Atherosclerosis is the most common pathological process that leads to CVD, including myocardial infarction and stroke( Reference Sherer and Shoenfeld 1 ). Consequently, progression of atherosclerosis occurs at the subendothelium by the accumulation of lipid-engorged macrophages, immune cells and smooth muscle cells( Reference Sherer and Shoenfeld 1 ). Oxidation of LDL enhances the recruitment of vascular cell adhesion molecules and chemokines. These macrophages then take up oxidised LDL-cholesterol (LDL-C) and become foam cells, which subsequently produce growth factors and cytokines that lead to the proliferation of vascular smooth muscle cells and the development of plaques. It is generally believed that increasing the uptake of hepatic LDL through the up-regulation of LDL receptors could be a target in drug therapy for lowering LDL-cholesterol levels. The reverse cholesterol transport (RCT) mechanism by HDL is known to remove cholesterol from peripheral tissues( Reference O'Connell and Genest 2 ). HDL has been shown to exert cardioprotective effects in endothelial and vascular smooth muscle cells by generating a cascade of intracellular signals including the activation of PI3K/Akt (phosphatidylinositol 3-kinase/v-akt murine thymoma viral oncogene homolog 1), ERK1/2 (extracellular-signal-regulated kinases), p38 mitogen-activated protein kinase and RhoA (ras homolog gene family, member A)( Reference Nofer and Assmann 3 , Reference Mineo, Yuhanna and Quon 4 ).
Paraoxonase, a multifunctional antioxidant enzyme tightly associated with HDL, has been shown to not only inhibit LDL oxidation and hydrolyse oxidised LDL, but also detoxify the homocysteine metabolite, homocysteine thiolactone( Reference Navab, Hama-Levy and Van Lenten 5 , Reference Aviram, Rosenblat and Bisgaier 6 ). A negative correlation of serum paraoxonase 1 (PON1) activity with the development of aortic lesion scores has been demonstrated previously in a mouse model when fed an atherogenic diet( Reference Shih, Gu and Hama 7 ). Thus, one of the major reasons for the anti-atherogenic property of HDL has been attributed to its protective effect against LDL oxidation catalysed by its component enzyme PON1( Reference Durrington, Mackness and Mackness 8 , Reference Aviram, Dornfeld and Rosenblat 9 ). PPARα controls the expression of a wide range of hepatic genes encoding proteins involved in fatty acid catabolism and lipoprotein metabolism( Reference Azhar 10 ). In addition, PPARα also regulates macrophage cholesterol homeostasis by promoting cholesterol efflux through the modulation of the expression of key proteins involved in this process( Reference Robinson and Grieve 11 , Reference Marx, Duez and Fruchart 12 ), as well as by reducing intracellular lipid accumulation in macrophages( Reference Marx, Duez and Fruchart 12 , Reference Chinetti-Gbaguidi, Rigamonti and Helin 13 ). Dietary and behavioural habit modifications need to be considered as the first step to sufficiently lower plasma lipid levels rather than pharmacological treatment that involves a search for new drugs capable of decreasing plasma LDL-C and total TAG levels.
‘Njavara’ (Oryza sativa L., variety ‘njavara’) is a unique, indigenous, medicinal rice variety. Njavara rice (with bran) is the major ingredient in ‘medicinal porridge’ recommended by Ayurvedic physicians to improve immune functions of the body during the rainy season. Much research has focused on the ability of rice bran oil to decrease plasma levels of total cholesterol (TC) and LDL-C in both hypercholesterolaemic and normal animals. In many experimental studies, it has been found that oryzanol, an unsaponifiable component of rice bran oil, has beneficial effects against the development of CVD by its cholesterol-lowering action. It has been reported that total oryzanol content was 2.7 and 7 times higher in Njavara rice bran than in Sujatha and palakkadan matta, other common rice varieties( Reference Mohanlal, Parvathy and Shalini 14 ). This is suggestive of the enhanced action of the extract at lower concentrations. However, the anti-atherogenic actions of Njavara rice bran oil (NjRBO) have not been defined. Therefore, it is important to determine whether changes in plasma and tissue lipids are related to modifications in the gene expression levels of ATP-binding cassette transporter A1 (ABCA1), apoA1, apoB, PPARα and PON1, which are the major regulators for maintaining whole-body cholesterol homeostasis, after supplementation of NjRBO in high-cholesterol diet (HCD)-fed rats.
The present study was conducted to convincingly demonstrate the cardioprotective abilities of NjRBO by evaluating its role in lipid metabolism via RCT and also by showing parallel positive alterations in the liver gene expression of PON1, which can provide scientific evidence for the traditional use of NjRBO in the treatment of CVD. An effective dose of NjRBO (100 mg/kg body weight) was fixed by conducting a dose-dependent study in rats with carrageenan-induced paw oedema.
Materials and methods
Chemicals and solvents
All biochemicals used in the present study were purchased from Sigma Chemical Company, and other chemicals and solvents of analytical grade were obtained from SRL Chemicals.
Extraction of oil
About 100 g of stabilised rice bran of Njavara black were defatted using 800 ml of the petroleum diethyl ether solvent for about 16 h in a Soxhlet extractor. The solvent from the extract was evaporated at 30°C by using a rotary evaporator (Laborota 4000-Heidolph). The analysis of oryzanol content by HPLC showed that the contents of each oryzanol component as well as total oryzanol were significantly higher in Njavara rice bran (Njavara black) than those in staple varieties and corresponding rice samples. The oryzanol content of Njavara black was determined to be 1·84 mg/g, which was used as NjRBO in the present study.
