Introduction
The Egyptian Vulture Neophron percnopterus is a medium-sized Old World vulture that mainly occurs in parts of southern Europe (especially Spain and France), the Middle East, India and sub-Saharan Africa. The species is widely affected by the human-vulture conflicts arising mainly from illegal poisoning activities (Hernández and Margalida Reference Hernández and Margalida2009, Margalida Reference Margalida2012) and strict sanitary lifestyles of developed countries (Tella Reference Tella2001, Donázar et al. Reference Donázar, Margalida, Carrete and Sánchez-Zapata2009, Ogada et al. Reference Ogada, Keesing and Virani2012a). These conflicts resulted in a substantial decline in the European population (nearly 50% over three generations) and the species is considered globally ‘Endangered’ since 2007 (BirdLife International 2012).
According to BirdLife International’s (2004) estimates, Turkey holds one of the largest Egyptian Vulture populations in the Western Palearctic, with 1,500 to 3,000 breeding pairs. This estimate is on par with the Spanish population (1,452–1,556 pairs; Del Moral Reference Del Moral2009), which is considered to be the largest Egyptian Vulture population in Europe. Prior to the beginning of this study (2010), Egyptian Vultures were never the subject of an extensive ecological study in Turkey. Many basic questions regarding population ecology of the species remain unanswered, including its exact population size, trends, survival rates, breeding success or nesting patterns, and its current distribution at the local and national levels. Therefore, research providing insight into any of those questions would present an important contribution to the global conservation of the species, not just at the national scale.
Natural and human induced factors operating at different scales coupled with the nesting behaviour of a raptor species are expected to create a specific nest site selection pattern (Liberatori and Penteriani Reference Liberatori and Penteriani2001, Krüger Reference Krüger2002, Martínez et al. Reference Martínez, Serrano and Zuberogoitia2003, Sarà and Vittorio Reference Sarà and Vittorio2003, Sergio et al. Reference Sergio, Pedrini and Marchesi2003, López-López et al. Reference López-López, García-Ripolĺs, Aguilar, García-López and Verdejo2006, Margalida et al. Reference Margalida, Donázar, Bustamante, Hernández and Romero-Pujante2008, Mateo-Tomás and Olea Reference Mateo-Tomás and Olea2009, Moreno-Opo et al. Reference Moreno-Opo, Fernández-Olalla and Margalida2012). This pattern may influence basic population parameters such as breeding success and population size (Newton Reference Newton1979). Several studies conducted in Spain and Italy, measuring either the deviation of nest sites from random expectations or the difference between active and extinct sites, found various factors affecting nest site selection patterns of Egyptian Vultures, including illegal use of poison baits (Carrete et al. Reference Carrete, Grande, Tella, Sánchez-Zapata, Donázar, Díaz-Delgado and Romo2007), elevation of the nest sites (Mateo-Tomás and Olea Reference Mateo-Tomás and Olea2009), presence of Mediterranean vegetation and urbanised landscapes (Sarà and Vittorio Reference Sarà and Vittorio2003), and nest exposure (orientation; Liberatori and Penteriani Reference Liberatori and Penteriani2001). Consequently, analysing nest site selection patterns of Egyptian Vultures in Turkey can provide useful information on many aspects of the species’ ecology that may shed light on future management options, and possibly identify focal points for future research.
Here, our main objectives were to: (1) Provide an estimate of population size of the Egyptian Vultures breeding in a relatively small area around Beypazarı, Ankara; and (2) analyse nest site selection patterns of this population.
Study area and methods
Beypazarı is 100 km west of Ankara and has a population of 47,014 people (Türkiye İstatistik Kurumu 2011). The region is known for its high biodiversity, with many rare, endemic or threatened species, and its immediate surroundings contain three Key Biodiversity Areas (Kirmir Valley, Sarıyar Reservoir and Nallıhan Hills; Eken et al. Reference Eken, Bozdoğan, İsfendiyaroğlu, Kılıç and Lise2006) and one Important Bird Area (Sarıyar Reservoir; BirdLife International 2013). The south-western part of the study area is mainly composed of steppe habitat with agricultural fields concentrating around the Sarıyar reservoir. The proportion of forested land - dominated by black pine Pinus nigra in the east, and Turkish pine P. brutia in the west - increases through the northern part. Numerous rivers and creeks have formed valleys with steep cliffs that provide nesting sites for raptors.
