older site. Additionally, while not diagnostic, the skeletal distribution of new bone lesions, particularly of the long bones (well preserved in both assemblages), were remarkably different between Ōta and Tsukumo, signalling a diachronic epidemiological shift. Unilateral and isolated new bone lesions were common in Ōta individuals—a pattern characteristic of localised disease or microtrauma rather than a systemic disease like the case of scurvy. In contrast, long bones from Tsukumo individuals clearly displayed the discrete and symmetrical new bone deposits associated with muscle attachment sites that are suggestive of repeated microtrauma in individuals with scurvy.
While we observed a significant increase in the prevalence of scurvy at Tsukumo compared with Ōta, it is important to note that the presence of scurvy indicates a considerable impact on the health of both populations. Suzuki (Reference Suzuki1998) notes that the Jōmon, regardless of time period, exhibited high levels of osteoporosis due to bone demineralisation. While not systematically recorded, we similarly observed macroscopically that the Jōmon trabecular bone appeared structurally compromised in comparison to other skeletal assemblages we have analysed. Low bone density was also apparent on the radiographs of individuals with scurvy. As vitamin C is needed to build new bone, the lack of this nutrient would have contributed considerably to accelerated bone loss with increasing age.
Overall, the Jōmon sedentary way of life may have increased the pressure on available resources within the local surrounds, particularly during the lean winters. The specific ecology of the Japanese islands and the distinct seasonality of the temperate climate contrasts with sedentary foragers from northern Vietnam. Unlike the Jōmon, these foragers were buffered by the rich tropical resources available to them year-round and show no evidence of scurvy (Vlok et al. Reference Vlok2023). The increase of scurvy morbidity over time in Japan indicates that, ultimately, environmental change with climate cooling substantially increased pressure on resources available to the Tsukumo Jōmon, widening the impact of scurvy.
While a shift in the morbidity of scurvy over time may be observed, there is no discernible relationship between the presence of scurvy and age at death. A general difference in the ages-at-death of the two assemblages does, however, indicate an increase in mortality between the time periods. Therefore, our results suggest that population decline was precipitated by an increase in mortality, but with no evidence of nutritional stress as a causal factor. An association between both increased mortality and increased scurvy prevalence would be required for such a causal relationship to be identified. As such, the evidence for an increase in scurvy by prevalence alone provides a false picture of diachronic disease trends. Our results indicate that caution should be taken in accepting lesion or disease frequencies at face value without consideration of the age-at-death profiles. Previous authors have similarly cautioned the application of such a simplistic interpretation (Wood et al. Reference Wood1992; DeWitte Reference DeWitte2010, Reference DeWitte2014a & Reference DeWitteb; DeWitte & Hughes-Morey Reference DeWitte and Hughes-Morey2012; DeWitte & Stojanowski Reference DeWitte and Stojanowski2015).
Our findings lead to another question: What are the reasons for a change in the morbidity of scurvy without any observed impact on mortality, particularly given that scurvy can be fatal? One possibility is that the Tsukumo Jōmon adapted their food sharing strategies in times of increased ecological stress. A lack of change in the non-specific stress markers including LEH and cribra orbitalia over time support this degree of resilience and social adaptability (Temple Reference Temple, Temple and Stojanowski2018; Robbins Schug et al. Reference Robbins Schug2023). Ethnographic accounts show that present-day foragers change their food-procurement and sharing strategies to accommodate fluctuations in resource returns. The Hadza foragers of northern Tanzania, for example, increase sharing of food provisions during times of resource scarcity (Hawkes et al. Reference Hawkes, O'Connell and Blurton Jones2014). The Hadza do not cultivate or store food, like the Jōmon did, and are reliant on the immediate use of resources (Berbesque et al. Reference Berbesque, Marlowe, Shaw and Thompson2014) but it is possible a similar foraging strategy was employed by the inhabitants of Tsukumo during periods of resource scarcity. The reduction in sex-related differences to carbon and nitrogen isotopes at Tsukumo compared to Ōta, may further support more homogenised diets in the latter site due to food sharing (Kusaka et al. Reference Kusaka, Hyodo, Yumoto and Nakatsukasa2010). Our sample was too small to assess sex-related differences in prevalence but a similar argument has been made at several Jōmon sites, where similar diets have been shared across different sexes and identities (Yoneda et al. Reference Yoneda, Yoshida, Yoshinaga, Morita and Akazawa1996; Temple et al. Reference Temple, Kusaka and Sciulli2011; Kusaka et al. Reference Kusaka, Yamada and Yoneda2018).
It is important to recognise that nutritional stress is but one avenue through which population decline may be mediated with climate change. While our study suggests that, at least for the western Jōmon, the change is not driven by mortality related to dietary change, other factors that increased mortality, such as increasing infectious disease levels and reduction of fertility, could have led to population decline with climate change. Further bioarchaeological work across more sites inclusive of palaeopathology, palaeodemography, isotope analysis and ancient DNA is needed to explore these potential impacts on Jōmon demography.
Conclusion
A definitive relationship between increased nutritional deficiency and population decline in the Jōmon was not supported by our research. Instead, we document diachronic changes in the morbidity of scurvy in the absence of concomitant changes in mortality, suggesting an impact on health with resource decline in the absence of causal population decline. We argue that assessment of age-at-death profiles is essential to the discussion on diachronic changes and that changes in disease prevalence alone do not present the whole picture of epidemiological change in the past. At Tsukumo, a strategy for the sharing of food resources during times of reduced resource return may have contributed to an increase in the prevalence of scurvy, while ultimately buffering the community from its fatal consequences. We conclude that alternative models for the correlation between climate change and population decline are needed, particularly for the western Jōmon.
Acknowledgements
We would like to thank Professor Masato Nakatsukasa from the University of Kyoto for allowing access to study these assemblages. We are ever so grateful to Dr Kazuhiro Sakaue and Ms Hikari Ishijima from the National Museum of Nature and Science, Tokyo who radiographed the remains.
Funding statement
This project was funded by a University of Otago Doctoral Scholarship and an Australasian Society of Human Biology Studentship.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.15184/aqy.2024.50.