It is critical for the establishment of the epigenome that during gametogenesis and embryogenesis the epigenome experience elimination and reconstruction twice, respectively( Reference Reik, Dean and Walter 1 ). Consequently, these two periods, which are vulnerable to the environment (such as nutrition, climate and so on), are the focus of epigenetics studies( Reference Cai, Jia and Lu 2 , Reference Skinner, Haque and Nilsson 3 ). Unlike in mammals, in poultry, embryonic development is mainly separated from the matrix. It is therefore difficult to estimate the effect of nutrients on embryogenesis if nutrients exhibit little or no accumulation in eggs. Currently, in ovo feeding (IOF) is gradually operational( Reference Kornasio, Halevy and Kedar 4 , Reference Zhai, Neuman and Latour 5 ) and it can thus be used to evaluate the effect of specific nutrients on epigenome reprogramming, providing an effective approach to the study of nutri-epigenetics.
Folic acid, a water-soluble B-complex vitamin, also known as pteroylglutamic acid, has a critical role in one-carbon metabolism( Reference Choi and Mason 6 , Reference Zhao, Chen and Chen 7 ). Similarly, folic acid is a central co-enzyme for both DNA methylation and DNA synthesis( Reference Bailey and Gregory 8 ). Methylenetetrahydrofolate reductase (MTHFR; EC 1.5.1.20), a key enzyme in folate metabolism, has a key role in both biological methylation and nucleotide synthesis, balancing these two pathways to preserve normal homoeostasis( Reference Zhao, Chen and Chen 7 , Reference Fox and Stover 9 ). In addition, methionine synthase reductase (MTRR), another pivotal enzyme, is essential for folate and homocysteine metabolism( Reference Sharp and Little 10 ).
As a methyl group, folate functions as a ground substance for the formation of methionine, which can be converted to S-adenosylmethionine (SAM)( Reference Bailey and Gregory 8 ). In the methionine metabolic cycle, SAM is further converted to S-adenosylhomocysteine, thereby donating the methyl group for DNA and protein methylation, and finally changing chromatin conformation( Reference Cai, Jia and Lu 2 ). Consequently, folate supplementation may modulate gene expression by modifying epigenetic marks such as histone methylation and chromatin conformation.
Folate insufficiency is associated with the clinical syndrome including CVD and intra-uterine growth retardation( Reference Boushey, Beresford and Omenn 11 , Reference Kalra 12 ), whereas folate supplementation may result in decreased abnormal embryologic development by increasing fetal folate concentrations and supporting normal embryonic DNA synthesis during gestation( Reference Thaler, Nelssen and Goodband 13 ). Folate, as a methyl donor, is able to reverse the epigenetic marks and thereby recover the metabolic disorders in offspring caused by prenatal or neonatal abnormal development( Reference Cai, Jia and Lu 2 , Reference Lillycrop, Rodford and Garratt 14 , Reference Weaver, Champagne and Brown 15 ). It has also been observed that folate supplementation can enhance the immunity of domestic animals( Reference Delaney, Garg and Fernandes 16 – Reference Protiva, Mason and Liu 18 ). However, although folate is fundamental for embryonic and fetal development, whether IOF of folic acid can influence the growth performance, folate metabolism and immune function of broilers has not been well investigated.
The present experiment was therefore undertaken to evaluate the effects of folic acid injection during incubation on the growth performance and immune function of broilers. Simultaneously, to explore the potential epigenetic mechanisms underlying such effects, we studied the splenic expression of immune effector molecules and determined the status of conformation and histone methylation on their promoters. The information obtained in this study can therefore be useful for understanding the role of folate on growth and immunity adjustment in poultry.
Methods
Animals and sampling
Incubation
A total of 400 fertilised broiler (Cobb 500) eggs were selected with an average weight of 65·7 g. Eggs were randomly assigned to four groups – 0, 50, 100 and 150 µg of folic acid – and each group was uniformly assigned to five incubator trays. The microcomputer automatic incubator (9TV-2A; Beijing LanTianJiao Electronic Technology Co., Ltd) was calibrated before hatching. The inner temperature of the incubator was controlled at 38·0–38·2°C from d1 to 10, at 37·8–38·0°C from d11 to 18 and at 37·5–37·8°C from d19 to 21. The humidity was maintained at 45–65 %. A 270° overturn of the eggs was continued for 3 min every 2 h until d19. On d3 and 10, all eggs were candled, after which infertile and dead eggs were removed. Levels of folic acid were injected into the yolk sac at embryonic age 11 d, and the injection volume of each egg was 0·1 ml. The incubation period was 21 d.
