Clinical studies have shown that the increase in postprandial TAG response may predict the incidence of coronary artery disease(Reference Hyson, Rutledge and Berglund1). The magnitude of the postprandial TAG response is determined by several factors such as fasting TAG concentration, age, sedentary lifestyle, sex(Reference Roche and Gibney2) and genetic isoforms in, for example, apoE(Reference Kobayashi, Saito and Taira3, Reference Cardona, Morcillo and Gonzalo-Marin4). Nutritional factors, including dietary fat composition and dose also affect the magnitude of the postprandial lipidaemic response(Reference Murphy, Isherwood and Sethi5–Reference Zampelas, Murphy and Morgan7). Recently, postprandial hypertriacylglycerolaemia has been reported to induce endothelial activation both in hypertriacylglycerolaemic subjects(Reference Giannattasio, Zoppo and Gentile8) and healthy men(Reference Berry, Tucker and Banerji9), partly due to the increased production of pro-inflammatory cytokines seen during the postprandial TAG phase(Reference van Oostrom, Rabelink and Verseyden10). Postprandial inflammation has thus emerged as a vital concept in human nutrition(Reference Margioris11, Reference Burdge and Calder12).
One of the primary steps in the atherosclerotic process involves adherence of circulating monocytes to the endothelium followed by secretion of proteins involved in the immune response(Reference Libby13). Chronic inflammation in the endothelium results in endothelial dysfunction and ultimately atherogenesis. Repeated exposure of pro-inflammatory cytokines and pro-oxidants to the blood vessel wall may damage the endothelium and promote atherogenesis(Reference Libby13).
Peripheral blood mononuclear cells (PBMC) include monocytes and lymphocytes and are central in inflammation. Activation of PBMC by different stimuli such as fatty acids will enable them to alter the expression and release of cytokines and thereby the atherosclerotic process. It is well known that fatty acids act as ligands for the nuclear receptors PPAR(Reference Kliewer, Sundseth and Jones14) or may modulate the activity of Toll-like receptors(Reference Erridge and Samani15, Reference Wong, Kwon and Choi16); both mechanisms involve the NK-κB pathway and can thereby modulate the expression of pro-inflammatory factors(Reference Hong and Tontonoz17).
Moderate or no effects on circulating inflammatory markers have been observed after the intake of a single meal with different fat quality(Reference Poppitt, Keogh and Lithander18–Reference Jimenez-Gomez, Lopez-Miranda and Blanco-Colio21). Gene expression profiling of PBMC from single-meal studies has demonstrated that different fat qualities influence oxidative stress(Reference Bouwens, Grootte and Jansen22) and inflammatory responses(Reference Jimenez-Gomez, Lopez-Miranda and Blanco-Colio21, Reference Camargo, Ruano and Fernandez23). PBMC may therefore be an appropriate model system to study postprandial inflammatory effects.
The aim of the present study was to examine the effect of consuming a single high-fat meal with different fatty acid composition on circulating inflammatory markers and gene expression in PBMC to further elucidate the role of fat quality on postprandial inflammation.
Subjects and methods
Subjects
In the postprandial study, sixteen healthy females were recruited among students at Akershus University College in October 2008. Due to events unrelated to the study, two dropped out after the first test day and they were therefore not included in the analysis. Of the subjects, three performed two of the three test days. The participants in the ex vivo experiments were recruited separately and consisted of eight healthy subjects (three females and five males). Baseline characteristics for both study populations are shown in Table 1. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Regional Committee of Medical Ethics in Norway. Written informed consent was obtained from all subjects.
Table 1 Baseline variables for participants in the postprandial study and the ex vivo experiment
(Medians and interquartile ranges (IQR))

Postprandial study design
In the postprandial study three different test meals were consumed in a fixed order and all test days were separated by 2 weeks. All the participants ate the same test meal on the same test day, but they were not informed which test meal they were eating. They were told not to change their diet during the study period. The subjects were served at the University College an identical standardised low-fat meal (22 % energy from fat) as dinner the day before each of the test days. In addition, the subjects were instructed to eat a light supper at home, no later than 20.00 hours. This meal was also identical before each test day. The subjects had to abstain from alcohol 24 h before the test meal, and did not eat anything 12 h before blood sampling. On the morning of each test day body weight was measured and a fasting blood sample was taken before the test meal. The test meals were served at 08.00–09.00 hours and were eaten within 20 min. Postprandial blood samples were taken 3 and 6 h after the beginning of the test meals. Subjects consumed nothing but water, kept their physical activity to a minimum and did not leave the college during the test meal day. The subjects were served a dinner after the last blood sample was taken.
