CVD is the most prominent lifestyle-dependent cause of morbidity and mortality in the Western world (WHO Health Report 2002). Development of vascular changes is thought to originate already in the prenatal and postnatal periods( Reference Barker 1 – Reference Eriksson 4 ). Impaired fetal growth from the middle to the late phases of gestation leads to disproportionate or reduced size at birth, which is associated with an increased risk of cardiovascular events later in life( Reference Barker 1 , Reference Banci, Saccucci and Dofcaci 3 ). Postnatal factors including infant feeding( Reference Owen, Whincup and Kaye 5 ), infant growth( Reference Eriksson 4 ) and childhood nutrition( Reference Kaikkonen, Mikkilä and Magnussen 6 ) may also play major roles in programming cardiovascular changes.
Breast-feeding in infancy is a potential determinant of cardiovascular risk factors and later CVD( Reference Owen, Whincup and Kaye 5 , Reference Martin, Ness and Gunnell 7 – Reference Owen, Whincup and Gilg 12 ). Studies vary in the duration of follow-up and have differences in the definition of breast-feeding. The reported effect of breast-feeding on cardiovascular health later in life has been controversial, as some groups have reported favourable effects( Reference Owen, Whincup and Kaye 5 , Reference Owen, Whincup and Gilg 12 , Reference Martin, Gunnell and Smith 13 ), some have found no association( Reference Verier, Duhamel and Beghin 14 – Reference de Jonge, van Osch-Gevers and Geelhoed 16 ) and some have reported an inverse relationship( Reference Leeson, Kattenhorn and Deanfield 17 ).
We previously evaluated the effect of breast-feeding on body composition( Reference Pirilä, Saarinen-Pihkala and Viljakainen 18 ). Our previous results suggested that breast-feeding has an indirect influence on body composition in adulthood. Longer duration of breast-feeding resulted in lower-fat accumulation during the first year( Reference Saarinen and Siimes 19 ), and this was positively tracked to central adiposity at 32 years of age. In the present study, our primary aim was to evaluate whether longer duration of breast-feeding reduces the risk of cardiovascular events in adulthood. The present cohort consisted of 32-year-old adults with no diagnosed cardiovascular events. We analysed the impact of breast-feeding on blood pressure, lipid profile, vascular properties and calculated clustered cardiovascular risk scores: the Framingham risk score (FRS)( Reference Touboul, Labreuche and Vicaut 20 , Reference Wilson, D'Agostino and Levy 21 ) and the metabolic syndrome criteria score (National Cholesterol Education Program's Adult Treatment Panel III, NCEP-ATPIII)( Reference Olufadi and Byrne 22 ).
Subjects and methods
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Ethics Committee of the Helsinki and Uusimaa Hospital District in Helsinki, Finland. Written informed consent was obtained from all the study participants.
Participants
In 1975, 238 healthy, full-term Finnish newborn babies at the Helsinki University Central Hospital were recruited to participate in a study examining the role of milk feeding in the Fe status of infants( Reference Saarinen 23 – Reference Saarinen and Siimes 26 ). According to the inclusion criteria, the participants' birth weight exceeded 3000 g. The mothers were recommended to breast-feed for as long as possible and all babies received breast milk for at least 2 weeks. When breast milk became insufficient, mothers were instructed to use a home-prepared cows' milk formula or a commercial milk formula (Bona®; Chymos Oy). All babies were introduced to solid food at 3·5 months with the same strict guidelines. The same physician (U. M. S.-P.) examined all the babies seven times during the first year: at 2 weeks and then at 1, 2, 4, 6, 9 and 12 months of age. Data on milk feeding and anthropometric measurements were collected at each visit. The mean duration of breast-feeding was 5·2 (sd 3·2) months, while, on average, in Finland in 1974, only 30 % were breast-fed for 3 months or longer( Reference Hultin, Opas and Sarna 27 ). The mean age at the introduction of weaning milk was 3·6 (sd 3·1) months.
Between 2006 and 2009, all the participants from the original cohort were traced through the Population Registry Centre in Finland, and invited to a 32-year follow-up assessment. From the original cohort, 188 participants were reached and 158 consented to participate and were examined at the outpatient clinic at the Children's Hospital, Helsinki University Central Hospital, Finland( Reference Pirilä, Saarinen-Pihkala and Viljakainen 18 , Reference Pirilä, Taskinen and Viljakainen 28 ). The participation rate was 66 %: women were more likely to participate (72 %) than men (60 %). The reasons for non-participation were lack of time or interest.