The hatchability of fertilised eggs (%) was calculated as follows: (the number of hatchlings/the number of fertilised eggs)×100.
Feeding
Each treatment was allocated to six replicates with 12 broilers/replicate after incubation. The composition of the experimental diet is shown in Table 1. All birds were placed in three-layer wired battery cages and housed in an environmentally controlled room maintained at 32–34°C for the first week and then reduced by 2–3°C/week. The relative humidity was set at 50 % throughout the study, and the lighting programme was 23 h of light for the first 2 weeks and 20 h thereafter. Birds were given free access to commercial diet and water. Feeds were offered as pellets in the whole phase, and a disc automatic feeding system was used to avoid feed spillage. The feeding period lasted for 42 d.
Table 1 Ingredients and nutrient composition of broiler diets on fed basis(52)
CP, crude protein; ME, metabolisable energy.
* Premix provided per kg of feed: vitamin A, 8000 IU; vitamin D3, 2500 IU; vitamin K3, 2·65 mg; vitamin B1, 2 mg; vitamin B2, 6 mg; vitamin B12, 0·025 mg; vitamin B12, 0·025 mg; vitamin E, 30 IU; biotin, 0·0325 mg; pantothenic acid 12 mg; niacin, 50 mg; Cu, 8 mg; Zn, 75 mg; Fe, 80 mg; Mn, 100 mg; Se, 0·15 mg; I, 0·35 mg.
Sampling
On d1, 21 and 42, birds were first weighed in bulk, and then one bird approximate to the average body weight (BW) was selected from each replicate and weighed after fasting for 12 h. Blood samples were taken from the wing vein, and the plasma samples were obtained by centrifugation at 2500 rpm for 10 min at 4°C and then frozen at −20°C for analysis. After the blood sampling, broilers were electrically stunned and executed by exsanguination and necropsied immediately. Spleen and liver were removed within 15 min postmortem, snap-frozen in liquid N2 and stored at −80°C until further analysis.
The experimental protocol was in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals (Ministry of Science and Technology, China, revised 2004) and approved by the Institutional Animal Care and Use Committee (College of Animal Science and Technology, Northwest A&F University, China).
Measurement of growth performance
The BW was recorded for each replicate on d1, 21 and 42; concomitantly, the feed intake was recorded. BW, average daily gain (ADG), average daily feed intake (ADFI) and feed conversion ratio (FCR) were then calculated.
Determination of hepatic folate content and plasma lysozyme activity, IgG and IgM concentration
A microbial method was used to determine the hepatic folate content( Reference Hoppner and Lampi 19 ). Briefly, approximately 200 mg of liver tissue was homogenised and diluted to 10 ml with phosphate buffer solution (PBS) (0·02 mol/l, pH 7·2) containing 50 mg of ascorbate. An aliquot of 0·2 ml of homogenate was autoclaved with buffer for 25 min, and then 1 ml of chicken pancreas extract (6 mg/ml) was added (Difco), further diluted to 10 ml with PBS and incubated overnight (37°C). After centrifugation (3000 rpm, 10 min), a further 5-fold dilution was made for the supernatant before addition to the assay tubes. The folate content in the culture medium has a direct relationship with the growth and propagation of Lactobacillus casei ATCC 7469, and bacterial multiplication is computed by the optical density (OD) value. After an overnight incubation at 37°C, the turbidity was measured at 540 nm. The folate contents were calculated and expressed as µg/g of liver tissue.
The lysozyme activity and the concentration of IgG and IgM in plasma were measured using commercially available kits (A050-1, E026 and E025, respectively; Nanjing Jiancheng Bioengineering Institute) according to the manufacturer’s instructions.