Test meals
The three test meals consisted of a 150 g chocolate cake which were prepared in three batches and stored at − 20°C until the day of consumption. Each cake was prepared in order to contain the same amount of energy and to contain the same percentage of energy from protein, total fat and carbohydrates (Table 2). The percentage of energy from fat was high (67–70 %). In general, the same amount and the same ingredients were used in the preparation of each test meal except that the amount of coconut fat, soyabean oil, linseed oil and cod liver oil differed in each cake, giving them different fatty acid compositions. Coconut fat was used as a source for saturated fat because of its high content of saturated fat (91–94 % SFA). In the coconut cake the amount of coconut fat was 29 g and the amount of soyabean oil was 11 g (in total 40 g fat). This cake had the highest content of coconut fat and had a high content of saturated fat and a low level of n-3 polyunsaturated fat. In the linseed cake the amount of coconut fat was 17 g and the amount of linseed oil was 23 g (in total 40 g fat). This cake had a lower level of saturated fat and 14 % energy from n-3 polyunsaturated fat, mainly from α-linolenic acid (ALA; 18 : 3n-3). In the cod liver oil cake the amount of coconut fat was 17 g, the amount of linseed oil was 8 g and the amount of cod liver oil was 15 g (in total 40 g). This cake had a lower level of saturated fat and 10 % energy from n-3 polyunsaturated fat in a combination of ALA, EPA (20 : 5n-3) and DHA (22 : 6n-3). The vitamin E content in the oils was 0·12 mg/g, 0·12 mg/g and 2 mg/ml in the soyabean oil, linseed oil and cod liver oil, respectively. The peroxide value in the baked cakes was 0·3, 0·4 and 0·7 mekv/kg in the coconut cake, linseed cake and cod liver oil cake, respectively. The anisidine value in the baked cakes was 1·9, 2·2 and 4·4 in the coconut cake, linseed cake and cod liver oil cake, respectively. The peroxide and anisidine values remained unchanged in the baked cakes compared with the raw cakes. These values are within the European Pharmacopeia recommendation for oxidation products in fish oils intended for human consumption(24). The fatty acid composition, the content of macronutrients and the anisidine and peroxide values were analysed by Eurofins Norsk Matanalyse (Oslo, Norway) in duplicate portions of each test meal.
Table 2 Nutritional values of the three test meals
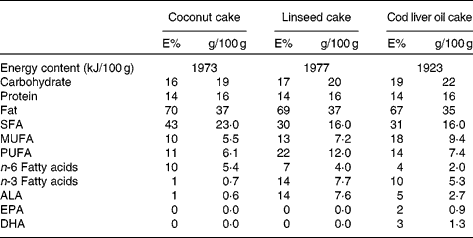
E%, energy percentage; ALA, α-linolenic acid.
Blood sampling and analysis
Blood from venepuncture was collected after an overnight fast ( ≥ 12 h) and at 3 and 6 h after consumption of the test meals. Serum was obtained from silica gel tubes (BD Vacutainer; Becton, Dickinson and Co., Franklin Lakes, NJ, USA), kept at room temperature for at least 30 min until centrifugation at 1300 g for 12 min at room temperature. Plasma was obtained from EDTA tubes (BD Vacutainer; Becton, Dickinson and Co.), kept on ice immediately and within 12 min centrifuged at 1500 g for 10 min at 10°C. Standard blood chemistry and lipid variables were measured in serum or plasma using routine laboratory methods at Oslo University Hospital. Serum levels of C-reactive protein (CRP) were measured by a high-sensitivity immunoturbidimetric assay (Roche Diagnostic, Indianapolis, IN, USA). The intra-assay and inter-assay variations for CRP were 1·34 and 5·3 %, respectively. The cytokine levels were analysed with Bio-Plex Pro Human Cytokine Assay as previously described utilising a Bio-Plex instrument based on Luminex xMAP technology (Bio-Rad Laboratories Inc., Hercules, CA, USA)(Reference Lehto, Niskanen and Herzig25). The assay conditions were standardised and pre-optimised to ensure optimal reproducibility of the assays, and the kit was performed according to the manufacturer's instructions. The plasma samples were centrifuged for 10 min at 13 300 g before the analysis and diluted 1:2 in an appropriate sample matrix. The results were automatically calculated with Bio-PlexManager Software™ version 4.3 with five-parameter logistic equations (Bio-Rad, Hercules, CA, USA). High-sensitivity range standard settings were utilised. The calculated intra- and inter-assay variations from additional reference samples were 5·13 and 24·4 % for the IL-6 analysis and 4·97 and 18·78 % for the IL-8 analysis, respectively.