All participants underwent physical examination by one of the authors (S. P.). Data were collected with a structured questionnaire on various lifestyle factors, such as current and previous physical activity, alcohol consumption, smoking, medical history (therapy for dyslipidaemia or hypertension) and education. The survey was completed by an interview during the clinical visit. Dietary intake of nutrients was assessed by 3 d food records. A licensed dietitian analysed the records with a computer program (software AIVO 2000-Diet32, version 1.4.6.2; AIVO Finland Ltd).
Blood pressure
Hypertension was defined as systolic blood pressure >130 mmHg, diastolic blood pressure >85 mmHg or by the treatment for hypertension. Blood pressure was recorded twice within 5 min after a supine rest of at least 10 min with an automatic device (Dinamap, Critikon; GE Medical Systems) and with a cuff size appropriate for the participants' left upper arm. The average of two measurements was used.
Ultrasonography
Vascular properties of the carotid artery were assessed with ultrasound by using a B-mode ultrasound imager (Vivid 7; GE Vingmed AS) with 12L vascular probe (GE Medical Systems). All measurements were performed by one of the authors (S. P.) who was blinded to the participants' breast-feeding history. Due to technical problems, images of only sixty-nine patients were available for detailed analysis at the end of the study. These measurements were regarded representative for the whole cohort as no differences in characteristics between those with and without ultrasonic examination were found (see the Supplementary table, available online). The examination setting was standardised, and started at 08.00 hours in the test office. The participants were examined after an overnight fast, and a 4 h tobacco and coffee restriction. Measurement for the right common carotid artery (CCA) was obtained 1–2 cm above bifurcation. A simultaneous echocardiogram recording was stored. The image frames of three subsequent beats were saved at the end diastole (ECG Q wave), and then CCA far-wall intima–media thickness (CCA-IMT) was measured and the mean value of the three measurements was used. Analyses were performed from digitally stored data with semi-automated analysing software AMS II, version 1.1331 (T Gustafsson; Chalmers University of Technology).
Biochemical measurements
Venous blood was drawn from the participants after an overnight fast. Serum total cholesterol, HDL-cholesterol, LDL-cholesterol and TAG concentrations were determined by the photometric enzymatic analysis. Insulin resistance was assessed with homeostatic model assessment of insulin resistance (HOMA-IR) and calculated using the following formula:
where 1 mIU insulin = 6 pmol insulin.
Framingham risk score and National Cholesterol Education Program's Adult Treatment Panel III
The FRS gives an estimated risk of an individual for CHD over a period of 10 years. It is based on the Framingham Heart Study( Reference Wilson, D'Agostino and Levy 21 ). We calculated the risk score for each participant. Points were given for both sexes with separate risk score tables( Reference Wilson, D'Agostino and Levy 21 ). The variables included were age, LDL-cholesterol, HDL-cholesterol, systolic or diastolic blood pressure (higher points were used), diabetes and smoking. According to the Framingham Heart Study, at the age of 30–34 years, the mean estimated risk of CHD (including angina pectoris) over 10 years is 3 % for men and < 1 % for women.
According to the NCEP-ATPIII, the presence of the metabolic syndrome is defined by the following five criteria, of which at least three should be present: waist circumference surrogating central fat ≥ 102 cm in men and ≥ 88 cm in women; blood pressure >130/85 mmHg; fasting TAG concentration ≥ 1·7 mmol/l in men and ≥ 1·3 mmol/l in women; fasting glucose concentration ≥ 5·6 mmol/l; HDL-concentration < 1·0 mmol/l in men and < 1·3 mmol/l in women( Reference Olufadi and Byrne 22 , Reference Grundy, Cleeman and Daniels 30 ).
