The prevalence of obesity continues to rise in the USA, and the Centers for Disease Control and Prevention estimate that more than one-third of US adults and approximately 12·7 million children and adolescents are obese(Reference Hurt, Kulisek and Buchanan1). The obesity epidemic has led to increasing rates of obesity-related pathological conditions, including CVD, type 2 diabetes, insulin resistance and certain cancers where disturbed lipid metabolism and chronic low-grade inflammation serve as the causal mediators in pathogenesis(Reference Hurt, Kulisek and Buchanan1,Reference Ruiz-Nunez, Pruimboom and Dijck-Brouwer2) . The accumulation of excess fat in adipose tissue promotes macrophage infiltration, which leads to the release of pro-inflammatory cytokines inducing low-grade inflammation(Reference Surmi and Hasty3,Reference Guilherme, Virbasius and Puri4) . There is an increasing need to identify food components with lipid-modulating and anti-inflammatory capabilities that effectively combat obesity-associated metabolic abnormalities and inflammation.
The consumption of fruits and vegetables has been suggested as a preventative measure of overweight and obesity(Reference Tetens and Alinia5). More specifically, anthocyanins, a type of polyphenols copiously present in certain berries with well-known antioxidant activity, have been reported to provide various health benefits for preventing obesity-related conditions, including insulin resistance, CVD and cancer(Reference Jennings, Welch and Spector6–Reference Thoppil, Bhatia and Barnes8). The American cranberry (CR; Vaccinium macrocarpon) is a rich source of polyphenols, including anthocyanins(Reference Côté, Caillet and Doyon9). Previous in vitro experiments suggest that CR may have anti-bacterial(Reference Huang, Nikolic and Pendland10), anti-carcinogenic(Reference Sun and Liu11) and anti-inflammatory properties(Reference McKay and Blumberg12). Kim et al.(Reference Kim, Ohn and Kim13) reported the potential anti-inflammatory ability of CR as the group observed reduced serum inflammatory markers in rats supplemented with freeze-dried CR powder. Anhê et al.(Reference Anhê, Roy and Pilon14) suggested CR extract might improve insulin sensitivity in diet-induced obese mice. However, CR research on metabolic dysfunctions and inflammation remains limited. In particular, there remains a gap in the evaluation of CR consumption on white adipose tissue morphology and functionality related to inflammation. Furthermore, although studies have shown that CR consumption increases plasma HDL-cholesterol concentrations in humans(Reference Ruel, Pomerleau and Couture15) and animals(Reference Kim, Jung and Kim16), mechanisms for the HDL-cholesterol-raising effect of CR have been limitedly understood. Therefore, the objective of this study was to assess lipid-modulating, with a primary focus on HDL metabolism, and anti-inflammatory effects of freeze-dried whole CR powder using mice expressing human apo A-I transgene (hApoAITg), which have similar HDL profiles to those of humans(Reference Rubin, Ishida and Clift17).
Materials and methods
Animal care and diet
hApoAITg mice (C57BL/6J background) were purchased from Jackson Laboratory. At 8 weeks of age, male hApoAITg mice were randomly assigned and fed ad libitum either a modified American Institute of Nutrition-93M high-fat/high-cholesterol control diet (16 % fat, 0·25 % cholesterol by weight, n 15) or the high-fat/high-cholesterol diet supplemented with CR (5 % whole CR powder by weight, n 16). The number of animals (n 15/group) was estimated based on the animal study that showed that 5 % of whole CR supplementation significantly increased plasma HDL-cholesterol levels(Reference Kim, Jung and Kim16) at an 80 % power with a two-tailed level of significance at P = 0·05. The standardised CR powder was kindly provided by Future Ceuticals. The experimental diet composition is listed in Table 1. The supplemental CR level (5 %) is equivalent to about 220 g of CR/d for humans(Reference Reagan-Shaw, Nihal and Ahmad18). Mice were housed under a 12 h light–12 h dark cycle with ad libitum access to food and water for 8 weeks of the feeding period. Body weight and food consumption were recorded weekly. At the end of the feeding period, mice were fasted for 4 h and subsequently anaesthetised by ketamine (110 mg/kg) and xylazine (10 mg/kg) (Henry Schein Animal Health). Blood was collected by performing cardiac puncture and centrifuged at 1500 g for 10 min for serum collection. Tissue samples, including the liver, small intestine, epididymal white adipose tissue (eWAT), soleus muscle and brown adipose tissue, were harvested and snap-frozen in liquid N2 for gene analysis or fixed in 10 % formalin for histological evaluations. Serum and tissue samples were stored at −80°C until use. The Institutional Animal Care and Use Committee at the University of Connecticut approved all animal procedures.