Peripheral blood mononuclear cell isolation and culturing
After blood collection, PBMC were isolated by using the BD Vacutainer Cell Preparation tubes according to the manufacturer's instructions (Becton, Dickinson and Co.) (postprandial study) or obtained by heparinised blood by gradient centrifugation in Isopaque-Ficoll (Lymphoprep; Nycomed, Oslo, Norway) (PBMC ex vivo study). Pellets were frozen and stored at − 80°C for further RNA isolation. For the ex vivo experiments the cells were incubated in flat-bottomed twenty-four-well trays (Costar, Corning Inc., Corning, NY, USA; 8 × 106 per ml; 250 μl per well) in medium (Roswell Park Memorial Institute (RPMI)-1640 with 2 mm-l-glutamine; Gibco; Invitrogen Corp., Carlsbad, CA, USA) supplemented with 5 % fetal calf serum (F7524; Sigma Aldrich, St Louis, MO, USA) with one of the following fatty acids: ALA or EPA (Sigma Aldrich) complexed to fatty acid-free bovine serum albumin at a molar ratio of 2·5:1 at a concentration of 60 μm or with medium and bovine serum albumin alone (unstimulated). Cell lysate and cell-free supernatant fractions were harvested after 24 h for further gene expression analyses and cytokine release experiments, respectively, and stored at − 80°C. The level of lipid peroxides in the conditioned medium as well as in the fatty acid stock solutions was measured in a pilot study before the ex vivo experiments. The level of lipid peroxides was below detection level (data not shown). In addition, no sign of toxicity in the cells after incubation with ALA or EPA, as measured by lactate dehydrogenase activity, was observed (data not shown). The level of endotoxins in the conditioned medium and in the fatty acid stock solutions was also measured and was below detection level ( < 10 pg/ml) (data not shown).
Gene expression analysis
Total RNA was isolated from all PBMC samples using an RNeasy mini kit (QIAGEN, Hilden, Germany) and lysis buffer containing β-mercaptoethanol according to the manufacturer's instructions and stored at − 80°C. RNA quantity and quality measurements were performed using the ND 1000 Spectrophotometer (Saveen Werner, Malmö, Sweden) and Agilent Bioanalyser (Agilent Technologies, Santa Clara, CA, USA), respectively. From two subjects in the postprandial study PBMC were not retrieved on all three test days. Therefore the numbers of subjects in the gene expression analyses were twelve, eleven and ten for the coconut, linseed and cod liver oil cakes, respectively. In addition, on test day 2, 3 h after intake of linseed oil cake, a problem occurred during the blood sampling and therefore not enough blood for PBMC isolation was retrieved from one participant. In the ex vivo experiment study not enough RNA was retrieved from one subject. All RNA samples had an RNA integrity number > 8. A quantity of 500 ng RNA (TaqMan single assays; Applied Biosystems, Foster City, CA, USA) or 400 ng RNA (TaqMan Low-Density Array cards; Applied Biosystems) from all samples was reverse transcribed by a high-capacity cDNA reverse transcription kit (catalogue no. 4387406; Applied Biosystems). Quantitative real-time PCR (Q-RT-PCR) was performed on an ABI PRISM 7900HT Sequence Detector System (Applied Biosystems). For Q-RT-PCR in the postprandial study, we used inventoried TaqMan gene expression assays (catalogue no. Hs00174103_m1, Hs00174097_m1 and Hs01115512_m1, for IL-8, IL-1β and PPARγ, respectively) and the following housekeeping genes glucoronidase β (GUSβ) and TATA box binding protein (TBP) (inventoried TaqMan gene expression assays catalogue no. Hs99999908_m1 and Hs00427620_m1, respectively; Applied Biosystems). In the ex vivo experiments custom-designed TaqMan Low-Density Array cards were used for Q-RT-PCR amplification of the target genes IL-8 (Hs99999034_m1), IL-6 (Hs00174131_m1), IL-1β (Hs00174097_m1), PPARγ (Hs00602622_ml), and the housekeeping genes TBP (Hs00427620_m1) and GUSβ (Hs99999908_m1) (inventoried TaqMan gene expression assays; Applied Biosystems). For both studies, carnitine palmitoyltransferase-1A (CPT1A) (Hs00912681_m1) was measured using TaqMan Low-Density Array cards. The relative mRNA level for each transcript was calculated by the ΔΔcycle threshold (Ct) method(Reference Livak and Schmittgen26). Briefly, the Ct values for each target gene were normalised against the mean of the Ct values for the two housekeeping genes GUSβ and TBP ( = ΔCt). ΔΔCt was then calculated as ΔCt at 3 or 6 h after fasting minus ΔCt at fasting level (0 h) or as ΔCt for stimulated cells minus ΔCt for unstimulated cells (PBMC ex vivo experiments). The fold change in mRNA expression was calculated as 2− ΔΔCt.