Animal experiments
For the present study, adult male rats (Sprague–Dawley strain, weighing 100–120 g) bred in the department animal house were used. The rats were kept in an environment with a controlled temperature (24–26°C), humidity (55–60 %) and photoperiod (12 h light–12 h dark cycle). A standard diet and tap water were provided ad libitum. The rats were treated humanely in accordance with the current institutional guidelines. All experiments were conducted according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (registration no. IAEC-KU-9/2012-13-BC-AH-PK A1) in accordance with the accepted principles of the Government of India for the use and care of laboratory animals (no. KU-12/2005–06).
Screening of different doses of Njavara rice bran oil in an acute model of inflammation in rats with carrageenan-induced paw oedema
Paw oedema was induced and the inhibition rate of oedema was calculated( Reference Viji and Helen 15 ).
A total of twenty-one adult male Sprague–Dawley (weighing 100–120 g) were used for the study. The rats were divided into seven groups, each consisting of three rats: group I served as the control (receiving saline only); group II received carrageenan alone; group III received carrageenan+NjRBO (25 mg/kg body weight); group IV received carrageenan+NjRBO (50 mg/kg body weight); group V received carrageenan+NjRBO (100 mg/kg body weight); group VI received carrageenan+NjRBO (200 mg/kg body weight); group VII received carrageenan+indomethacin (3 mg/kg body weight).
NjRBO was administered orally, with doses of 25, 50, 100 and 200 mg/kg. After 1 h, 0·1 ml of 1 % carrageenan suspension in 0·9 % NaCl solution was injected into the sub-plantar tissue of the right hind paw. Paw volume was measured before the injection and at the 3rd and 5th hours after the injection of carrageenan using a plethysmometer. Indomethacin (3 mg/kg body weight), a non-steroidal anti-inflammatory drug, was used as the positive control. The swelling degree of paw and the inhibition rate of oedema were calculated as follows:
where V c and V t are the average oedema volume of the control and the test, respectively. For dose-dependent studies, test drugs of 25–200 mg/kg body weight were administered.
Experimental design and treatment protocol for Njavara rice bran oil in chronic inflammation
A total of twenty-seven albino rats (Sprague–Dawley strain, weighing 100–120 g) were randomly divided into three groups of nine rats each, and fed a standard diet formula based on the AIN-93M maintenance diet, containing 590 g maize starch, 200 g casein, 15 % soyabean oil and 10 g α-cellulose as fibre/kg diet. Choline, cysteine, minerals and vitamins were added based on the formulation of AIN-93M( Reference Reeves, Nielsen and Fahey 16 ). Group I served as the control and fed the standard diet. Group II received a HCD (standard diet+1·5 % cholesterol and 0·5 % cholic acid). Group III received the HCD plus NjRBO (100 mg/kg body weight) mixed in the diet. The duration of the experiment was 60 d. Body weights were recorded weekly during the experimental period.
Sampling procedures
At the end of the experimental period, rats were deprived of food for 16 h, and then anaesthetised with diethyl ether inhalation and killed by decapitation. Blood samples were collected into tubes without an anticoagulant, kept at room temperature for 1 h, and serum was separated by centrifugation for 20 min at 1500 g . Serum was stored in − 80°C until analysed. Tissues from each animal were analysed separately.
Biochemical estimations
TC, TAG and HDL-cholesterol (HDL-C) levels in serum were measured with a test kit method (Agappe Diagnostics), based on a reported enzymatic method( Reference Bachorik and Ross 17 , Reference Allain, Poon and Chan 18 ). The procedures given by the supplier was followed without any modifications for the determination of cholesterol, HDL-C and TAG levels, whereas LDL-C levels were calculated using Friedewald's equation( Reference Warnick, Knopp and Fitzpatrick 19 ). The atherogenic index (AI) was calculated by the following formula:
Total lipids from the liver and aorta were extracted using chloroform/methanol according to the method described by Folch et al. ( Reference Folch, Lees and Sloane-Stanley 20 ). Aliquots of these samples were used for the estimation of TC, phospholipid, TAG and NEFA levels( Reference Carr and Drekter 21 – Reference Falholt, Lund and Falholt 24 ). Lipid peroxides (thiobarbituric acid-reactive substances (TBARS)) in tissue homogenates were estimated using TBARS by the method of Ohkawa et al. ( Reference Ohkawa, Ohishi and Yagi 25 ). C-reactive proteins levels (CRP) in plasma were determined by using an immunoturbidimetric kit (DiaSys Diagnostics).
Measurement of hepatic 3-hydroxy-3-methylglutaryl-CoA reductase, plasma lecithin cholesterol acyl transferase and lipogenic enzymes activities
For the measurement of lipogenic enzyme activities, the rat liver was minced and homogenised in glycyl-glycine buffer in an ice-cold condition. Homogenates were centrifuged at 9000 g at 40°C for 20 min and the supernatant fraction was used for the measurement of various enzyme activities. The activity of reductase (3-hydroxy-3-methylglutaryl (HMG)-CoA reductase) was measured, as described by Rao et al. ( Reference Rao and Ramakrishnan 26 ), by determining the ratio of HMG-CoA:mevalonate acid. Plasma lecithin cholesterol acyl transferase (LCAT) activity was assayed as described by Schoenheimer et al. ( Reference Schoenheimer and Sperry 27 ). The activities of glucose-6-phosphate dehydrogenase (G6PDH) and isocitrate dehydrogenase (IDH) were measured by the method of Kornberg et al. ( Reference Kornberg, Horecker and Smyrniotis 28 ). The activity of malic enzyme (ME) was measured by the method described by Ochoa( Reference Ochoa 29 ). Fatty acid synthase activity was measured according to the method of Nepokroeff et al. ( Reference Nepokroeff, Lakshamanan and Porter 30 )and Moibi et al. ( Reference Moibi, Ekpe and Christopherson 31 ). Acetyl-CoA carboxylase (ACC) activity was measured using a discontinuous spectrophotometric assay as described by Willis et al. ( Reference Willis, Omar and Sambanthamurthi 32 )
Measurement of paraoxonase and arylesterase activities
Activities of paraoxonase and arylesterase (ARE) were measured in serum as described by Erdem et al. ( Reference Erdem, Karatay and Yildirim 33 )
Protein assay
Protein was assayed by the method of Lowry et al. ( Reference Lowry, Rosebrough and Farr 34 ).