Table 1. Composition of the modified American Institute of Nutrition (AIN)-93M high-fat and high-cholesterol diet (HF/HC) and HF/HC supplemented with 5 % cranberry (CR) powder (g/kg diet)

Serum chemistry
Serum total cholesterol (TC) and TAG concentrations were enzymatically determined using Cholesterol Reagent (Pointe Scientific) and L-Type TG M kit (Wako Chemical), respectively, as we previously reported(Reference Kim, Ku and Pham19). Serum HDL-cholesterol concentration was measured after the precipitation of apoB-containing lipoproteins by using HDL-cholesterol precipitating Reagent Set (Pointe Scientific), and serum non-HDL-cholesterol was calculated by subtracting HDL-cholesterol from TC. Precinorm L (Roche) was used as a control. Serum levels of human apoA-I were measured using a human apoA-I ELISA kit (Thermo Scientific). Fluorometric lecithin–cholesterol acyltransferase (LCAT) and phospholipid transfer protein activity assay kits (Sigma Aldrich) were used to determine serum LCAT and PLTP activity, respectively.
Quantitative real-time PCR
Total RNA was extracted from tissue samples using TRIzol reagent (Invitrogen), and quantitative real-time PCR analysis was performed using SYBR Green and CFX96 real-time PCR detection system (Bio-Rad) as we previously described(Reference Yang, Seo and Nguyen20,Reference Ku, Pham and Park21) . Glyceraldehyde 3-phosphate dehydrogenase served as an internal control.
Histological analysis of the liver and epididymal white adipose tissue
Formalin-fixed liver and eWAT tissue sections were embedded in paraffin and cut into 5 μm sections for haematoxylin–eosin staining. All staining procedures were conducted at the Connecticut Veterinary Medical Diagnostic Laboratory (CVMDL). Tissue images were then viewed at various magnifications using an Axio Observer A1 microscope equipped with an AxioCam MRc camera (Carl Zeiss).
Statistical analysis
Unpaired t tests were conducted using GraphPad Prism 6.0 (GraphPad Software) to determine significant differences between groups. All data were considered statistically significant when the P value was <0·05 and are presented as mean values with their standard errors.
Results
The effect of cranberry supplementation on body weight and serum lipids
Control and CR-fed mice showed a gradual increase in body weight, but their body weights did not significantly differ between groups throughout the feeding period (Fig. 1(a)). Food intake was not different between groups (data not shown). Serum TC, non-HDL-cholesterol and TAG levels were significantly higher in the CR group than the control group, while there was no significant difference in serum HDL-cholesterol levels from each other (Fig. 1(b) and (c)). Also, serum apoA-I levels did not significantly differ between groups (Fig. 1(d)). While serum LCAT activity was significantly lower in the CR group than the control, serum phospholipid transfer protein activity was not significantly different between groups (Fig. 1(e) and (f)).

Fig. 1. Effect of cranberry (CR) supplementation on body weight and serum lipids in human apo A-I transgene (hApoAITg) mice. Male mice were fed a high-fat/high-cholesterol control or a high-fat/high-cholesterol diet containing 5 % CR by weight for 8 weeks. (a) Body weight of mice during the experimental period. ![]() , Control;
, Control; ![]() , CR. (b) Serum concentration of total cholesterol (TC), non-HDL-cholesterol (non-HDL-C) and HDL-cholesterol (HDL-C). (c) Serum TAG levels. (d) Serum apoA-I. (e) Serum lecithin–cholesterol acyltransferase (LCAT) activity. (f) Serum phospholipid transfer protein (PLPT) activity. Data are mean values with their standard errors (n 15–16 per group). * Significantly different from control (P < 0·05).