Statistical analysis
In the postprandial study, each subject consumed three test meals and was used as their own reference. Non-parametric statistics were used throughout the study due to the low number of participants. Data are given as median (interquartile range). The significance of the difference between the meals at 3 and 6 h and between time points for each test meal was assessed with Friedman's ANOVA, followed by the Wilcoxon matched-pairs test using Δ serum changes (between meals) or exact serum values (between time points) and regarding mRNA, fold change from fasting values (between meals) or fold change compared with housekeeping genes (between time points). Probability values (exact, two-tailed) were considered significant at values of P < 0·05. All calculations were performed using SPSS (version 17.0; SPSS, Inc., Chicago, IL, USA). Missing values in the Wilcoxon matched-pairs test analysis were excluded test-by-test.
In the ex vivo experiments the data are given as median (interquartile range). The significant of difference between groups was assessed with the Wilcoxon matched-pairs test using fold change in mRNA expression compared with unstimulated cells or percentage change compared with unstimulated cells. Probability values (exact, two-tailed) were considered significant at values of P < 0·05. All calculations were performed with the use of SPSS (version 16.0; SPSS, Inc.).
Results
Postprandial changes in lipids and glucose
All three test meals significantly increased the plasma TAG concentration at 3 h (Table 3), whereas no significant difference in the TAG concentration at 6 h after intake of either of the three test meals was observed compared with fasting. Furthermore, the increase in plasma TAG concentration at 3 h did not differ between the meals. No changes in serum glucose were observed after 3 h (Table 3); however, serum glucose was significantly reduced at 6 h compared with at 3 h after intake of the linseed cake. The reduction in serum glucose at 6 h after intake compared with fasting differed significantly between the coconut cake and the linseed cake (Table 3). Finally, there was a significant increase in plasma total cholesterol at 6 h after intake of the coconut cake and cod liver oil cake compared with both fasting levels and 3 h after meal consumption (Table 3). The increase in plasma total cholesterol did not differ between the meals.
Table 3 Biochemical variables at baseline and after intake of coconut cake, linseed cake or cod liver oil cake¶
(Medians and interquartile ranges (IQR))

* P < 0·05 v. baseline in linseed cake.
† 3 h v. 6 h.
‡ 0 h v. 6 h.
§ 0 h v. 3 h.
∥ Change 6 h v. 0 h between coconut cake and linseed cake.
¶ P values between meals are calculated on Δ values (change from fasting 0 h).
Postprandial changes in inflammatory markers
To investigate whether intake of a single high-fat meal could elicit different inflammatory responses, we measured circulating levels of CRP, IL-6 and IL-8. No significant differences were observed in the circulating levels of CRP and IL-8 either between the meals or after different time points (Table 3). However, the plasma concentration of IL-6 was significantly increased 6 h compared with 3 h after intake of linseed cake. No other significant changes in plasma IL-6 were observed (Table 3).
Gene expression changes in peripheral blood mononuclear cells
Circulating PBMC may alter gene expression as a response to the acute change in the environment. We therefore analysed the mRNA expression level of the pro-inflammatory cytokines IL-8, IL-6 and IL-1β. The mRNA level of IL-8 was significantly increased 6 h after intake of the cod liver oil cake compared with fasting and 3 h (Fig. 1(a)). This increase in mRNA after 6 h compared with fasting was significantly different from the effect observed after intake of linseed cake. No significant changes in the mRNA level of IL-1β and IL-6 were observed either between the meals or after different time points (Fig. 1(b) and (c)). We also measured the mRNA expression of the transcription factor PPARγ, known to be activated by lipids. No change in PPARγ mRNA after intake of any of the cakes was observed (Fig. 1(d)). The mRNA level of the PPARγ target gene, CPT1A, was measured. CPT1A mRNA was significantly increased 6 h compared with 3 h after intake of cod liver oil cake (Fig. 1(e)). No significant differences in CPT1A were observed between the meals.