RT-PCR and ELISA
RNA was extracted with TRI Reagent (Sigma Life Science) and quantified at 260 nm. Reverse transcription was performed with the Reverse Transcription System (Eppendorf Mastercycler), according to the manufacturer's protocol. Glyceraldehyde-3-phosphate dehydrogenase served as a control. The primer sequences for ABCA1, apoA1, apoB, PON1 and PPARα are listed in Table 1. A total reaction volume of 25 μl contained 6 μl of reverse transcription product, 1·5 mm-MgCl2, 2·5 U Taq DNA polymerase, 100 μm-deoxyribonucleotide triphosphate (dNTP), 0·1 μm-primer and 1 × Taq DNA polymerase Mg-free buffer. The reaction mixture was incubated in a thermocycler (Eppendorf Mastercycler) programmed to predenature at 105°C for 2 min, denature at 94°C for 30 s, anneal at 59°C for 30 s, and extend at 72°C for 60 s, for a total of thirty cycles. The last cycle was followed by incubation at 72°C for 5 min and cooling to 4°C. The PCR products were electrophoresed on 1·5 % agarose gel and stained with ethidium bromide. The image of the gel was captured in digital format.
Table 1 Primer sequences

GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ABCA1, ATP-binding cassette transporter A1; PON1, paraoxonase 1.
ELISA was performed to quantify the amount of different proteins using specific antibodies. ApoA1, ABCA1 and apoB antibodies were purchased from Abcam and Santa Cruz Biotechnology, Inc.
Western blotting
Liver samples were homogenised in lysis buffer (1 % Triton X-100, 0·5 % sodium deoxycholate, 0·1 % SDS, 150 mm-NaCl, 2 mm-EDTA and 50 mm-Tris–HCl, pH 7·5) containing freshly added proteinase inhibitors (P8430; Sigma) and phosphatase inhibitors (P2850 and P5726; Sigma). After centrifugation at 20 000 g for 30 min at 4°C, the supernatant was used for Western blotting and the pellet was discarded. Protein extracts were separated on a 4–15 % SDS PAGE gel and transferred to nitrocellulose membranes (Bio-Rad 162-0113). The membranes were blocked with 5 % skimmed milk, probed with an appropriate primary antibody (anti-ABCA1 antibody ab151685, Abcam and anti-apoA-I rabbit polyclonal antibody (FL-267): sc-30 089), incubated with an appropriate horseradish peroxidase-conjugated secondary antibody (anti-rabbit IgG, Abcam (ab97051)), and then developed with the addition of a substrate. The bands were then quantified.
Assessment of atheroscleortic plaques in the aorta
After in situ perfusion with cold PBS and subsequently with cold buffered formalin, the arch and thoracic portion of the dorsal aorta were dissected free from the thoracic cavity and heart. The entire aorta was isolated from the arch to the aortic iliac bifurcation. After removing the adventitia and adipose tissue, the aorta was placed in 10 % neutral buffered formalin overnight. The aorta was then opened lengthwise, and pinned flat in a wax-bottomed dissecting pan. The tissue was stained for 15 min with 0·5 % Sudan IV solution in acetone and 70 % ethanol (1:1). The tissue was decolourised for 5 min using 80 % ethanol, and then washed gently with water for several minutes. The en face preparations were digitally photographed( Reference Smith, Tan and Tawfik 35 ).
Histopathological evaluation of the aorta
After killing the rats, tissues were removed and fixed in 10 % buffered formalin and then decalcified for 7 d. The tissues were then processed for paraffin embedding, sectioned at 5 μm thickness, and subsequently stained with Ehrlich's haematoxylin and eosin for examination under a light microscope.
Statistical analysis
Results were analysed using the statistical program SPSS/PC+, version 11.0 (SPSS, Inc.). The experiments with the treatment groups of six rats each were replicated with similar results for a total of six times. Statistical evaluation was done using the one-way ANOVA, and significant difference was determined using Duncan's test of P< 0·05.
Results
Effect of Njavara rice bran oil on the development of carrageenan-induced paw oedema
Various doses of NjRBO were screened for their anti-inflammatory activity, and selection of an effective dose was made using the carrageenan-induced paw oedema assay. In the present study, carrageenan induction significantly increased paw volume, and maximum oedema was observed at the 5th hour after the induction of carrageenan. The effect of pretreatment of the experimental rats with NjRBO on a series of doses was observed to evaluate significant inhibition in the development of carrageenan-induced paw oedema at the 3rd and 5th hours. Percentage inhibition by NjRBO showed maximum activity at doses of 100 and 200 mg/kg body weight. The administration of indomethacin (3 mg/kg) showed oedema inhibition by 40 and 68 % at the 3rd and 5th hours, respectively (Table 2). From these results, it was evident that NjRBO at the dose of 100 mg/kg body weight possesses maximum anti-oedematous activity in the carrageenan-induced acute inflammatory model. Therefore, for a further study, a dose of 100 mg/kg body weight was used as the minimum dose and maximum effect.