, CR. (b) Serum concentration of total cholesterol (TC), non-HDL-cholesterol (non-HDL-C) and HDL-cholesterol (HDL-C). (c) Serum TAG levels. (d) Serum apoA-I. (e) Serum lecithin–cholesterol acyltransferase (LCAT) activity. (f) Serum phospholipid transfer protein (PLPT) activity. Data are mean values with their standard errors (n 15–16 per group). * Significantly different from control (P < 0·05).
The effect of cranberry supplementation on lipid metabolism in the liver and small intestine
Although liver weight tended towards an increase in the CR group compared with the control group, these two groups showed similar hepatic lipid accumulation (Fig. 2(a) and (b)). Hepatic expression of cholesterol metabolism-associated genes, such as LDL receptor (Ldlr), scavenger receptor class B type I, apoA-I and Lcat, was significantly reduced by CR supplementation, but no significant difference in ATP-binding cassette transporter A1 (Abca1) mRNA levels between groups was found (Fig. 2(c)). Also, the hepatic expression of sterol regulatory element-binding transcription factor 1c (Srebf-1c) showed a trend towards lower expression in the CR group than control, but there were no significant differences in the mRNA abundance of other lipogenic genes, such as fatty acid synthase (Fas), and stearoyl CoA desaturase 1, as well as genes related to fatty acid β-oxidation, for example, carnitine palmitoyltransferase 1α (Cpt-1α) and acyl-CoA oxidase 1 (Acox-1) (Table 2). Furthermore, the expression of genes responsible for intestinal cholesterol and lipoprotein metabolism, such as apoA-I, liver X receptor α, Abca1, Niemann-Pick C1-like 1, 3-hydroxy-3-methylglutaryl coenzyme A reductase and Ldlr, was not significantly altered by CR supplementation (Table 2).
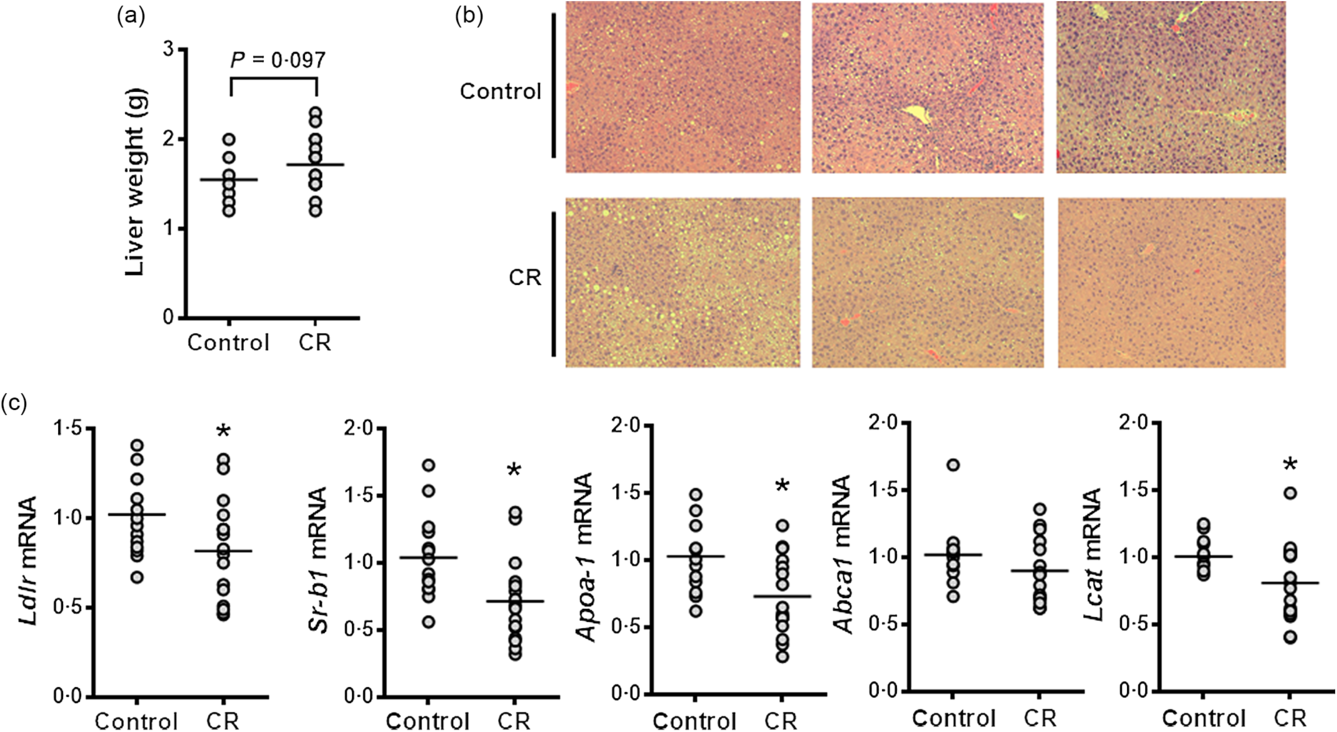
Fig. 2. Effect of cranberry (CR) supplementation on hepatic lipid accumulation and gene expression in human apo A-I transgene (hApoAITg) mice. Male mice were fed a high-fat/high-cholesterol control or a high-fat/high-cholesterol diet containing 5 % CR by weight for 8 weeks. (a) Liver weight. (b) Representative images of liver sections