Locating nests and pairs
The Egyptian Vulture is a territorial and cliff nesting species. It migrates to its breeding quarters in early or mid-March and lays eggs in the following one or two months (Cramp and Simmons Reference Cramp and Simmons1980). Potential nesting cliffs were checked for the presence of Egyptian Vulture pairs early in the breeding season. Pairs showing territorial behaviour such as aerial displays or copulations were tracked to find the exact nest locations and to confirm the start of incubation through April and the beginning of May. The monitoring of breeding sites varied from short visits to two hours depending on the visibility of the potential nest site. Nests with unknown status during the early breeding period were visited repeatedly to determine if the pair had laid eggs. In total 38 field days were spent locating nests and pairs in 2010 and 2011. Located nest sites were monitored during the rest of the breeding season (until August) with five visits on average (min = 2, max = 8) to each site as part of another study.
With the help of an observation point and cliff photographs, an approximate location for the nest site was determined on Google Earth. The potential error in this nest location approximation was assumed to be negligible at the scale of the study.
Data collection
To compare with the nest sites identified, 350 random points with 200-m radius circles were generated throughout the study area using ArcGIS 9.3.1. Those points were then filtered out using the following rules and after the locations were checked on Google Earth:
-
• If there were no cliffs within 200 m of the point, it was discarded. If there was a cliff within (and even if that cliff had an actual nest on it), the point was moved to the nearest spot on that cliff.
-
• If the point was inside a known territory of a pair but with an unknown nest that was not included in the study, it was discarded.
-
• If the point was in a valley or on a cliff, which was not regularly checked, it was discarded for the fact that there could be an unknown pair and nest nearby.
After this filtering process, a total of 70 points remained for the analysis. The locations of those points were then checked in the field to make sure that the cliffs they are on had visible structures for Egyptian Vultures to nest, such as ledges or caves. This left 30 points that we used in the analysis. It is important to note that the exact location of those points does not necessarily indicate a suitable cave or a ledge where the Egyptian Vulture can nest but rather they represent a random point on a suitable cliff that the species may use to breed. If the nest site selection pattern of the Egyptian Vulture in the area is non-random, then the statistical models should be able to differentiate between actual nests and random points.
We selected 20 habitat variables to include in the statistical models (Table 1). These were chosen in parallel to previous Egyptian Vulture studies conducted in Spain and Italy (Liberatori and Penteriani Reference Liberatori and Penteriani2001, Sarà and Vittorio Reference Sarà and Vittorio2003, Carrete et al. Reference Carrete, Grande, Tella, Sánchez-Zapata, Donázar, Díaz-Delgado and Romo2007, Mateo-Tomás and Olea Reference Mateo-Tomás and Olea2009). All variables were calculated using ArcGIS 9.3.1. One-kilometre radius circles were used to calculate the landscape variables. The 2.5 and 5-km circles that were used by Mateo-Tomás and Olea (Reference Mateo-Tomás and Olea2009) caused a high amount of overlap between nest sites due to the small study area and a very high nearest neighbour distance (NND). These overlaps would cause significant spatial autocorrelation. Also, frequently used variables in similar studies such as nest type or nest cover were not included in the analysis as they cannot be estimated for random points because they do not necessarily include a cave or a ledge.
Table 1. Variables used in the modeling process. Elevation, aspect, slope and relief variables were calculated using a digital elevation model with 100 meters resolution. Habitat cover variables were classified according to Corine Land Cover 2006 raster data Version 15.
Statistical models
Statistical modelling of nest site selection of a certain species can be considered as a special case of species distribution models (SDM) in which the focus is not on where the species is observed but on certain locations (trees, caves, cliff ledges etc.) where the species is known to nest. Appropriate choice of modelling methods is an essential part of constructing SDMs and Generalized Linear Modelling (GLM) has been the preferred method until recently (Rushton et al. Reference Rushton, Ormerod and Kerby2004, Hirzel and Le Lay Reference Hirzel and Le Lay2008). However, advances in machine learning methods provide a wide array of new tools that are more effective than more traditional approaches such as GLM in classifying non-linear and highly dimensional data (Kampichler et al. Reference Kampichler, Wieland, Calme, Weissenberger and Arriaga-Weiss2010). Random Forests is one such method that has been shown to be a powerful classifier (Cutler et al. Reference Cutler, Edwards, Beard, Cutler, Hess, Gibson and Lawler2007).