Statistical methods
The main outcome variables in the present study were the FRS (scale from − 17 to 5 for women and from − 8 to 5 for men) and the NCEP-ATPIII scoring (scale based on fulfilled criteria present, ranging from 0 to 5), and the secondary variables were systolic and diastolic blood pressure, total cholesterol, LDL-cholesterol, CCA-IMT and HOMA-IR. We used means and standard deviations to describe continuous variables, Student's t test to compare the differences between groups and χ2 test for categorised variables. We used linear regression to test the effect of the duration of breast-feeding on each outcome variable. As possible confounders for the association between breast-feeding and risk factors of CVD, we tested sex, physical activity level (scale from 1 – no exercise, 2 – occasional physical activity, 3 – regular light physical activity such as gardening, 4 – regular light exercise such as walking to 5 – hard training more than twice per week), current dietary habits (from food records), current smoking (number of cigarettes smoked per d) and alcohol consumption (scale from 1 – no alcohol, 2 – alcohol less than once per month, 3 – up to twice per month, 4 – up to twice per week, 5 – three or four times per week to 6 – daily alcohol intake), level of education (scale from 1 – primary school to 6 – university degree), current anthropometric measurements and infancy-related factors (birth weight). We ended up using proportional intake of fat in the diet to distinguish between high- and low-fat diets accompanied with the intake of vitamin C, a rough estimate of the amount of vegetables and fruits in the diet because data on micro-level were found to be more biased. Preliminary analyses were conducted to ensure no violation of the assumptions of normality, linearity and multicollinearity. Logarithmic transformations were used when needed. Linear regression was performed to evaluate the impact of the duration of breast-feeding on continuous variables (cardiovascular risk factors and FRS risk score). In model 1, crude values were used. Model 2 was adjusted for sex, and model 3 was additionally adjusted for current lifestyle factors: physical activity; proportion of fats in the diet; vitamin C; smoking; alcohol consumption. Logistic regression was performed to assess the impact of the duration of breast-feeding on the probability to fulfil at least three criteria of the metabolic syndrome (NCEP-ATPIII). The same models were used as in linear regression analyses. SPSS Statistics 19.0 (IBM) was used for all analyses. A P value < 0·05 was considered as statistically significant.
Results
Characteristics of the participants
At 32 years of age, 158 participated in the present study (seventy-six males and eighty-two females), 66 % of the original cohort. No differences in the duration of breast-feeding between the participants (5·2 (sd 3·2) months) and non-participants (4·7 (sd 3·4) months) were detected (Student's t test, P= 0·36), but, interestingly, birth weight (P= 0·035) and length (P= 0·003) were higher among non-participants. However, by 1 year of age, these differences attenuated between the participants and non-participants (P= 0·11 and P= 0·14, respectively; data not shown).
In the present study, three participants (2 %), all women, were underweight (BMI < 18·5 kg/m2), while 31 % were overweight (BMI 25–30 kg/m2) and 10 % were obese (BMI >30 kg/m2). According to the NCEP-ATPIII criteria, 14 % of men and 18 % of women had central obesity. All anthropometric measures were higher in men (Table 1).
Table 1 Baseline demographic and clinical characteristics of the study cohort (Mean values and standard deviations; percentages and 95 % confidence intervals)

* Based on Student's t test.
† 95 % CI for the difference.
Alcohol consumption more than twice per week was reported by 13 % of the participants. Compared with women, men had significantly higher alcohol consumption (Student's t test, P= 0·006; Table 1). The frequency of the regular usage of tobacco products was 33 %. Of the participants, 73 % reported current physical activity at least twice per week. Participants were highly educated as 78 % had graduated from high school and 57 % had a university degree. Of the women participants, 52 % had at least one child. According to the 3 d food records, men received on average 34 % of their daily energy from fats and 18 % from proteins, while the corresponding estimates in women were 35 and 17 %, respectively. The mean intakes of vitamin C were 104 mg in men and 113 mg in women.
Characteristics of the cardiovascular outcomes and risk scores
None of the participants used lipid-lowering medication, although 26 % had total cholesterol concentration above 5·0 mmol/l, 23 % had LDL-concentration above 3·0 mmol/l, 7 % had TAG concentration above 1·7 mmol/l, and 9 % of men had HDL-concentration below 1·0 mmol/l and 6 % of women below 1·3 mmol/l (NCEP-ATPIII cut-off points). The measure of insulin resistance HOMA-IR was higher in men than in women (Table 2).
Table 2 Risk factors and vascular properties (Mean values and standard deviations)

HOMA-IR, homeostatic model assessment of insulin resistance; CCA, common carotid artery; NCEP-ATPIII, National Cholesterol Education Program's Adult Treatment Panel III.
* P values refer to the difference between sexes.