Table 2 Effect of Njavara rice bran oil (NjRBO) on rat carrageenan-induced hind paw oedema
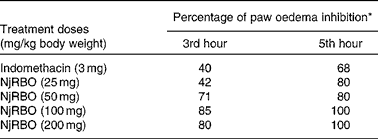
* Percentage of oedema inhibition was calculated as described in the Materials and methods section.
Serum lipid profile and atherogenic index
To study the anti-atherogenic effect of NjRBO on HCD-fed rats, the lipid profile in serum and the atherogenic index were determined after treatment for 60 d. The supplementation of the HCD progressively increased the TC, TAG and LDL-C levels, and the atherogenic index compared with the control, but the supplementation of NjRBO decreased their levels. HDL-C concentration in the HCD-fed group showed a significant decrease compared with the control; however, supplementation of NjRBO significantly increased (P≤ 0·05) the HDL-C levels (Table 3).
Table 3 Changes in the serum lipid profile and atherogenic index (Mean values with their standard errors; n 6 rats per group)
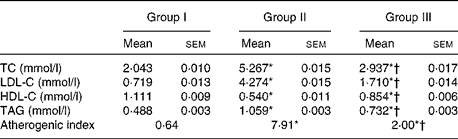
Group I, control rats fed the standard diet; group II, rats fed the high-cholesterol diet (HCD) (standard diet+1·5 % cholesterol and 0·5 % cholic acid); group III, rats fed the HCD with Njavara rice bran oil (100 mg/kg body weight) mixed in the diet; TC, total cholesterol; LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol.
* Mean value was significantly different from that of group I (P< 0·05).
† Mean value was significantly different from that of group II (P< 0·05).
Concentration of tissue lipids
Lipids were extracted from the liver and aorta using chloroform/methanol. The concentrations of cholesterol, TAG, phospholipids and NEFA were estimated, and the results are presented in Table 4. Lipid contents in the liver and aorta were significantly reduced in rats treated with NjRBO compared with rats fed with the HCD (P< 0·05).
Table 4 Concentrations of cholesterol, TAG and phospholipids in tissues (Mean values with their standard errors; n 6 rats per group)
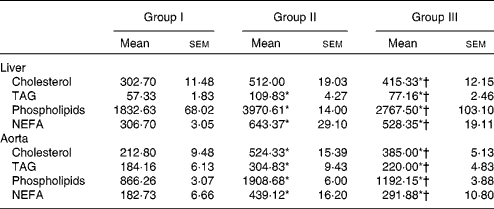
Group I, control rats fed the standard diet; group II, rats fed the high-cholesterol diet (HCD) (standard diet+1·5 % cholesterol and 0·5 % cholic acid); group III, rats fed the HCD with Njavara rice bran oil (100 mg/kg body weight) mixed in diet.
* Mean value was significantly different from that of group I (P< 0·05).
† Mean value was significantly different from that of group II (P< 0·05).
Activities of lecithin cholesterol acyl transferase, 3-hydroxy-3-methylglutaryl-CoA reductase and lipogenic enzymes
The activity of HMG-CoA reductase was significantly increased in rats fed the HCD. The hypocholesterolaemic effects of NjRBO were observed characterised by reduced hepatic HMG-CoA reductase in HCD-fed rats treated with NjRBO. The activity of LCAT was found to be decreased in HCD-fed rats and was significantly increased in the NjRBO-treated group (Table 5). The HCD significantly increased the activities of the lipogenic enzymes (G6PDH, IDH, ME, ACC and fatty acid synthase) examined in the liver, but these activities were decreased after treatment with NjRBO (Table 6).
Table 5 Activities of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver and lecithin cholesterol acyl transferase (LCAT) in plasma (Mean values with their standard errors; n 6 rats per group)
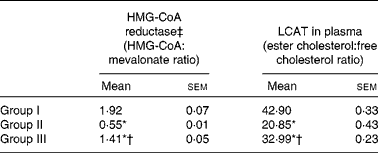
Group I, control rats fed the standard diet; group II, rats fed the high-cholesterol diet (HCD) (standard diet+1·5 % cholesterol and 0·5 % cholic acid); group III, rats fed the HCD with Njavara rice bran oil (100 mg/kg body weight) mixed in the diet.
* Mean value was significantly different from that of group I (P< 0·05).
† Mean value was significantly different from that of group II (P< 0·05).
‡ Lower ratio indicates higher enzyme activity.
Table 6 Changes in the activities of lipogenic enzymes in the liver‡ (Mean values with their standard errors; n 6 rats per group)
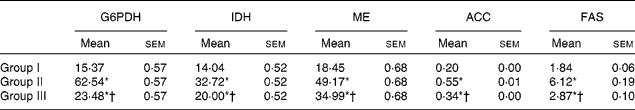
Group I, control rats fed the standard diet; group II, rats fed the high-cholesterol diet (HCD) (standard diet+1·5 % cholesterol and 0·5 % cholic acid); group III, rats fed the HCD with Njavara rice bran oil (NjRBO) (100 mg/kg body weight) mixed in the diet.
* Mean value was significantly different from that of group I (P< 0·05).
† Mean value was significantly different from that of group II (P< 0·05).
‡ Activity for glucose-6-phosphate dehydrogenase (G6PDH), isocitrate dehydrogenase (IDH) and malic enzyme (ME) is expressed as μmol of NADPH (or NAD) produced/min per g wet weight of tissue, for acetyl-CoA carboxylase (ACC) as μmol of acetyl-CoA consumed/min per g dry tissue weight, and for fatty acid synthase (FAS) as μmol of NADPH utilised/min per g tissue at 37°C.