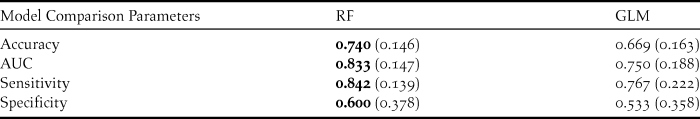
Therefore, GLM and Random Forests were both employed to elucidate the relationship between the occurrence of a nest (random point vs actual nest) and the explanatory variables. Due to the small sample size (39 nests and 30 random points), the data were not divided into training and test sets. All model results were obtained from the original data set. These two models were compared using accuracy (error rate of the model when predicting cross validated data), AUC (the area under the receiving operator curve; ROC), sensitivity (rate of correctly classified presences) and specificity (rate of correctly classified absences). AUC produces values between 0.5 and 1, with better fitted models having higher values closer to 1. These classification measures were calculated by 10-fold cross validation using caret package (Kuhn Reference Kuhn2013) in R statistical software. Spatial autocorrelation (see Legendre and Legendre Reference Legendre and Legendre1998) was investigated by calculating the Moran’s I values for the Random Forests model residuals using ape package (Paradis et al. Reference Paradis, Claude and Strimmer2004) in R. All statistical models were performed using R statistical software (R Core Team 2013).
Generalized Linear Models (GLM)
Generalized Linear Models were employed with a binomial error distribution and a logit link function (McCullagh and Nelder Reference McCullagh and Nelder1989). A hierarchical modelling procedure similar to those of Mateo-Tomás and Olea (Reference Mateo-Tomás and Olea2009) and Carrete et al. (Reference Carrete, Grande, Tella, Sánchez-Zapata, Donázar, Díaz-Delgado and Romo2007) were followed when constructing GLMs. The variables were first divided into nest site and landscape scales, and then the landscape was divided into five categories (Table 1). Variables were first modelled in their respective categories. Selected variables from the five categories were again modelled at the landscape scale. Finally, combined models were constructed with selected variables at nest site and landscape scales. In every step of the modelling procedure, only models with ΔAICc < 2 from the best model (lowest AICc value) were considered. Further model filtering was carried out by removing complex models with more variables that did not improve upon simpler models (Richards Reference Richards2008). Selected models in the final hierarchical step were averaged for multi-model inference (Burnham and Anderson Reference Burnham and Anderson2002) resulting in a final averaged model.
MuMIn package (Bartoń Reference Bartoń2013) in R statistical software was used to compare multiple variable permutations of GLMs.
Random Forests
Random forests (RF) method is an improvement over Classification and Regression Trees (CART; Breiman Reference Breiman2001). Instead of growing just one tree, RF grows a very high number of trees and makes its predictions on a majority-vote basis from every tree in the forest. Out of bag (OOB) error rate is the default accuracy measure calculated when building random forest models. This measure can be affected by two parameters of a random forest model: the number of trees in the forest (ntree) and mtry (number of randomly selected variables to use in each split in a tree). It has been shown that higher number of trees in a forest leads to better variable importance score stability (Genuer et al. Reference Genuer, Poggi and Tuleau-Malot2010), thus every forest was constructed with 2,000 trees. The tuneRF function in the randomForest package in R was used to find the best mtry value which gave lowest OOB error rate. This function, starting with a pre-determined value of mtry, multiplies or divides mtry by a factor (two in this study) and builds forests until OOB error rate does not decrease.
The Initial Forest was grown for variable selection. In order to additionally improve the OOB error rate of the forests, variables which had a negative variable importance score was discarded from the model. A negative score means that a random permutation of a variable’s values among cases performs better than its original combination, indicating that the variable is increasing the OOB error rate. After discarding these variables, 10 forests were constructed using the best mtry value (indicated by tuneRF) and the forest with the lowest OOB-error rate was chosen to be the Final Forest.
Partial dependence plots were employed to illustrate the individual effects of the variables on the probability of a point being a nest (Hastie et al. Reference Hastie, Tibshirani and Friedman2009). The randomForest package (Liaw and Wiener Reference Liaw and Wiener2002) in R was used to construct the forests. See the online supplementary material for methodological details of the CART and Random Forests methods.
Results
Population size
The presence of 45 territorial pairs was confirmed by the end of the breeding season in 2011. 39 of those pairs’ nests were found and used in the nest site selection analysis (Figure 1). Additionally, eight potential pairs were located which were not monitored regularly in the study period. Even if only the 45 confirmed pairs are considered, the density of the Beypazarı population is extremely high: 6 pairs per 100 km2.