stained with haematoxylin and eosin. (c) Hepatic mRNA expression of genes involved in cholesterol metabolism. n 15–16 per group. * Significantly different from control (P < 0·05). Ldlr, LDL receptor; Sr-b1, scavenger receptor class B type I; Abca1, ATP-binding cassette transporter A1; Lcat, lecithin–cholesterol acyltransferase.
Table 2. Gene expressions in the liver, small intestine and brown adipose tissue (BAT) of human apo A-I transgene (hApoA-ITg) mice fed high-fat/high-cholesterol diet (HF/HC) and HF/HC supplemented with cranberries (HF/HC-CR) for 8 weeks (fold of control)
(Mean values with their standard errors)
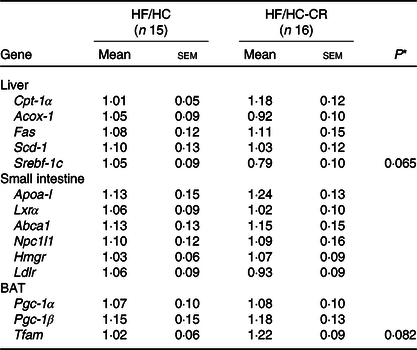
Cpt-1α, carnitine palmitoyltransferase 1α; Acox-1, acyl-CoA oxidase 1; Fas, fatty acid synthase; Scd-1, stearoyl CoA desaturase 1; Srebf-1c, sterol regulatory element-binding transcription factor 1c; Apoa-I, human apo A-I; Lxrα, liver X receptor α; Abca1, ATP-binding cassette transporter A1; Npc1l1, Niemann-Pick C1-like 1; Hmgr, 3-hydroxy-3-methylglutaryl coenzyme A reductase; Ldlr, LDL receptor; Pgc-1α, PPARγ coactivator; Tfam, mitochondrial transcription factor A.
* P values indicate significant difference from HF/HC control (P < 0·05).
The effect of cranberry supplementation on lipid metabolism and inflammation in epididymal white adipose tissue
While eWAT weight was significantly higher in the CR group than the control, the expression of genes involved in fatty acid synthesis, such as Srebf-1c and Fas, in the eWAT was significantly reduced by CR supplementation (Fig. 3(a) and (b)). Also, the expression of fatty acid β-oxidation genes, such as Cpt-1α, and Acox-1, was significantly lower in the CR group compared with the control mice (Fig. 3(c)). Mice on the CR diet had significantly lower eWAT mRNA levels of F4/80, a macrophage marker, than those of the control, although the expression of M1 macrophage marker, for example, chemokine (C-C motif) ligand 2 (Ccl-2), was not altered by CR supplementation (Fig. 3(d)). Furthermore, histological analysis of eWAT revealed more macrophage infiltration in the control group than the CR group (Fig. 3(e)).