Activities of paraoxonase 1 and arylesterase in serum
The HCD significantly decreased the activities of both PON1 and ARE; however, treatment with NjRBO (100 mg/kg per d) for 60 d significantly increased the activity of PON1 in serum compared with the control rats (Table 7).
Table 7 Plasma levels of C-reactive protein (CRP), serum paraoxonase (PON) and arylesterase (ARE) (Mean values with their standard errors; n 6 rats per group)
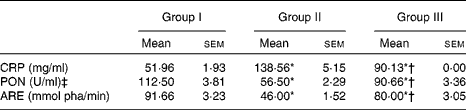
Group I, control rats fed the standard diet; group II, rats fed the high-cholesterol diet (HCD) (standard diet+1·5 % cholesterol and 0·5 % cholic acid); group III, rats fed the HCD with Njavara rice bran oil (100 mg/kg body weight) mixed in the diet; pha, phenylacetate.
* Mean value was significantly different from that of group I (P< 0·05).
† Mean value was significantly different from that of group II (P< 0·05).
‡ 1 U = enzyme quantity that disintegrates 1 μmol of paraoxon substrate in 1 min.
Gene expression of paraoxonase 1 in the liver
The present study shows that NjRBO has a transcriptional role in the up-regulation of the expression of PON1. The rats supplemented with NjRBO showed significantly increased (P< 0·05) expression of PON1 compared with HCD-fed rats that exhibited decreased expression of PON1. These data suggest that the observed effects on PON1 were consistent with the results of serum HDL-C and liver apoA1 expression (Fig. 1).

Fig. 1 mRNA expression in the liver of rats. (a) Western blotting was performed to determine the mRNA expression in the liver. The relative amount of mRNA expression of (b) ATP-binding cassette transporter A1 (ABCA1), (c) apoB, (d) PPARα, (e) apoA1 and (f) paraoxonase 1 (PON1) were estimated by semi-quantitative RT-PCR. PCR were quantified by densitometry and normalised to the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the control. Intensity was measured and expressed as arbitrary units. Values are means (n 6 rats per group), with their standard errors represented by vertical bars. * Mean value was significantly different from that of group I (P< 0·05). † Mean value was significantly different from that of group II (P< 0·05). Group I, control rats fed the standard diet; group II, rats fed the high-cholesterol diet (HCD) (standard diet+1·5 % cholesterol and 0·5 % cholic acid); group III, rats fed the HCD with Njavara rice bran oil (100 mg/kg body weight) mixed in the diet. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Up-regulation of ATP-binding cassette transporter A1 and apoA1 expression and down-regulation of apoB expression by Njavara rice bran oil
The HCD down-regulated the mRNA expression of ABCA1 and apoA1. The 60 d treatment with NjRBO up-regulated ABCA1 mRNA levels in the liver, indicating that NjRBO may promote cholesterol efflux from lipid-loaded cells. In addition, the transcriptional levels of apoA1 were enhanced similarly in HCD-fed rats supplemented with NjRBO. The HCD-fed rats showed the overexpression of apoB, indicating the increase in circulating LDL levels. The treatment with NjRBO decreased the expression of apoB in the liver (Fig. 1). These results were confirmed by determining their respective protein expression by ELISA, which was consistent with the results of mRNA expression (Fig. 2(a)–(c)). The Western blot analysis of ABCA1 and apoA1 also showed their increased expression in group III and decreased expression in group II (Fig. 2(d) and (e)).

Fig. 2 Protein expression of (a) ATP-binding cassette transporter A1 (ABCA1), (b) apoB and (c) apoA1 in the liver: the presence of ABCA1, apoB and apoA1 antigens in the liver was determined by ELISA. (d) Western blotting was performed to determine the protein expression of ABCA1 and apoA1 in the liver. The protein expression study showed a marked decrease in group II and increased expression in group III compared with the control. β-Actin was used as a loading control. Liver samples were homogenised in lysis buffer. Protein extracts (10 mg) were subjected to 4–15 % SDS–PAGE gel, and the blot was then probed with the anti-ABCA1 antibody and anti-apoA-I antibody. (e) Densitometry results (arbitrary units) of the Western blots. Values are means (n 6 rats per group), with their standard errors represented by vertical bars. Representative Western blots for each protein are also shown. * Mean value was significantly different from that of group I (P< 0·05). † Mean value was significantly different from that of group II (P< 0·05), optical density; group I, control rats fed the standard diet; group II, rats fed the high-cholesterol diet (HCD) (standard diet+1·5 % cholesterol and 0·5 % cholic acid); group III, rats fed the HCD with Njavara rice bran oil (100 mg/kg body weight) mixed in the diet. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Relative PPARα mRNA expression in the liver
To investigate the influence of PPARα in ABCA1-mediated cholesterol efflux, the expression of PPARα mRNA was examined. The HCD-fed rats supplemented with NjRBO showed significantly increased expression of PPARα, which was decreased in HCD-fed rats (Fig. 1).
Effect of Njavara rice bran oil on serum C-reactive proteins and thiobarbituric acid-reactive substances in the liver
The serum levels of CRP, an important biomarker for various inflammatory diseases, were significantly higher in HCD-fed rats than those of the control rats. Supplementation of NjRBO significantly (P< 0·05) decreased serum CRP concentrations (Table 7). TBARS levels, an indicator of lipid peroxidation, were significantly increased in the liver of rats fed the HCD, but supplementation of NjRBO significantly (P≤ 0·05) decreased their levels (Fig. 3).