Figure 1. Study area and the location of random points (n = 30) and nest sites (n = 39).
Nest site selection patterns
GLM
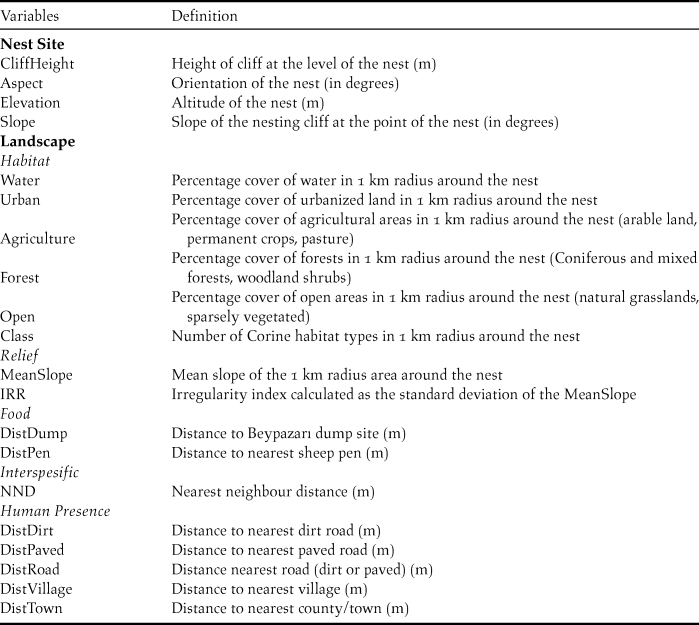
Selected GLMs for the nest site scale included Elevation and Aspect, while the landscape scale included DistVil and MeanSlope as variables. The combined models were constructed using these four variables and their every permutation. Table 2 shows the models within ΔAICc <2 range of the best model and their respective Akaike weights. After model filtering only two models were left: Aspect+Elevation and Elevation. These two models were averaged and variable coefficients showed that increasing Elevation (-0.0057) and Aspect (-0.0050) had a negative effect on nesting probability.
Table 2. Combined models using selected variables from nest site and landscape scales. Only models within the range of ΔAICc <2 from the best model is presented. After the filtering process, models in bold were used for model averaging.
Random Forests
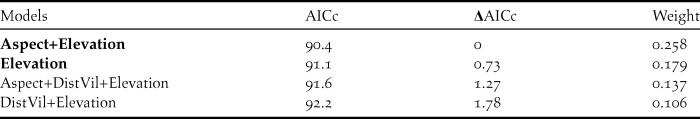
The default value of mtry = 4 was selected for the Initial Random Forest model according to tuneRF function. The OOB-error rate of the Initial Forest was 42.03% and 12 variables had negative variable importance scores (Table 3). These variables were discarded from the Final Forest model along with Open and DistDirt which was correlated with DistVil (0.516; P = 0.0001) and DistRoad (0.886; P = 0.0001), respectively, but had lower importance scores. Once more, mtry = 4 was selected for the Final Forest construction by tuneRF. The lowest OOB-error rate among the ten constructed Final Forests was 27.54%. The model was able to correctly classify 33 nests and 17 random points while misclassifying 6 nests and 13 random points.
Table 3. Variable importance scores of the Initial and Final Random Forest models. Variables in bold were selected for the Final Forest construction.
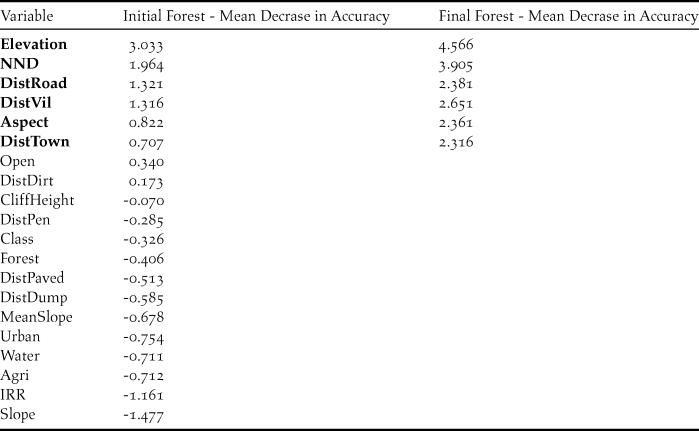
As with GLM, Elevation and Aspect were selected by RF as important variables which determine the nest site selection of Egyptian Vultures (Table 3; Figure 2). Some variables that were “missed” by GLM were deemed important by RF such as NND, DistTown, DistVil and DistRoad. Partial dependence plots in Figure 3 show the detailed relationship between an explanatory variable used in the Final Forest and a measure of probability of a point being a nest site. The probability of nesting declined with increasing elevation. Also, the effect of aspect was clearer when compared to GLM as the probability was highest for nests facing southeast. Plots also showed that pairs in Beypazarı preferred to nest at some distance from the nearest human settlements, whether small or large. This effect was also apparent in the distance to nearest road as the species preferred not to nest too close to roads. In addition, the probability of nesting increased with nearest neighbour distance until NND reached 1.5 km after which it showed a slight decline. There was no significant autocorrelation at the α = 0.05 level in the Final Random Forests model (Moran’s I = 0.026 and P = 0.286).