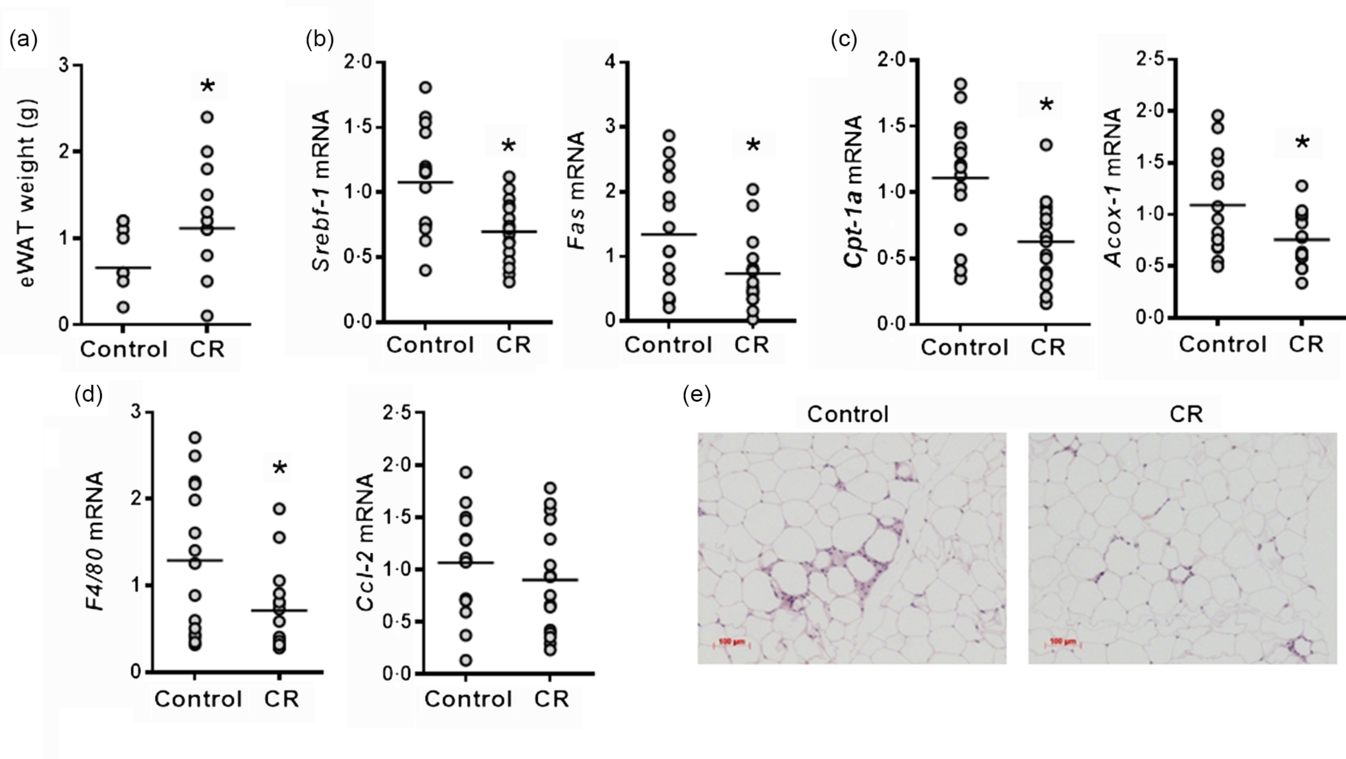
Fig. 3. Effect of cranberry (CR) supplementation on lipid metabolism and inflammation in epididymal white adipose tissue (eWAT) of human apo A-I transgene (hApoAITg) mice. Male mice were fed a high-fat/high-cholesterol control or a high-fat/high-cholesterol diet containing 5 % CR by weight for 8 weeks. (a) eWAT weight. (b) eWAT mRNA expression of genes related to fatty acid synthesis. (c) eWAT mRNA expression of fatty acid β-oxidation genes. (d) eWAT mRNA expression of inflammatory genes. (e) Representative images of eWAT sections stained with haematoxylin and eosin. n 15–16 per group. * Significantly different from control (P < 0·05). Srebf-1c, sterol regulatory element-binding transcription factor 1c; Fas, fatty acid synthase; Cpt-1a, carnitine palmitoyltransferase 1α; Acox-1, acyl-CoA oxidase 1; Ccl-2, chemokine (C-C motif) ligand 2.