At 32 years of age, the mean systolic blood pressure was 120 mmHg and the mean diastolic blood pressure was 73·5 mmHg. According to the NCEP-ATPIII cut-off points, 25 % had systolic blood pressure above 130 mmHg and 10 % had diastolic blood pressure above 85 mmHg. Furthermore, two participants with elevated diastolic blood pressure had systolic pressure below 130 mmHg. Only one participant reported the use of antihypertensive medication; however, despite the treatment, he was hypertensive. The CCA-IMT tended to be higher in men (Table 2).
According to the FRS, twenty-two (29 %) men had at least 3 % risk of CHD over 10 years. For the remaining men, the risk was lower. None of the women had a notably elevated risk compared with the average risk at this age. The criteria for the metabolic syndrome according to the NCEP-ATPIII were met by two women and twelve men (Fig. 1). Nine of these also had an elevated risk of CHD within 10 years.
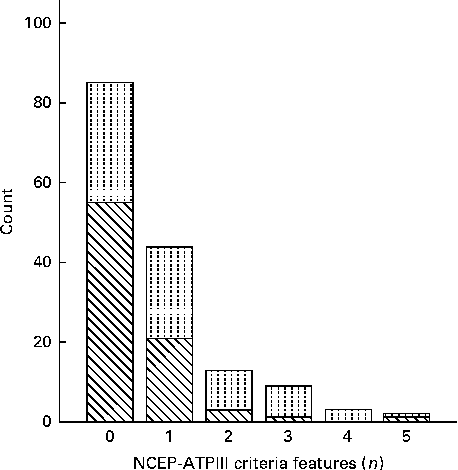
Fig. 1 Clustering of metabolic criteria features in the participants defined according to the National Cholesterol Education Program's Adult Treatment Panel III (NCEP-ATPIII). The metabolic syndrome requires the presence of three or more features. The observed features were central obesity, glucose intolerance or diabetes, hypertension and dyslipidaemia (HDL and TAG). Sex: ![]() , male;
, male; ![]() , female.
, female.
Breast-feeding and cardiovascular risk factors
Pearson's correlation showed that the duration of breast-feeding did not have significant associations with FRS, NCEP-ATPIII scoring, vascular variables, blood pressure or lipid profile as the correlations were within the range of 0·01–0·06. Table 3 presents the associations between the duration of breast-feeding and all the outcomes as analysed with linear regression. Model 1 represents crude values. The results are shown in relation to the absolute duration of breast-feeding. Again, the duration of breast-feeding did not have significant associations with FRS, blood pressure, CCA-IMT or cholesterol levels (P>0·5 for all). In model 2, the values were adjusted for sex, which seems to be the most important predictor of CVD risk. In model 3, values were additionally adjusted for current physical activity, dietary proportion of fat and vitamin C, number of cigarettes per d and alcohol consumption. These adjustments did not influence the results. After all the adjustments, model 3 explained 85 % of the variance in the FRS.
Table 3 Various cardiovascular risk variables* (Regression coefficients and 95 % confidence intervals)

FRS, Framingham risk score; CCA-IMT, common carotid artery intima–media thickness; HOMA-IR, homeostatic model assessment.
* The value of the coefficient characterises the impact of 1-month increase in the duration of breast-feeding on cardiovascular risk variables.
† For HOMA-IR, logarithmic transformation (natural logarithm) was utilised.
‡ Model 1 shows crude values.
§ Model 2 is adjusted for sex.
∥ Model 3 is same as model 2 plus adjusted for current physical activity, proportion of fats in the diet, dietary vitamin C, alcohol consumption and number of cigarettes per d (smoking is used as a covariate in all the outcome variables except the FRS which already includes smoking as one of its components).
Due to a skewed variation of the NCEP-ATPIII in the cohort (Fig. 1), the association with the duration of breast-feeding was tested using logistic regression. Subjects who fulfilled at least three of the NCEP-ATPIII criteria were recoded as 1 (n 14, increased risk, multiple risk factors) and others with 0. In model 1, the OR characterising the impact of a 1-month increase in the duration of breast-feeding was 0·96 (95 % CI 0·8, 1·1), in model 2 adjusting for sex, OR was 0·95 (95 % CI 0·8, 1·1) and in model 3 containing all confounders, OR was 0·95 (95 % CI 0·8, 1·1).
Discussion
We demonstrate here that cardiovascular risk in adulthood is not influenced by the duration of breast-feeding in subjects born at term and appropriate for gestational age. The present findings were not modified by the current lifestyle factors. Male sex was the most important independent predictor of CVD. Clustered FRS, an estimate of 10-year CHD risk, was not related to the duration of breast-feeding.