Real-time PCR for mRNA quantification
The total RNA was extracted from tissue samples using a total RNA extraction kit (9767; Takara) according to the manufacturer’s protocol. Total RNA was quantified using the NanoDrop® ND-1000 spectrophotometer (Thermo Scientific) with the OD value at 260 nm, and the purity was assessed by determining the OD260:OD280 ratio and formaldehyde-agarose gel electrophoresis. The complementary DNA (cDNA) was synthesised with a PrimeScript® RT reagent Kit (Takara) according to the protocols provided by the manufacturer. All cDNA samples were stored at −20°C until use. The gene (β-actin, MTHFR, MTRR, IL-2, IL-4 and IL-6) expressions were performed with SYBR® Premix Ex TaqTM II (RR8R; Takara). Briefly, a 25-µl PCR mixture was prepared as follows: 12·5 µl of SYBR® Premix Ex Taq II (2×), 1 µl of forward primer (10 µm/l), 1 µl of reverse primer (10 µm/l), 1 µl of cDNA and 9·5 µl of double-distilled water. Primers for real-time PCR were synthesised by Sangon Biotech, and they are listed in Table 2. The PCR was conducted on an iCycler iQ5 multicolour real-time PCR detection system (Bio-Rad Laboratories) programmed as follows: 95°C for 10 min; 40 cycles of 95°C for 10 s, 60°C for 30 s, 72°C for 30 s; and 72°C for 5 min. All samples were run in triplicate, and the average cycle threshold (C
t
) values were used for quantification using the
![]() $$2^{{{\minus}{\triangle}{\triangle}C_{t} }} $$
method(
Reference Livak and Schmittgen
20
).
$$2^{{{\minus}{\triangle}{\triangle}C_{t} }} $$
method(
Reference Livak and Schmittgen
20
).
Table 2 Primer sequences for quantitative real-time PCR analysis

MTHFR, methylenetetrahydrofolate reductase; MTRR, methionine synthase reductase; MNase, micrococcal nuclease; ChIP, chromatin immunoprecipitation; qPCR, quantitative PCR.
Micrococcal nuclease quantitative PCR assay for promoter conformation
Briefly, the cell nuclei were extracted from spleen tissue using a nucleus extraction kit (CN1100; Solarbio) first, and then a suitable dose of micrococcal nuclease (MNase) (N3755; Sigma) was selected to digest these cell nuclei. Next, the histone was digested by proteinase K to obtain DNA. Last, the PCR was conducted with a 20-μl mixture system containing the following: 10 µl of SYBR® Premix Ex Taq II (2×), 1 µl of forward primer (10 µm/l), 1 µl of reverse primer (10 µm/l), 1 µl of DNA and 7 µl of double-distilled water. The primers used are listed in Table 2. The PCR cycle conditions were set as follows: 95°C for 15 min, followed by 10 cycles of 95°C for 15 s, 70°C for 30 s and 72°C for 45 s, and then followed by another 35 cycles of 94°C for 15 s, 60°C for 30 s and 72°C for 45 s. All samples were run in triplicate, and the analytical method used for MNase-quantitative PCR data was previously reported( Reference Ling and Waxman 21 , Reference Shu, Gruissem and Hennig 22 ).
Chromatin immunoprecipitation assay for histone methylation
DNA combined with the dimethylation of histone H3 lysine 4 (H3K4me2) or H3K9me2 was prepared using the EpiQuikTM Tissue Methyl-Histone H3K4 (H3K9) chromatin immunoprecipitation (ChIP) kit (P2009 and P2008, respectively; Epigentek). Next, quantitative PCR was performed to determine the accessibility to promoters of genes (IL-2, IL-4 and IL-6). A reaction system (25 μl) was as follows: Quantitect (2×), 10 μl; primer mix (5 µm/l), 2 μl; DNA, 2 μl; and double-distilled water, 11 μl. Primer sequences used were all obtained from GenBank and are listed in Table 2. The reaction protocol was as follows: 95°C for 15 min, followed by 10 cycles of 95°C for 15 s, 70°C for 30 s and 72°C for 45 s, followed by another 35 cycles of 94°C for 15 s, 60°C for 30 s and 72°C for 45 s. All samples were run in triplicate, and a fold enrichment method (http://www.lifetechnologies.com/) was used to analyse the ChIP-qPCR data.
Statistical analysis
The hatchability of fertilised eggs was analysed using binary logistic regression with the SPSS statistical software (version 18.0, SPSS Inc.). All other data were analysed by one-way ANOVA and polynomial regression analysis using the general liners model with the SPSS statistical software (version 18.0). Pearson’s correlation analysis test (SPSS statistical software; version 18.0) was used to analyse the relationship between the expression of IL-2, IL-4 and IL-6 with that of in vivo immune response and epigenetic changes. Significant differences between the treatments were determined using Fisher’s least significant difference test. Results were presented as means with their standard errors. Differences in treatment means were considered significant at P<0·05, and instances in which 0·05<P<0·10 were considered trends.