The effect of cranberry supplementation on the expression of energy utilisation genes in soleus muscle and brown adipose tissue
Fatty acid oxidation and mitochondrial biogenesis in skeletal muscle and brown adipose tissue have a substantial impact on energy expenditure along with eWAT(Reference Zhao, Huang and Du22). Therefore, we next evaluated the effect of CR on the expression of genes related to fatty acid β-oxidation and mitochondrial biogenesis in soleus muscle and brown adipose tissue. The mRNA abundance of fatty acid β-oxidation genes, that is, Cpt-1a, Cpt-1b, Acox-1 and mitochondrial uncoupling protein 3 (Ucp-3) was significantly higher in the CR group than the control group, while Ucp-2 mRNA tended towards an increase in the CR group compared with the control group in the soleus muscle (Fig. 4(a)). The mRNA levels of Ppara and PPARδ (Ppard), transcription factors involved in fatty acid oxidation and uncoupling, were significantly higher in the mice fed CR than control mice (Fig. 4(b)). Lipoprotein lipase (Lpl), an enzyme that hydrolyses TAG in lipoproteins so that NEFA can be taken into muscle, showed a significantly higher expression in the CR group than the control group (Fig. 4(c)). While the expression of a mitochondrial biogenesis gene, mitochondrial transcription factor A (Tfam), in soleus muscle was significantly increased by CR compared with the control group, there were no significant differences in the expression of PPARγ coactivator 1α (Ppargc-1a) and Pgc-1β (Ppargc-1b), which are genes important for mitochondrial biogenesis(Reference Villarroya, Iglesias and Giralt23) (Fig. 4(d)). Serving as transcriptional regulators to UCP-2 and UCP-3 expression, the PPAR promote lipid metabolism in WAT.

Fig. 4. Effect of cranberry (CR) supplementation on energy utilisation genes in the soleus muscle of human apo A-I transgene (hApoAITg) mice. Male mice were fed a high-fat/high-cholesterol control or a high-fat/high-cholesterol diet containing 5 % CR by weight for 8 weeks. (a) mRNA expression of genes for fatty acid β-oxidation and uncoupling. (b) Pparα (Ppara) and Pparδ (Ppard) mRNA. (c) Lipoprotein lipase (Lpl) mRNA. (d) mRNA abundance of genes involved in mitochondrial biogenesis. n 15–16 per group. * Significantly different from control (P < 0·05). Cpt-1a, carnitine palmitoyltransferase 1α; Cpt-1b, carnitine palmitoyltransferase 1β; Acox-1, acyl-CoA oxidase 1; Ucp-2, uncoupling protein 2; Ucp-3, uncoupling protein 3; Ppargc1a, PPARγ coactivator 1α; Ppargc1b, PPARγ coactivator 1β; Tfam, mitochondrial transcription factor A.
In the brown adipose tissue, Tfam expression showed a trend towards an increase in the CR group compared with the control group, but mRNA levels of Pgc-1α and Pgc-1β were not significantly different between the two groups (Table 2).
Discussion
The development of pathological conditions, including CVD, is an apparent manifestation of an obese state due to high lipid burden at adipose tissue and consequent low-grade inflammation(Reference Bastien, Poirier and Lemieux24). To meet a growing need for the identification of foods capable of preventing and/or ameliorating dyslipidaemia and chronic inflammation, the first objective of the current study was to assess the effects of CR consumption on HDL metabolism of hApoAITg mice. Also, our second goal was to determine the potential anti-inflammatory effects of CR in metabolically active tissues, including the liver, eWAT and skeletal muscle in hApoAITg mice. Although CR consumption has been shown to increase plasma HDL-cholesterol levels in humans(Reference Ruel, Pomerleau and Couture15) and animals(Reference Kim, Jung and Kim16), we did not find the noteworthy effects of CR on HDL metabolism. However, CR consumption mitigated obesity-induced inflammation in WAT.