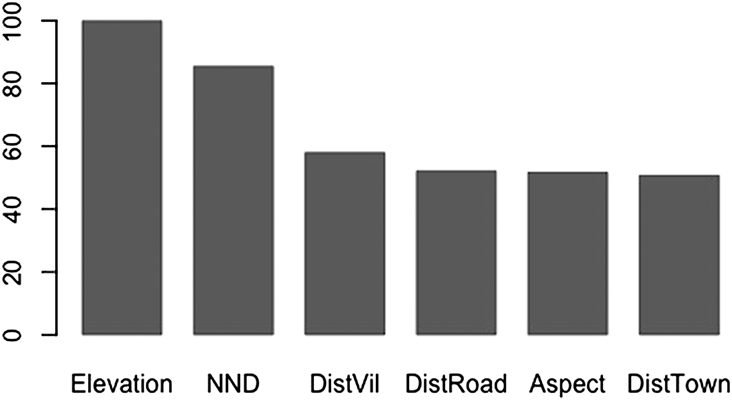
Figure 2. Variable importance scores of the Final Random Forest. Scores were re-scaled so that the maximum value would be 100 for easier interpretation.
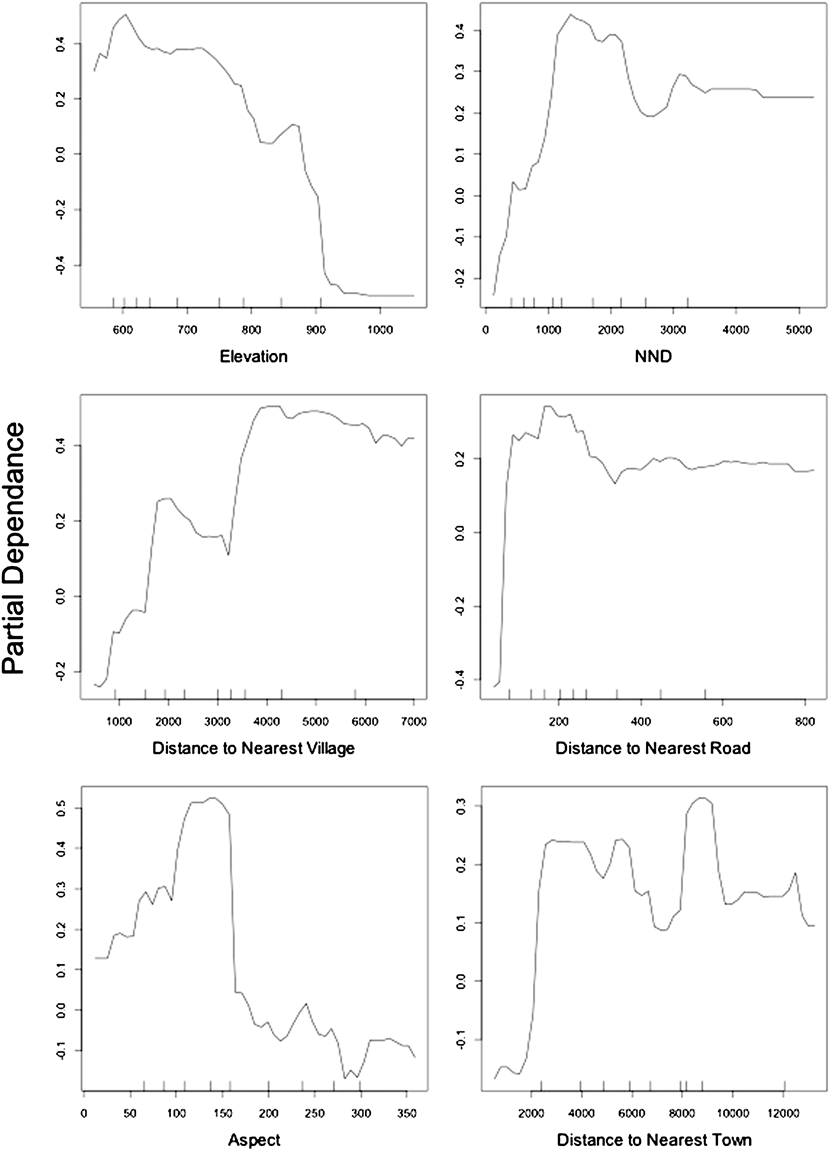
Figure 3. Partial dependence plots of the variables included in the Final Random Forest model. Y axis is half of the log of probability of presence (probability of a point being a nest in this study) when there are only two classes (nests and random points); see the supplementary materials for details. X axis is the related variable’s values.