Fig. 3 Effect of Njavara rice bran oil on lipid peroxidation. Lipid peroxidation product, thiobarbituric acid-reactive substance (TBARS) concentration, was measured using the method of Ohkawa et al. ( Reference Ohkawa, Ohishi and Yagi 25 ). Values are means (n 6 rats per group), with their standard errors represented by vertical bars. * Mean value was significantly different from that of group I (P< 0·05). † Mean value was significantly different from that of group II (P< 0·05). Group I, control rats fed the standard diet; group II, rats fed the high-cholesterol diet (HCD) (standard diet+1·5 % cholesterol and 0·5 % cholic acid); group III, rats fed the HCD with Njavara rice bran oil (100 mg/kg body weight) mixed in the diet. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Effect of Njavara rice bran oil on atherosclerotic lesions in the aorta
The analysis of haematoxylin and eosin-stained cross-sections of the aortic arch from rats fed the HCD revealed non-uniform aortic wall thickening with moderate endothelial damage accompanied by the thickening of the subendothelial layer. Degenerative changes were found in the whole vascular wall with a loss of normal arrangement of elastic lamellae of the media. In rats fed with the HCD supplemented with NjRBO, the aortic wall was of uniform thickness and endothelial damage was accompanied by a mild thickening of the subendothelial layer. No degenerative changes were observed. The elastic lamellae in the tunica media showed a normal wavy structure. The histopathological data of the aorta are shown in Fig. 4.

Fig. 4 Haematoxylin and eosin-stained cross-sections of the aorta. Group I, the aorta of the control rat; group II, the aorta of rats fed the high-cholesterol diet (HCD); group III, the aorta of rats fed the HCD diet+100 mg/kg Njavara rice bran oil. TI, tunica intima; TM, tunica media with elastic fibres; TA, tunica adventitia. (40 × magnification). A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
To compare the effects of NjRBO on atherosclerotic lesions, the Sudan IV-stained thoracic aorta was compared in all the experimental groups. The atherosclerotic lesion showed a marked increase in HCD-fed rats. The lesion area was decreased in NjRBO-treated rats. The respective images of the aorta from rats exhibiting atherosclerotic lesions are shown in Fig. 5. Atherosclerotic progression was decreased after treatment with NjRBO.

Fig. 5 Effect of Njavara rice bran oil (NjRBO) on atherosclerotic progression, the aorta from the root to iliac bifurcation was dissected free, fixed overnight, spread and stained with Sudan IV. The en face preparations of the aorta from (a) the control group, (b) high-cholesterol diet-fed group and (c) NjRBO-treated group are presented. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Discussion
Njavara is one of the important Indian medicinal rice varieties grown in South India, and is used mainly for the purpose of Ayurvedic treatments( Reference Goffman and Bergman 36 , Reference Nam, Choi and Kang 37 ). It is regarded as a special rice variety with beneficial properties for the circulatory, respiratory, digestive and nervous systems according to the Indian indigenous system of medicine or Ayurveda( Reference Goffman and Bergman 36 ). Njavarakizhi and Navaratheppu are the two major treatments in Ayurveda for arthritis, paralysis, neurological disorders, degeneration of muscles and tuberculosis( Reference Akiri Rao, Sareddy Reddy and Phanithi Babu 38 ). ‘Njavara’ contains significant amounts of oryzanol components, phenolic acids, flavonoids, proanthocyanidins and phytic acid compared with staple varieties, especially the contents of each of the oryzanol components as well as total oryzanol have been found to be significantly higher in Njavara rice bran than those in staple varieties( Reference Mohanlal, Parvathy and Shalini 14 ). As the body of evidence increases, anti-inflammatory and immunomodulatory strategies have now become emerging treatments for targeting the root causes of diseases( Reference Geng and Jonasson 39 ).
It is now generally recognised that atherosclerosis is a chronic inflammatory disease that can lead to acute clinical events following plaque rupture and thrombosis( Reference Steffens, Veillard and Arnaud 40 ). The underlying pathogenesis involves imbalanced lipid metabolism and a maladaptive immune response entailing chronic inflammation of the arterial wall( Reference Weber and Noels 41 ). Therefore, its anti-inflammatory properties and its use in various inflammatory diseases lead to a hypothesis that NjRBO supplementation would alter lipid-related inflammatory and atheroprotective processes critical for limiting the progression of atherosclerosis. Therefore, the objective of the present study was to evaluate the hypolipidaemic properties of NjRBO. The anti-inflammatory effect and dose of NjRBO was assessed by a carrageenan-induced acute inflammatory rat model. Significant inhibition of oedema at the 3rd hour was observed at a dose of 100 mg/kg body weight. Therefore, for subsequent studies, this dose was selected owing to its potent anti-inflammatory property. The anti-atherosclerotic effects were then tested in rat model by HCD supplementation for 60 d. Following the 60 d study, HCD-fed rats showed a significant decrease in HDL-C levels and increased the levels of serum TC, TAG and LDL-C and atherogenic index. A previous study from our laboratory has reported a significant increase in TC, TAG and LDL-C levels after induction with a HCD( Reference Ratheesh, Shyni and Sindhu 42 ). However, their concentrations were significantly decreased in the NjRBO treated group and HDL-C concentration was significantly increased. The concentrations of cholesterol, TAG, NEFA and phospholipids in tissues were significantly higher in HCD-fed rats, but was decreased in the NjRBO-treated group. The decrease in lipids may be due to the decreased synthesis of lipids or increased degradation of lipids. To examine the effect of NjRBO on lipid metabolism and lipoprotein function, activities of some enzymes critical to lipogenesis and lipoprotein metabolism were investigated.