Results
Growth performance
The hatchabilities of folic acid treatments (0, 50, 100 and 150 µg) were 79·2, 79·4, 90·9 and 89·6 %, respectively, and the χ 2 value and P value of the logistic model were 9·339 and 0·025, respectively. Compared with the control group, 50 µg of folic acid treatment has no effect (P=0·971) on hatchability, whereas IOF of 100 µg of folic acid increased (P=0·025) the hatchability and an increasing trend (P=0·051) was found in the 150-µg folic acid group.
Although there were no significant differences on ADFI in the different feeding periods, an increasing trend (P=0·061) was observed during d21–42 with the increased amount of folic acid additive (Table 3). During d21–42 and d1–42, ADG showed an increase (P<0·05) and FCR had a significant decrease (P<0·05) in treatment groups injected with 100 and 150 µg of folic acid. It was interesting that the injection of a higher amount of folic acid (150 µg) gave rise to an improved BW on d21 (P<0·05), which was not observed on d42.
Table 3 Effect of in ovo feeding (IOF) of folic acid on the performance of broilers (Mean values with their standard errors)

P A , P value of one-way ANOVA; P L , P value of linear analysis; BW, body weight; ADFI, average daily feed intake; ADG, average daily gain; FCR, feed conversion ratio.
a,b Mean values (n 6) within a row with unlike superscript letters were significantly different (P<0·05).
Folate content in the liver
Compared with the control group, in ovo injection of 50, 100 and 150 µg of folic acid all increased the hepatic content of folate on d21 (P<0·05), and an increase was also observed in the 100- and 150-µg groups on d42 (P<0·05) (Table 4).
Table 4 Effects of in ovo feeding (IOF) of folic acid on folate content in liver of broilers (μg/g) (Mean values with their standard errors)
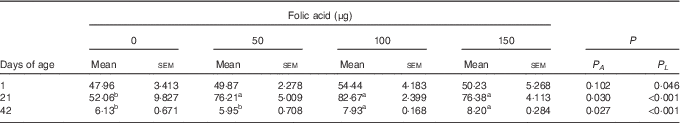
P A , P value of one-way ANOVA; P L , P value of linear analysis.
a,b Mean values (n 6) within a row with unlike superscript letters were significantly different (P<0·05).
Hepatic expression of methylenetetrahydrofolate reductase and methionine synthase reductase
The expression of MTHFR was greater in the 150-µg group on d1 and 42 and in the 50-µg group on d1 (P<0·05) (Fig. 1(a), (b) and (c)), and the mRNA expression level of MTRR exhibited a significant increase in the 100- and 150-µg treatments on d21 (P<0·05) (Fig. 1(b)). However, no differences were found in the expression of MTHFR and MTRR on the other days (P>0·05).

Fig. 1 Effects of in ovo feeding of folic acid on the expression of MTHFR and MTRR on d1 (A), d21 (B) and d42 (C) in livers of broilers. Values are means (n 6), with standard errors represented by vertical bars. Mean values with unlike superscript letters were significantly different (P<0·05). The P values gained by linear analysis (P
A
) of MTHFR on d1, 21 and 42 were <0·001, 0·171 and 0·253, respectively. The P
A
values of MTRR on d1, 21 and 42 were 0·096, <0·001 and 0·275, respectively. MTHFR, methylenetetrahydrofolate reductase; MTRR, methionine synthase reductase. ![]() , 0 mg;
, 0 mg; ![]() , 50 mg;
, 50 mg; ![]() , 100 mg;
, 100 mg; ![]() , 150 mg.
, 150 mg.
Lysozyme activity, and the concentration of IgG and IgM in plasma
The lysozyme activity in plasma exhibited an increase (P<0·05) with increasing folic acid concentrations on d1, 21 and 42 (Table 5). Compared with the saline group, the IgG concentration was substantially improved (P<0·01) in the 100- and 150-µg folic acid groups on d1, 21 and 42. Similarly, on d21 and 42, folate treatments (50, 100 and 150 µg) all showed an increase (P<0·05) in the plasma content of IgM; however, no effect was found on d1.
Table 5 Effects of in ovo feeding of folic acid on plasma parameters of broilers (Mean values with their standard errors)

P A , P value of one-way ANOVA; P L , P value of linear analysis.
a,b Mean values (n 6) within a row with unlike superscript letters were significantly different (P<0·05).