Model comparison
Random Forest model had the highest scores among all classification measures. Both models were better at predicting presences than absences, with higher scores for sensitivity than specificity (Table 4).
Table 4. Comparison of the modeling techniques using 4 classification measures obtained by cross validation. Values in bold are the highest scores. Standard deviations are in brackets
Discussion
Density and distribution
The density of the Beypazarı Egyptian Vulture population is very high, with six pairs per 100 km2. This finding is comparable to some of the Spanish populations with the highest density. For example, in the Bardenas Reales region of the Ebro Valley, Spain, Donázar and Ceballos (Reference Donázar and Ceballos1990) reported one pair per 14.5 km2 which is equivalent to 6.9 pairs per 100 km2. Even though it is a rather old record, the authors had acknowledged this population (40 pairs) as “one of the densest in Europe”. In a more recent study comprising a wider area in northern Spain, Mateo-Tomás and Olea (Reference Mateo-Tomás and Olea2009) found a much lower density with only 0.14 territories per 100 km2. However, they indicated that this low density increases to six territories per 100 km2 in certain areas with a high concentration of breeding pairs.
When compared to other populations in Europe, the exceptional nature of the Beypazarı population stands out even more. In the Italian peninsula, Egyptian Vultures faced a sharp decline from 29 pairs to 9 pairs between 1970 and early 1990s (Liberatori and Penteriani Reference Liberatori and Penteriani2001). A similar situation was observed for the island of Sicily, where the number of pairs declined from 29 in 1980 to 13 in 2002 (Sarà and Vittorio Reference Sarà and Vittorio2003). While France and Portugal are estimated to have 87 and 90 pairs of Egyptian Vultures, respectively, Balkan populations are relatively small with 30–35 pairs for Macedonia, 40–45 pairs for Bulgaria and 30–50 pairs for Greece (Iñigo et al. Reference Iñigo, Barov, Orhun and Gallo-Orsi2008). Considering these estimates, we can argue that the Beypazarı population of Egyptian Vultures is one of the densest local populations in the Western Palearctic.
It is also important to note that these estimates are conservative because we have included only the intensively monitored pairs in the population estimate. As indicated above, there were an additional eight potential pairs that were not monitored regularly. In case they are confirmed to be breeding, population size and density will increase even further. It is also possible that there are pairs that we “missed” during our surveys of the study area, since the population has only been studied for two years. The exact size of the population can only be determined through extensive and regular monitoring in the future.
Nest site selection patterns
Elevation
Elevation of a nest site was the most important variable determined by the Final Random Forests model, having twice as much contribution to the model’s accuracy (OOB-error rate) than other variables (except NND) included in the model (Figure 2). The negative trend outlined by the partial dependence plot shows that the species has a strong preference for nesting at lower elevations (Figure 3). The probability of a point being a nest reaches its highest value around 600 m and there are no nest sites above 900 m, even though the maximum elevation of the study area is 1,800 m.