HMG-CoA reductase, is a key enzyme in the endogenous synthesis of cholesterol through two post-transcriptional actions: increasing the controlled degradation of reductase protein and decreasing the efficiency of the translation of HMG-CoA reductase mRNA( Reference Cicero and Gaddi 43 ). The activity of HMG-CoA reductase was found to be increased in the HCD-fed group, but the enzyme activity decreased after treatment with NjRBO. LCAT converts free cholesterol into cholesteryl esters on HDL by a transesterification reaction involving the transfer of a fatty acid at the sn-2 position of phosphatidylcholine, or lecithin, to the free hydroxyl group of cholesterol, and is an important driving force behind the RCT pathway stimulating HDL-mediated efflux. The activity of the enzyme was decreased in the HCD-fed group, but was increased after treatment with NjRBO. These data provide evidence for the ability of NjRBO to help eliminate the accumulated increased amounts of lipid by both inhibiting cholesterol synthesis and enhancing the HDL-mediated removal of excess cholesterol from peripheral tissues, and its delivery to the liver for excretion, resulting in catabolism in a manner that influences atherogenicity.
Lipogenic enzymes are responsible for converting glucose to TAG( Reference Sanz, Diez-Ferncindez and Valverde 44 ). The de novo synthesis of lipids in the cytoplasm of vertebrate tissues requires sources such as acetyl-CoA and reducing equivalents (NADPH) produced by one or more of cytoplasmic dehydrogenases (G6PDH, ME and IDH). ACC1 and fatty acid synthase are in the liver and fatty tissues, and are involved in fatty acid biosynthesis. Therefore, activities of these lipogenic enzymes were studied. G6PDH is a key enzyme that catalyses the first reaction in the pentose phosphate pathway, leading to the production of reducing power in the form of NADPH for reductive biosynthesis. ME, another NADPH-generating enzyme that catalyses the oxidative decarboxylation of l-malate to yield CO2, pyruvate and NADPH, is considered to be a lipogenic enzyme whose activity correlates with de novo fatty acid synthesis. IDH is an enzyme that catalyses the oxidative decarboxylation of isocitrate, producing α-ketoglutarate and CO2, thereby generating NADPH needed for fatty acid synthesis. The activities of liver lipogenic enzymes were significantly affected by diet-induced hypercholesterolaemia. The rats fed the HCD had higher activities of G6PDH, ME, IDH, ACC and fatty acid synthase, indicating the availability of NADPH for the biosynthesis of lipids than the rats treated with NjRBO. Therefore, the results of the experiment showed that supplementation of NjRBO to HCD-fed rats for 60 d decreased the plasma levels of TC, TAG and LDL-C in serum and tissues, suggesting that it might have a role in altering cholesterol metabolism by lowering cholesterol biosynthesis.
CRP, an acute-phase protein, has been suggested to directly induce the inflammatory response leading to the progression of atherosclerosis( Reference Bhaskar, Kumar and Krishnan 45 ) by modulating endothelial function, and its concentration is known to predict cardiovascular events( Reference Kalsait, Khedekar and Saoji 46 ). An extensively used method for evaluating lipid peroxidation is the analysis of tissue TBARS, a product of lipid peroxidation( Reference Ratheesh, Shyni and Sindhu 47 ). Plasma CRP and tissue TBARS levels were significantly enhanced in hypercholesterolaemic-induced rats when compared with the control group. Treatment with NjRBO showed a significant decrease in the levels of CRP and TBARS compared with HCD-fed rats. This shows that NjRBO protects from free radical formation and thereby reduce inflammation. PON1 has been reported to have lipophilic antioxidative properties, which is associated with the ability of the enzyme to protect LDL and HDL from oxidation, to decrease lipid peroxidation caused by free radicals on cell membranes and lipoproteins, and to slow the development of atherosclerosis( Reference Yildirim, Kisa and Karadeniz 48 ). PON1 has three known enzymatic molecules, including paraoxonase, ARE, and dyazoxonase, of which both PON1 and ARE are esterase enzymes that have lipophilic antioxidant characteristics( Reference Erdem, Karatay and Yildirim 33 ). Therefore, PON1 and ARE activities were measured in serum. The HCD significantly decreased the activities of both PON1 and ARE in serum, but were increased after supplementation with NjRBO compared with the control group. These results show that NjRBO supplementation has a role in decreasing oxidative stress by enhancing free radical-scavenging system.
Of the various processes elucidating anti-atherogenic properties, the RCT mechanism relates well to the results presented above with a significant increase in the concentration of HDL-C in groups administered with NjRBO. RCT, a mechanism whereby excess cholesterol is effluxed from lipid-loaded cells, is a key atheroprotective event that counteracts cholesterol uptake( Reference Park, Kim and Lee 49 ). Therefore, we hypothesised that the molecular mechanisms underlying the protective effects of NjRBO may involve the regulation of a set of genes important in RCT. Elevated concentration of apoB-100, the only protein component of LDL-C, is recognised as a risk factor for the development atherosclerotic coronary artery disease( Reference Young 50 ). In the present study, increased concentrations of apoB and LDL-C were also observed in the HCD-fed group, but treatment with NjRBO restores the concentration to a normal level, showing decreased progression of atherosclerosis. Previous reports have suggested that the most effective and direct way to prevent or treat atherosclerosis is to decrease subendothelial apoB-LP retention by lowering apoB-LP in the blood through lifestyle changes and drugs( Reference Brewer, Remaley and Neufeld 51 ). ABCA1 is an essential membrane protein for the initial step of HDL biogenesis by facilitating the efflux of cellular free cholesterol and phospholipid to extracellular lipid-free apoA-I, forming nascent HDL particles( Reference Chung, Sawyer and Gebre 52 ). In the present study, HCD-fed rats showed decreased expression of ABCA1, apoA1 and increased expression of apoB. Hepatic ABCA1 transporter and apoA-I are the major determinants of the plasma levels of α-HDL-C as well as poorly lipidated apoA-I, which interact with ABCA1 transporters on peripheral cells in the process of RCT( Reference Gaidukov and Tawfik 53 ). The major findings of the present study also revealed a significant increase in ABCA1 and apoA1 mRNA and protein expression in the liver of rats administered with NjRBO as well as lower TC, TAG and LDL-C levels in serum and tissues, suggesting increased cholesterol efflux. ABCA1 and apoA1 expression correlates well with serum HDL-C concentration that was found to be increased in rats administered with NjRBO. These findings suggest enhanced an effect of NjRBO in mediating the efflux of cholesterol, thereby enhancing the cardioprotective effect.