Expression of IL-2, IL-4 and IL-6 in the spleen
In ovo, 150-µg folic acid injection on embryonic age 11 d increased (P<0·05) the expression of IL-2 while decreasing (P<0·05) the expression of IL-6 on d21 and 42 (Fig. 2(b) and (c)) in the spleen. The expression of IL-4 exhibited an increase (P<0·05) in the 100- and 150-µg folic acid treatment groups (Fig. 2(a), (b) and (c)).

Fig. 2 Effects of in ovo feeding of folic acid on the expression of IL-2, IL-4 and IL-6 on d1 (A), 21 (B) and 42 (C) in spleens of broilers. Values are means (n 6), with standard errors represented by vertical bars. Mean values with unlike superscript letters were significantly different (P<0·05). The P values gained by linear analysis (P
A
) of IL-2 on d1, 21 and 42 were 0·955, <0·001 and <0·001, respectively. The P
A
values of IL-4 on d1, 21 and 42 were 0·177, <0·001 and <0·001, respectively. The P
A
values of IL-6 on d1, 21 and 42 all were 0·036, <0·001 and <0·001, respectively. ![]() , 0 mg;
, 0 mg; ![]() , 50 mg;
, 50 mg; ![]() , 100 mg;
, 100 mg; ![]() , 150 mg.
, 150 mg.
Chromatin immunoprecipitation quantitative PCR for the histone methylation of IL-2, IL-4 and IL-6 promoters in the spleen
On both d21 and 42, more enriched H3K4me2 mark (P<0·05; Fig. 3(a)) was detected in the IL-2 promoter in the spleens of broilers hatched to 150-µg folic acid-injected eggs. Meanwhile, in ovo, 100- and 150-µg folic acid injections resulted in an enrichment (P<0·05; Fig. 3(b)) of H3K4me2 mark in the IL-4 promoter. In contrast, the levels of H3K4me2 in the IL-6 promoter exhibited a decrease (P<0·05; Fig. 3(c)) on both d21 and 42 in groups supplemented with 100 and 150 µg of folic acid. Compared with the saline group, all of the folic acid groups, except 50- and 100-µg groups on d21, showed a decrease (P<0·05; Fig. 4(a)) of H3K9me2 mark in IL-2 promoters. All of the folic acid groups showed a decrease (P<0·05; Fig. 4(b)) of H3K9me2 mark in IL-4 promoters. In contrast, the enrichment of H3K9me2 in the IL-6 promoter exhibited a pronounced increase (P<0·05; Fig. 4(c)) with the IOF of folic acid on d21 and 42.

Fig. 3 Effects of in ovo feeding of folic acid on the H3K4me2 enrichment ratio in IL-2, IL-4 and IL-6 promoters on d1 (A), 21 (B) and 42 (C) in spleens of broilers. Values are means (n 6), with standard errors represented by vertical bars. Mean values with unlike superscript letters were significantly different (P<0·05). The P values gained by linear analysis of IL-2, IL-4 and IL-6 on d21 and 42 all were <0·001. H3K4me2, histone H3 lysine 4 dimethylation. ![]() , 0 mg;
, 0 mg; ![]() , 50 mg;
, 50 mg; ![]() , 100 mg;
, 100 mg; ![]() , 150 mg.
, 150 mg.

Fig. 4 Effects of in ovo feeding of folic acid on the H3K9me2 enrichment ratio in IL-2, IL-4 and IL-6 promoters on d1 (A), 21 (B) and 42 (C) in spleens of broilers. Values are means (n 6), with standard errors represented by vertical bars. Mean values with unlike superscript letters were significantly different (P<0·05). The P values gained by linear analysis of IL-2, IL-4 and IL-6 on day 21 and 42 all were<0·001. H3K9me2, histone H3 lysine 9 dimethylation. ![]() , 0 mg;
, 0 mg; ![]() , 50 mg;
, 50 mg; ![]() , 100 mg;
, 100 mg; ![]() , 150 mg.
, 150 mg.
Micrococcal nuclease quantitative PCR for IL-2, IL-4 and IL-6 promoters in the spleen
Folic acid injection during incubation had no effect on the promoter conformation of IL-6 on d21 and 42 (Fig. 5(c)). As shown in Fig. 5(a) and 5(b), however, the tightness of the IL-2 and IL-4 promoter conformation was improved (P<0·05) in the 150-µg treatment group on d21 and 42.