The effect of CR consumption on circulating TAG levels is controversial. In a double-blind, placebo-controlled, parallel-arm human study performed by Novotny et al. (Reference Novotny, Baer and Khoo25), serum TAG levels were significantly lower in the treatment group than in the placebo group after having consumed 240 ml of low-energy CR juice twice daily for 8 weeks. However, in a similar study lasting for 28 d, Caron et al. (Reference Caron, Kautza and Wilson26) did not find alterations in TAG levels in the low-energy CR juice treatment group. In the present study, we did not observe a hypolipidemic effect of CR powder. Instead, significantly greater serum TAG were found in the CR group than in the control mice. Our results show apo-B-containing cholesterol was significantly higher in the CR-fed mice than in the control mice without significantly altering TC. Kim et al.(Reference Kim, Jung and Kim27) reported that rats fed an atherogenic diet supplemented with 5 % freeze-dried CR powder tended to have greater TAG levels compared with the control animals. They also observed a tendency towards higher LDL-cholesterol levels in CR-fed rats than in control, although no difference in TC was found between the groups. The discrepancy between studies on the effects of CR on serum TAG, TC and LDL-cholesterol suggests that CR may have different effects on serum lipid profile depending on the amount supplemented, the form of consumption, and animal species.
The prevention of monocyte recruitment and consequent inflammation in WAT is essential to preventing the development of obesity-related pathological conditions, including insulin resistance(Reference Guilherme, Virbasius and Puri4). Despite no changes in body weight, CR supplementation prevented eWAT inflammation, as the degree of macrophage infiltration was lower compared with the control group. In the CR group, mRNA level of macrophage marker F4/80 was significantly reduced, while a trending decrease and increases in the mRNA levels of Ccl-2 and an anti-inflammatory cytokine, IL-10 (data not shown), were observed. Macrophage infiltration may be morphologically determined by the presence of crown-like structures in WAT(Reference Murano, Barbatelli and Parisani28). Crown-like structures obtain their name from the manner in which macrophages surround metabolically stressed adipocytes and consume dying cells. Macrophage infiltration was detected in both the CR-fed and control mice; however, the infiltration tended to be more severe in the control mice, which is in accordance with the F4/80 and Ccl-2 expression levels. These results were observed without a significant difference in average adipocyte diameter between the groups (data not shown), possibly suggesting that WAT inflammation may be partly influenced by factors additional to adipocyte hypertrophy, including the rate of hyperplasia or overall storage capacity of WAT(Reference Lee, Wu and Fried29).
We previously demonstrated that altered expression of metabolic genes in skeletal muscle might promote an anti-inflammatory condition in WAT by decreasing macrophage infiltration through increased energy utilisation at the muscle(Reference Benn, Kim and Park30). In the soleus muscle, we found that mRNA abundances of genes involved in β-oxidation, including Cpt-1a, Cpt-1b and Acox-1, were significantly greater in mice fed CR than the control mice. Furthermore, inductions of Ppara, Ppard and Lpl expression were greater in the CR-fed mice compared with the control. Ucp-3 expression significantly increased in the CR-fed mice, with Ucp-2 tending to increase in the CR group compared with the control mice. Uncoupling proteins disrupt the coupling of electron transport and oxidative phosphorylation and therefore stimulate substrate oxidation with energy dissipated as heat rather than supporting ATP synthesis(Reference Jia, Zhang and Ge31). The benefits of uncoupling are reported by Clapham et al.(Reference Clapham, Arch and Chapman32) that demonstrated protection against obesity with lower fasting serum glucose and insulin in mice overexpressing Ucp-3 in skeletal muscle. The expression of genes involved in mitochondrial biogenesis was also measured with Tfam expression significantly increased in the CR-fed mice and a tendency towards an increase in Pgc-1α and Pgc-1β compared with the control mice. Also, we found that the expression of metabolic genes, including Fas, Srebf-1c, Acox-1 and Cpt-1a, was significantly down-regulated in the CR-fed mice in WAT. Although further investigations are required to directly attribute CR consumption to increases in the expression of metabolic genes in skeletal muscle, these results suggest CR may have the potential to influence WAT inflammation via energy modulation in skeletal muscle.