Despite the fact that the elevation at which this species nests is highly variable at a global scale (Cramp and Simmons Reference Cramp and Simmons1980), the general negative trend between the probability of nesting and elevation was attributed to adverse climatic conditions at high altitudes (Mateo-Tomás and Olea Reference Mateo-Tomás and Olea2009). Elevation was also deemed important for a population of Bearded Vulture Gypaetus barbatus in Spain which preferred to nest at mid-elevations avoiding low or high altitudes (Donázar et al. Reference Donázar, Hiraldo and Bustamante1993). Bearded Vultures were reported to avoid lower elevations due to dense forested areas devoid of foraging habitat. A similar trend was also reported by Margalida et al. (Reference Margalida, García and Cortés-Avizanda2007) in which Bearded Vultures tended to have higher population density with low tree cover. This may partially explain the reason why Egyptian Vultures prefer to nest at lower altitudes in Beypazarı. In our study area dense forests are concentrated at higher elevations, in contrast with the Bearded Vulture population studied in Spain. The southern and lower part of the study area is mainly an open steppe habitat providing the necessary foraging opportunities due to extensive animal husbandry as well as the presence of a large rubbish dump.
The relationship between food sources (for instance sheep and goats) and the density of Egyptian Vultures has been indicated before in north-east Spain (Margalida et al. Reference Margalida, García and Cortés-Avizanda2007). While Donázar and Ceballos (Reference Donázar and Ceballos1990) argued that there is no direct relationship between availability of food sources and population density of the species, the Beypazarı population is likely to be more comparable to north-east Spain in terms of food source dynamics. As indicated by Margalida et al. (Reference Margalida, García and Cortés-Avizanda2007) for north-east Spain, Turkey does not have vulture restaurants (except for two feeding stations; one in western and the other in eastern Turkey). Whether municipal rubbish dumps can be a replacement for vulture restaurants is a future research question. In any case, Egyptian Vultures of Beypazarı rely mostly on livestock farming which leads them to open ground at low elevations rather than forested areas at higher elevations in the study area.
Another important point is that Egyptian Vultures carry food only in their beaks and therefore can only carry a small amount (Cramp and Simmons Reference Cramp and Simmons1980). Several feeding trips per day between the nest and foraging area might be necessary. When nests are located at high elevation away from foraging areas, the energy requirements of these trips might actually exceed the energy gained during foraging (Bergier and Cheylan Reference Bergier and Cheylan1980).
Even though habitat cover variables were included in the model, none of them (except Open) were selected by the random forest model since they had a negative effect on the model’s accuracy. One might expect that, if the arguments above are true, the Forest variable should have been selected by the model perhaps showing a similar trend with Elevation. We argue that a 1-km radius around a nest site is not representative of the species’ home range as has been shown by other studies (Carrete et al. Reference Carrete, Grande, Tella, Sánchez-Zapata, Donázar, Díaz-Delgado and Romo2007, Mateo-Tomás and Olea Reference Mateo-Tomás and Olea2009), and if the study area is extended, especially towards north, enabling the use of wider areas (2.5, 4 or 8 km) around nest sites, the Forest variable might also be selected by the final model.
Human disturbance
Because human settlements tend to be located at lower elevations, nest site preference of the population in Beypazarı may have an increased risk of human conflict (Mateo-Tomás and Olea Reference Mateo-Tomás and Olea2009). This is apparent in the final Random Forest model which has three out of six variables related to human disturbance (DistRoad, DistTown and DistVil).
All these variables show a similar trend in partial dependence plots, stabilising after a sharp increase in probability of a point being a nest (Figure 3). The highest probabilities for nesting in relation to human disturbance are attained at 150–200 m from the nearest road, 2 km from the nearest town and 4 km from the nearest village. These results clearly indicate that Egyptian Vultures nesting in the study area prefer cliffs at a certain distance from human presence or activity. This trend has been noted before as Egyptian Vultures in Sicily also chose nest sites where human settlements are underrepresented (Sarà and Vittorio Reference Sarà and Vittorio2003). Even though the species does not appear to be disturbed by, and may be indifferent to, constant human presence, dogs or construction machinery during feeding at the Beypazarı dump site (pers. obs.), it is apparent that their behaviour is rather different at the nest site/territory, with the birds preferring a higher degree of seclusion.
Margalida et al. (Reference Margalida, García and Cortés-Avizanda2007) report that in north-eastern Spain Egyptian Vultures have higher density populations where human presence is low. They indicate, however, that this is a different pattern to that observed by Ceballos and Donázar (Reference Ceballos and Donázar1989) in which Egyptian Vultures tolerated proximity of human activity. The Beypazarı population, in this regard, is similar to the population in north-western Spain. This study, however, demonstrates that even when regional populations might show differences in terms of proximity of nesting sites to human presence, within a region that has high human presence there is still a tendency to nest away from anthropogenic factors.