Fig. 5 Effects of in ovo feeding (IOF) of folic acid on chromatin conformation in IL-2, IL-4 and IL-6 promoters on d1 (A), 21 (B) and 42 (C) in spleens of broilers. Values are means (n 6), with standard errors represented by vertical bars. Mean values with unlike superscript letters were significantly different (P<0·05). The P values gained by linear analysis of IL-2, IL-4 and IL-6 on d21 and 42 all were <0·001. ![]() , 0 mg;
, 0 mg; ![]() , 50 mg;
, 50 mg; ![]() , 100 mg;
, 100 mg; ![]() , 150 mg.
, 150 mg.
Polynomial regression analysis
The results of polynomial regression analysis indicated that all of the testing indexes, except IL-2 and IL-4 mRNA expression on d1, MTHFR mRNA expression on d21 and 42, MTRR mRNA expression on d1 and 42, BW on d1 and RCR during d1–21, showed a linear change (P<0·05) with the increased amount of folic acid additive.
Correlation analysis between the expression levels of IL-2, IL-4 and IL-6 with that of in vivo immune response and epigenetic changes
The results of correlation analysis proved that the expression levels of IL-2 and IL-4 were positively correlated with in vivo immune response (the lysozyme activity and the concentration of IgG and IgM in plasma), as well as H3K4me2 on their promoters, whereas they were negatively correlated with H3K9me2 and chromatin conformation on their promoters (Table 6). In contrast, the expression of IL-6 was negatively correlated with in vivo immune response and H3K9me2 and chromatin conformation on its promoter, whereas it was positively correlated with H3K4me2 on its promoter (Table 6).
Table 6 Correlations between the expression of IL-2, IL-4 and IL-6 with that of in vivo immune response and epigenetic changes

* Correlation is significant at the 0·05 level (two-tailed).** Correlation is significant at the 0·01 level (two-tailed).
Discussion
In the present study, IOF of folate caused a significant increase in hepatic expression of MTHFR and MTRR in broilers. MTHFR participates in one-carbon metabolism by converting 5, 10-methylenetetrahydrofolate to 5-methylenetetrahydrofolate, which is the primary circulating form of folate( Reference Choe, Lee and Jung 23 , Reference Tomita, D’Almeida and Villa 25 ). In addition, MTRR is essential in maintaining adequate intracellular folate pools. Simultaneously, MTRR participates in catalysing the re-methylation of homocysteine to methionine and is required for the production of SAM( Reference Sharp and Little 10 ). These two enzymes both serve as central roles in folate metabolism. Therefore, the incremental expression of MTHFR and MTRR observed in this study indicates that in ovo folic acid supplementation may speed up the circulation of folate in broilers.
Lysozyme, as a nonspecific immunity factor, has an important role in maintaining the physiological equilibrium of organisms through strengthening the phagocytosis of macrophages and leucocytes( Reference Krusteva, Hristova and Damyanov 26 ). Similar to lysozyme, Ig is also vital for humoral immunity. The results presented herein demonstrate that, in plasma, at all of the concentrations of lysozyme IgG and IgM exhibited an increase in folic acid-exposed broilers, suggesting that in ovo folic acid supplementation improves the immunity of broilers. In addition, treatment with 1·2 mg of folate daily for 12 weeks was associated with higher levels of proteins (serum albumin, IgM and complement protein) involved in the activation and regulation of immune function in humans( Reference Duthie, Horgan and de Roos 27 ).