ApoA-I is the major protein component of HDL and is primarily expressed in the liver and small intestine(Reference Haas, Mazza and Wong33). It plays a key role in reverse cholesterol transport by serving as the initial acceptor of unesterified cholesterol exported by ABCA1 from peripheral cells, including foam cells(Reference Curtiss, Valenta and Hime34). The importance of apoA-I is highlighted by the finding that low levels of apoA-I associate more strongly with CVD risk than HDL-cholesterol levels(Reference Florvall, Basu and Larsson35). Moreover, overexpression of apoA-I has proven to promote macrophage-specific reverse cholesterol transport(Reference Zhang, Zanotti and Reilly36). Interestingly, we found that hepatic apoA-I expression was significantly decreased in the CR-fed mice, while no significant difference was found in the serum apoA-I level. To the best of our knowledge, we are the first that reports an effect or lack thereof of whole CR powder on circulating apoA-I levels. We attribute the lack of modification in the serum apoA-I level to an insufficient length and/or amount of treatment. Although administered in a different form, Shidfar et al. (Reference Shidfar, Heydari and Hajimiresmaiel37) reported a significant increase in serum apoA-I in male type 2 diabetic patients who had received a cup of CR juice per d for 12 weeks. Therefore, another investigation with increased dose or duration using whole CR powder may be required to observe a potential increase in serum apoA-I. The decrease in apoA-I expression in the liver of CR-fed mice is unlikely to result from CR directly and is more plausibly related to the inflammatory response. The reciprocal effects of apoA-I on WAT metabolism and function, including its anti-inflammatory effects, are generally accepted(Reference Sultana, Cochran and Tabet38,Reference Vanella, Li and Kim39) . Umemoto et al. (Reference Umemoto, Han and Mitra40) demonstrated anti-inflammatory effects of apoA-I using cultured adipocytes and adipocytes from obesity-induced hApoAITg mice. It is possible that the decreased expression of hepatic apoA-I in the CR-fed mice could have resulted from a decreased necessity for apoA-I due to less adipocyte stress. Alternatively, the decreased expression of apoA-I in the liver of CR-fed mice may be explained by the adipocyte’s ability to recycle apoA-I. Several investigators have reported the adipocyte’s ability to internalise and secrete apoA-I(Reference Verghese, Arrese and Howard41,Reference Howard, Verghese and Arrese42) . Although the clinical significance of apoA-I recycling by adipocytes remains unclear, apoA-I may be present in adipocytes in the absence of its mRNA abundance(Reference Wang, Peng and Yi43). This peripheral tissue-dependent transcription of apoA-I in the liver (and possibly small intestine) is supported by the fact that the main function of apoA-I is to mediate reverse cholesterol transport from peripheral tissues. Therefore, the decreased expression of apoA-I in the CR-fed mice of the present study may have resulted from a decreased demand in adipocytes mediated by apoA-I recycling.
This study is among the first to investigate the potential health benefits of CR supplementation by altering HDL metabolism and chronic inflammation. The effects of CR supplementation on HDL metabolism were minimal in our study. However, we found that CR consumption prevents chronic inflammation in WAT, although exact mechanisms of action are not clear. Future investigations are required to identify the underlying mechanism of actions by which CR exerts such an anti-inflammatory effect in other diet-induced obesity mouse models, such as mice fed a diet rich in fat and sucrose.
Acknowledgements
This work was supported by a Nutricia Research Foundation grant to J. L.
C. C., M. B., T. X. P., Y. L., S. H., E. N. O. and B. K. carried out experiments and performed data analysis; C. C., M.-B. K., Y.-K. P. and J.-Y. L. wrote the manuscript and J.-Y. L. designed and led the study. All authors read the manuscript.
All authors claim no conflicts of interest.
Supplementary material
For supplementary materials referred to in this article, please visit https://doi.org/10.1017/S0007114520004080