The present study population is a unique birth cohort that has been followed up particularly closely during the first 12 months by the same physician, and the information on milk feeding has been obtained during frequent visits in infancy, thus avoiding recall bias. The prospective follow-up period of 32 years can be considered to be exquisitely long. The additional strength of the present study is the carefully collected data on confounding factors, such as current lifestyle factors, diet and physical activity, which were obtained in a structured fashion with a questionnaire and completed by interview by one of the authors. Originally, the collected cohort was homogeneous, as all babies were born at the same delivery hospital at term, their birth weight was above 3000 g and complementary foods were introduced to all at 3·5 months with the same guidelines. Also, the environments, including public primary and secondary schools, were similar for all participants, as they were native Finns with a similar cultural background. In our cohort, no cardiovascular events were diagnosed, as myocardial infarction or angina pectoris is still very rare by 32 years of age. Therefore, our approach was to investigate the association of infant feeding with the risk of cardiovascular events within 10 years. For our purposes, the FRS and NCEP-ATPIII metabolic syndrome criteria points (which exploit multiple CVD risk factors) were suitable and novel compared with prior studies.
Our cohort consisted mostly of relatively well-educated participants, which may have influenced the results. Breast-feeding is known to associate with higher socio-economic status and healthier lifestyle overall, and, accordingly, the results may not be representative for all social classes. In addition, the cohort size was limited. Therefore, some smaller potential effects of breast-feeding might not become notable in our cohort. However, even if the end points of the CI may be over 10-fold when compared with the coefficient of breast-feeding duration β itself (Table 3), they are still sufficiently narrow to enable considering the effect of the duration of breast-feeding as minor. Considering the FRS for model 3, the lower endpoint of the CI is − 0·19; hence, over 5-month increase in the duration of breast-feeding would be required to decrease the sum of the FRS by one point, which corresponds to about 1 % decrease in the 10-year CHD risk.
The role of breast-feeding as a potential determinant of individual cardiovascular risk factors has been of interest in several studies. A meta-analysis by Owen et al. ( Reference Owen, Whincup and Odoki 31 ) included thirty-seven studies on the effect of infant feeding mode on total cholesterol and LDL-cholesterol. Breast-fed infants were compared with bottle-fed infants, and no differences were detected in their total cholesterol or LDL-cholesterol levels at adolescence, but at adulthood (>17 years of age), those who received breast milk had a lower mean cholesterol level of 0·18 mmol/l( Reference Owen, Whincup and Odoki 31 ). Other studies with an extended follow-up up to middle age have reported no associations between breast-feeding and later blood pressure or lipid profile at 41 years of age( Reference Rudnicka, Owen and Strachan 15 , Reference Parikh, Hwang and Ingelsson 32 ). These reports support the present findings with no clinically relevant association discovered between infant feeding and the risk of CVD at 32 years of age.
In developed countries, higher socio-economic class, mature maternal age, lower maternal BMI and, at the same time, the choice of healthier lifestyle in general are known to associate with the mother's intention to breast-feed and also to the actual duration of breast-feeding( Reference Ludvigsson and Ludvigsson 33 – Reference Oddy, Li and Landsborough 35 ), and, in turn, the same factors contribute to the offspring's health. Accordingly, numerous confounders confuse the interpretation of the effect of breast-feeding on adult cardiovascular health. Therefore, to evaluate the true effect of infant feeding, we collected data on lifestyle factors and dietary habits, and used these confounders as covariates in statistical analysis. The Promotion of Breast-feeding Intervention Trial (PROBIT) has been designed to anticipate the control of confounders. This intervention study was scheduled by bringing the WHO Baby Friendly Hospital Initiative training to promote breast-feeding at randomised birth hospitals in Belarus. PROBIT has increased the duration of exclusive breast-feeding in these hospitals. Only breast-fed infants were included to PROBIT, and in their recent evaluation (2013) at 11 years of age, no difference has been reported in body composition and obesity between those exclusively breast-fed for 3 months and those with longer breast-feeding( Reference Martin, Patel and Kramer 36 ).