Cytokines, as immune regulatory proteins, have an important role in the immune system( Reference Wu, Cui and Peng 28 ) as well. Among these proteins, IL-2, produced by T helper (Th1) cells, regulates cellular immune responses, and IL-4, produced by Th2 cells, participates in mediating the humoral immune response( Reference Scott 29 ). IL-6 was originally identified as a B lymphocyte differentiation factor that regulates the immune response, haematopoiesis and inflammation( Reference Ishihara and Hirano 30 ). Dietary methyl-donor supplementation has been shown to diminish IL-6 levels in plasma while elevating IL-2 concentrations in mice( Reference Delaney, Garg and Fernandes 16 ). In agreement with these previous findings, we found a predominant up-regulation of IL-2 and IL-4 and a pronounced down-regulation of IL-6 in the spleens of the folate-exposed groups. IL-2 and IL-4 have key roles in anti-inflammation, and IL-6 is a pro-inflammatory cytokine. The dynamic balance of anti-inflammation and pro-inflammatory cytokine levels is critical for the health maintenance within animals. Previous researches revealed an attenuated expression of IL-6 in lipopolysaccharide-treated mice( Reference Zhao, Chen and Chen 7 , Reference Zhao, Chen and Dong 31 ). In addition, the combination of melatonin and folate was reported to reduce the markers of liver injury by CCl4 and the levels of inflammatory cytokines( Reference Ebaid, Bashandy and Alhazza 32 ). These previous findings are consistent with the effect of folate on immune maintenance and inflammation suppression( Reference Feng, Zhou and Xia 17 , Reference Gonda, Kim and Salas 33 ), and changes in folate delivery will create substantial differences in gene expression in immune response and human colorectal carcinogenesis( Reference Protiva, Mason and Liu 18 ). Moreover, the results of correlation analysis indicated that the discrepant splenic expression of IL-2, IL-4 and IL-6 may cooperate with the elevatory plasma concentrations of lysozyme, IgG and IgM on immunity enhancement of folic acid-exposed broilers.
The results presented above only demonstrate the different regulation of immune effector molecules at the transcription level in the spleens of folic acid-exposed broilers. Gene expression and function are also modulated through epigenetic modification( Reference Waterland and Michels 34 ). Research indicates that maternal dietary exposure can lead to subtle variations in the epigenetic regulation of immune genes expression, which can potentially lead to more profound effects on subsequent immune function, clinical phenotype and disease risk( Reference Hollingsworth, Maruoka and Boon 35 ). Histone modifications are vital mechanisms involved in the transcriptional regulation of genes( Reference Yun, Wu and Workman 36 ). For instance, H3K4me2 participates in euchromatin formation and ongoing gene expression( Reference Pekowska, Benoukraf and Zacarias-Cabeza 37 ). In the present experiment, higher H3K4me2 levels were found on the promoters of IL-2 and IL-4 in the spleens of broilers exposed to folate, and these levels coincided with the up-regulation of these two genes at the mRNA level; furthermore, the diminished IL-6 gene expression was in accordance with less enriched H3K4me2 on its promoter. Contrary to H3K4me2, H3K9me2 is a repressive histone mark that negatively regulates transcription by promoting a compact chromatin structure( Reference Pekowska, Benoukraf and Zacarias-Cabeza 37 ). The current study, indeed, revealed more enriched H3K9me2 on the IL-6 promoter. Concomitantly, the decreased H3K9me2 implies the possible involvement of the transcriptional regulation of IL-2 and IL-4 genes in the spleens of broilers in response to embryonic folate exposure. Therefore, it appears that IOF of folic acid regulates the expression of cytokines by adjusting the histone modification status on their promoters, thus promoting the immune function of broilers.
According to our understanding, the chromatin remodelling caused by the histone modification status of genes’ promoters may affect the binding of specific transcriptional factors, thereby regulating the transcriptional levels of these genes( Reference Lee, Erdjument-Bromage and Tempst 38 ). Furthermore, the positive regulation of transcription is associated with a more loose promoter conformation with higher accessibility( Reference Beato and Eisfeld 39 , Reference Biddie, John and Sabo 40 ), and vice versa. To our surprise, in the current study, we found an incongruity between gene expression and promoter conformation for IL-2, IL-4 and IL-6. Interestingly, the up-regulation of IL-2 and IL-4 mRNA expression was accompanied by its reduced promoter accessibilities, which showed a negative correlation. In addition, no differences were found among different treatments on IL-6 chromatin conformation. Epigenetics is an advanced biological system that selectively uses genomic information and is involved in various fundamental phenomena, among which exist extensive functional interactions( Reference Holliday 41 ). Thus, a complex physiological process may be regulated partially or altogether by these mechanisms( Reference Goldberg, Allis and Bernstein 42 ), even leading to an abnormal phenomenon caused by diverse action times and/or action sites, which is beyond our comprehension at first glance. For instance, choline is a methyl donor, but choline-deficient embryos exhibited global and Igf2 DMR2 DNA hypermethylation in a regulatory CpG within the Dnmt1 gene concomitant with the induction of Dnmt1 expression in rats, and the prenatal choline supply increased Igf2 mRNA levels rather than inhibiting gene expression through methyl supply( Reference Kovacheva, Mellott and Davison 43 ).