The therapeutic efficiency of NjRBO in advanced established atherosclerosis was also confirmed by histological quantification of atherosclerotic lesions and Sudan staining in the aorta after 60 d of HCD induction and treatment. Atherosclerotic lesions were clearly detected in the aortic roots of HCD-fed rats. More advanced vascular lesion formation was observed in rats fed the HCD, but progression of atherosclerotic lesions was significantly reduced in the NjRBO-treated group. Endothelial injury, characterised by atherosclerotic progression, was observed in HCD-fed rats. Reduced vascular lesion and endothelial damage was observed after treatment with NjRBO. Similar results were also observed on Sudan staining of the aorta. This suggests that lipid accumulation was greater in HCD-fed rats, which was reduced in the NjRBO-treated groups.
The transcriptional factor PPARα is highly expressed in tissues with high rates of fatty acid catabolism, and PPARα activators increase RCT by accelerating the efflux of cholesterol from peripheral cells and by increasing its uptake into the liver through a pathway involving increased vascular expression of HDL-C receptors, apoA1, ATP-binding cassette transporter-I and scavenger receptor class-B type-I( Reference Steinberg, Glass and Witztum 54 – Reference Ghanbari-Niaki, Ghanbari-Abarghooi and Rahbarizadeh 56 ). The present investigation attempted to confirm that whether induction of PPARα was responsible for up-regulation of ABCA1. PPARα expression was enhanced in the liver of rats treated with NjRBO, whereas the expression was decreased in rats fed the HCD. This indicates that increased PPARα expression in the NjRBO-treated group shows increased oxidised LDL uptake, and increased ABCA1 indicates the metabolic fate of the LDL taken in. Considerable evidence also shows that PPAR up-regulates PON1 expression in a variety of clinical and experimental situations( Reference Paragh, Harangi, Seres, Mackness, Mackness, Aviram and Paragh 57 , Reference Camps, García-Heredia and Rull 58 ). Several experimental data show that the physiological function of PON1 is to hydrolyse oxidised lipids and, therefore, function as an antioxidant enzyme( Reference Paragh, Harangi, Seres, Mackness, Mackness, Aviram and Paragh 57 , Reference Camps, García-Heredia and Rull 58 ). The present study also showed increased PON1 expression after treatment with NjRBO and decreased after the induction with HCD. In these experiments, we found that NjRBO supplementation enhanced the expression of PPARα and PON1 along with decreased lipid peroxidation. This was confirmed by the decrease in the concentration of TBARS in tissues. Therefore, we hypothesise that the increased PPAR gene expression observed in the present study in the NjRBO-treated group would be associated with increased HDL synthesis, which accords with increased hepatic PON1 gene expression, suggesting that cardioprotective nature of NjRBO could be due to its effect on transcriptional factors associated with cholesterol efflux. The supplementation of NjRBO through the diet clearly reduced atherosclerotic progression, exerting its potent hypocholesterolaemic activity. The depletion of apoB and enhanced apoA1 in the circulation clearly explains the impact of NjRBO on lipid metabolism, which is supported by reduced plaque, endothelial damage and lipid accumulation in the NjRBO-treated group. The protective effect of Njavara in various inflammatory conditions is known, but no scientific data are available on the molecular mechanisms by which the plant offers protection. This is the first report on molecular mechanisms of cardioprotection by NjRBO. The present data support treatment of the atherosclerotic condition by NjRBO from the medicinal rice in imparting a healthy lifestyle.
Conclusion
In summary, this is the first report of the regulation of genes of RCT, providing a plausible explanation for the hypolipidaemic effect of NjRBO in hypercholesterolaemic rats, owing protection against progression and development of atherosclerosis. This is also supported by the decreased lipid profile and biosynthesis of lipids in tissues evidenced by decreased activities of lipogenic enzymes. The present data presented exhibit the possible hypolipidaemic mechanisms that explain the cardioprotective properties of NjRBO. Since there is a strong relationship between lipid accumulation and inflammatory cytokines in atherogenesis, there is a need to explore the action of NjRBO on inflammation and hence atherosclerosis.
Acknowledgements
The present study was supported by a UGC-funded project (UGC file no. 40/196/2011 (SR)).
The authors' responsibilities were as follows: C. K. P. and A. H. designed and conducted the research; G. S. and V. S. helped in conducting the research; R. P. and A. J. helped with the extraction of the oil; C. K. P. and A. H. wrote the paper and performed the statistical analysis of the data. All authors read and approved the final manuscript.
None of the authors has a conflict of interest to declare.