Nearest neighbour distance (NND)
One of the regulators of the density of a raptor population in any given area is food supply (Newton Reference Newton1979). The abundance in food sources might alter a raptor’s territorial behaviour in terms of reduced aggressiveness and increased attacking distance when an intruder bird is present within the territory, leading to decrease in NND (Newton Reference Newton1979). Therefore, for territorial species, we can consider NND both as a proxy for population density and habitat quality.
When compared with other European populations (Bulgaria: 2,750 m, Pyrenees: 6,830 m, Catalonia: 7,000 m, Italian peninsula: 24,511 m; see references in Liberatori and Penteriani Reference Liberatori and Penteriani2001) NND of the Beypazarı population is much lower, with a mean value of 1,510 m. The partial dependence plot shows that probability of nesting is highest when NND is between 1,000 and 2,000 m (Figure 3).
Low NND and high population density in Beypazarı emphasise high habitat quality with respect to Egyptian Vulture nesting habits. Whether this is a result of food abundance, availability of nest sites or other habitat variables is a point of interest for future research. The increasing probability of nesting up to 2,000 m in partial dependence plot reflects the territorial behaviour of the species. Apparently, even when habitat quality allows a high density, Egyptian Vultures keep a minimum distance to neighbouring nests.
Aspect
The nests of Egyptian Vultures were reported to either have a mixed orientation (Grubac Reference Grubac, Meyburg and Chancellor1989), or to be predominantly exposed toward southern aspects (Vlachos et al. Reference Vlachos, Papageorgiu, Bakaloudis, Meyburg and Chancellor1998, Liberatori and Penteriani Reference Liberatori and Penteriani2001). In our study area, the probability of nesting was highest when Aspect was between 100 and 150 degrees, indicating a south-eastern exposure of the nest site (Figure 3). The possible reasons for this preference are not discussed extensively in the raptor literature, but might be related with the optimal use of sunlight (Carlon Reference Carlon1992).
Implications for conservation management
Vultures provide several ecosystem services through consumption of carrion, in the form of sanitation and nutrient recycling (Sekercioğlu et al. Reference Sekercioğlu, Daily and Ehrlich2004, Moleón et al. Reference Moleón, Sánchez-Zapata, Margalida, Carrete, Owen-Smith and Donázar2014). The widespread decline in vulture numbers at a global scale has a direct impact on human communities either economically or through sanitary issues. The absence of vultures prolongs carcass decomposition time and increases the number of mammals feeding on carcasses, resulting in higher rates of disease transmission, such as rabies. This in turn endangers the well-being of not only human populations but also wildlife and livestock animals (Ogada et al. Reference Ogada, Torchin, Kinnaird and Ezenwa2012b). In addition, Margalida et al. (Reference Margalida, Carrete, Sánchez-Zapata and Donázar2012) report that vultures remove 9,900 tons of carcasses per year in Spain alone. The removal of carcasses through natural means and not through industrial destruction saves costs for farmers of up to 20 € per animal. This might correspond to a more than 200 million € saved every year. Since Turkey has the second biggest vulture population in Europe (Birdlife International 2004), conservation of vulture species breeding in Turkey becomes imperative.
In this study we showed that Turkey hosts one of the largest local Egyptian Vulture populations in Europe. The statistical models suggest that human disturbance is limiting the distribution of this population through altering nest site selection patterns. Considering the species’ vulnerability to human presence in breeding territories (Zuberogoitia et al. Reference Zuberogoitia, Zabala, Martínez, Martínez and Azkona2008), it is unknown whether its selection of nest sites at a certain distance from human settlements will allow the Beypazarı population to sustain itself in the near future. Unfortunately, human disturbance is not only manifested through residential areas such as villages or towns. Beypazarı is a quickly developing trade centre and construction of small-scale hydroelectric power plants, mines and roads are becoming commonplace. In fact, some of these constructions are taking place only a few hundred meters from some of the known nest sites. To truly measure the impact of growing human communities in Beypazarı to the population of Egyptian Vultures, extensive monitoring of the species is essential.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0959270916000411
Acknowledgements
Funding was provided by the Royal Society for the Protection of Birds. We thank Ivaylo Angelov and his team from BSPB for their support during field work. Stefen Oppell’s advice on statistical analysis was invaluable. This was a Doğa Derneği (DD) project and would never have come to fruition if it was not for the valuable contributions of the whole DD team.