In reported studies, the association of breast-feeding with cardiovascular risk factors has been controversial, as variables showing beneficial effects vary between studies: they might have reported beneficial effects on one and no effect on another risk factor and vice versa with no consistency( Reference Lawlor, Riddoch and Page 8 , Reference Parikh, Hwang and Ingelsson 32 , Reference Ravelli, van der Meulen and Osmond 37 ). Large cohorts enable the detection of small significant changes between study groups. Typically, this raises a question about the clinical importance or even the meaning to public health. Based on two large simultaneous meta-analyses involving seventeen and twenty-four studies and altogether >17 000 participants, breast-feeding reduced systolic blood pressure by 1·1–1·4 mmHg later in life when compared with those formula fed in infancy. The reduction in diastolic blood pressure was moderate (0·5 mmHg). The authors have suggested that studies of small cohorts (n< 300) reported larger mean differences between infant-feeding groups, and these studies are subject to a chance finding( Reference Owen, Whincup and Gilg 12 , Reference Martin, Gunnell and Smith 13 ). Similarly, in the Mater-University Study of Pregnancy and its outcomes in Australia with nearly 4000 participants, the authors have reported a 0·69 mmHg lower systolic blood pressure at age 5 years among participants breast-fed over 6 months, compared with those who were breast-fed for a shorter duration( Reference Lawlor, Najman and Sterne 9 ). The European Youth Heart Study of over 2000 participants aged 9–15 years showed a 1·7 mmHg reduction in systolic blood pressure in favour of breast-feeding over formula feeding( Reference Lawlor, Riddoch and Page 8 ).
The effect of breast-feeding on serum cholesterol and TAG has been studied in the Framingham Third Generation Study: being breast-fed as an infant was associated with higher HDL-cholesterol levels at the age of 41 years, but no difference in other lipid levels or blood pressure was recognised, as mentioned earlier( Reference Parikh, Hwang and Ingelsson 32 ). Khan et al. ( Reference Khan, Green and Forsyth 38 ) reported improved microvascular function in 10–14-year-olds who had been breast-fed. In a Finnish study, men aged 24–39 years who had been breast-fed had better brachial endothelial function compared with those initially formula fed; in women, no differences were observed( Reference Järvisalo, Hutri-Kähönen and Juonala 10 ). Finally, only a few studies have reported the non-beneficial effects of breast-feeding, which may reflect publication bias. For example, Leeson et al. ( Reference Leeson, Kattenhorn and Deanfield 17 ) reported reduced arterial distensibility with longer breast-feeding. Non-invasive ultrasonic measurements of arterial distensibility, flow-mediated endothelial function and the thickening of arterial IMT are potential hallmarks of subclinical CVD( Reference Lorenz, Markus and Bots 39 ). These measurements are mostly used by researchers and are not yet in clinical practice.
Conclusion
In the present long-term prospective study from infancy to adulthood, we found a very weak association between the duration of breast-feeding and cardiovascular risk factors in adulthood. The present findings were not modified by the current lifestyle factors, including physical activity, dietary habits, smoking and alcohol consumption. Male sex seems to be the most predictive determinant of the increased risk of CVD. The associations in previous studies have been weak as well, and the clinical significance of the effects is questionable at both the individual and public health levels. As breast-feeding has many other beneficial aspects to infants, we, however, do recommend mothers to breast-feed their babies.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114513003346
Acknowledgements
The authors acknowledge adjunct Professor Hannu Rita from the Department of Forest Resource Management (Statistics and Methodology), University of Helsinki for statistical expertise. The authors also thank Minna Savolainen, RN, for the help in the data collection and Ulla Vesander, RD, for analysing the food records, both from the Children's Hospital, Helsinki University Central Hospital.
The present study was supported by grants from the following organisations in Helsinki, Finland: Foundation for Pediatric Research; Academy of Finland; Folkhälsan Research Foundation; Helsinki University Central Hospital Research Funds; Sigrid Juselius Foundation; Ida Montin Foundation; Yrjö Jahnsson Foundation. The founders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
The authors' contributions are as follows: S. P., M. Ta., O. M., M. K., U. M. S.-P. and M. Tu. took part in the planning of the study design; S. P. was responsible for the data collection at the 32-year follow-up; S. P., M. Ta., H. V. and M. Tu. were responsible for the analysis and interpretation of the data; S. P. wrote the manuscript with the assistance of M. Ta., H. V. and M. Tu. All authors took part on the manuscript preparation and contributed intellectually to the writing. Each author read and approved the contents of the manuscript.
None of the authors has any conflict of interest.