Obviously, the current study has some limitations. First, folic acid has a vital role in the maintenance of normal patterns of DNA methylation, which is important for cellular homoeostasis( Reference Choi and Mason 6 , Reference Siegfried and Simon 44 ). The present study did not investigate DNA methylation on related immunogenes. Second, epigenetics is involved in various fundamental phenomena; therefore, further studies are necessary to reveal the internal cause of the incongruity between gene expression and promoter conformation caused by embryonic folate supplementation.
In addition, we also found that IOF of folic acid led to a significant improvement in BW, ADG and FCR at the late growing stage of broilers. Folate is a potential antioxidant with the capacity for oxyradical scavenging( Reference Letelier, Jara-Sandoval and Molina-Berríos 45 ) and is essential for immunity maintenance( Reference Duthie, Horgan and de Roos 27 ), and these attributes would help birds cope with more immunological and oxidative stress during the late period. Furthermore, folate has an important role in poultry viability and growth, and folate insufficiency may affect the survival and growth of poultry( Reference Lee, Belcher and Miller 46 ). Therefore, more folate is needed to promote high-intensity growth and overcome more stress in the late stage of broilers.
In the current study, folic acid-exposed eggs also demonstrated a higher hatchability. Researches have shown that the hatchability and poultry weight are associated with folate content in the eggs( Reference Hebert, House and Guenter 47 ), and higher supplemental folic acid levels may be required for rapid embryonic development( Reference Snetsinger, Waibel and Siccardi 48 , Reference Robel 49 ). Although commercial poultry breeder diets contain supplemental folic acid, we do not consider folate as a limiting factor for embryonic development or hatchability, as this inference fails to consider the transport of folate to the egg( Reference Robel 49 ). IOF can directly convey and deposit nutrients into eggs, avoiding a plateau value of folate levels within the eggs( Reference Hebert, House and Guenter 47 ). In the present experiment, the concentration of folate in the liver positively responded to increasing levels of IOF folic acid (0–150 µg). Moreover, hepatic folate content on d42 showed a greater decrease than that on d1 and 21, indicating that folate was efficiently used in the late growing period of birds, and this could be associated with the marked improvement in performance only during the second stage of broilers.
The chicken (Gallus gallus) is an important animal model that bridges the mammals and vertebrates in evolution and has long been used as a model species for the study of embryology, immunology, behaviour and reproduction( Reference Burt and Pourquie 50 , Reference Li, Li and Hu 51 ). Although mostly descriptive, the results presented herein provide the first evidence that in ovo folic acid injection causes various changes in the splenic expression of immune effector molecules in broilers, with the involvement of epigenetic modifications including histone methylation and chromatin conformation. To some extent, these findings may help in the understanding of the role of embryonic folate supplementation on embryology and immunology in humans.
In summary, in ovo folic acid injection can improve the hatchability and growth performance in the late growth stage of broilers. The increase of hepatic folate concentration, as well as the expression of MTHFR and MTRR, demonstrates the promotion of folate metabolism. IOF of folate also enhances immune function via increasing plasma lysozyme activity and IgG and IgM concentrations, increasing the splenic expression of IL-2 and IL-4, and down-regulating the expression of IL-6, and the differential expression of immune effector molecules can be regulated by the histone modification of H3K4me2 and H3K9me2 and chromatin conformation in their promoters. Moreover, within the ranges of this research, the 150-µg treatment group was the best considering comprehensively all of the testing indexes.
Acknowledgements
This study was supported by the Natural Science Foundation of China (No.31272464), the Programme for New Century Excellent Talents (NCET-12-0476) and the Program for Shaanxi Youth Scientific Talents (2012KJXX-18). None of these funders had a role in the design, analysis or writing of this article. The authors are grateful to the staff at Animal Nutrition and Feed Science of Northwest A&F University for their assistance in conducting the experiment.
We express our sincere gratitude to Dr Kai Liu and Dingkui Qin for their suggestions and revisions of the paper.
The authors’ contributions are as follows: S. L., L. Z., X. Y. and J. Y. designed the research; S. L., L. Z., Y. L., J. S. and L. L. performed the research and analysed the data; S. L. and L. Z. wrote the manuscript; X. Y. and J. Y. participated in the revision of the manuscript. S. L. and L. Z. contributed equally to this work, and all authors contributed to the data interpretation and approved the final version of the manuscript.
The authors declare that no conflict of interest exists.