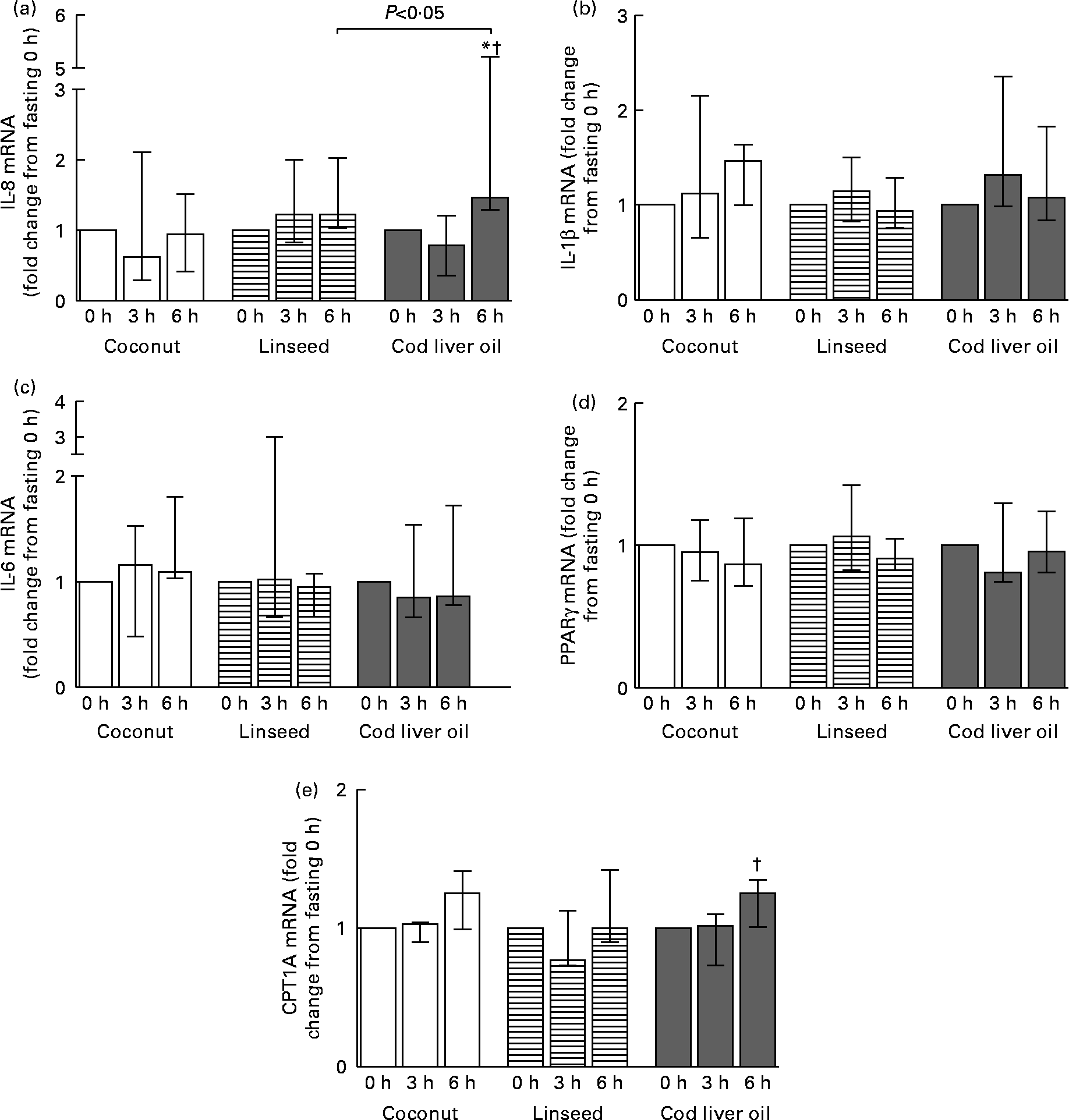
Fig. 1 mRNA levels of IL-8 (a), IL-1β (b) IL-6 (c) PPARγ (d) and carnitine palmitoyltransferase-1A (CPT1A) (e) in peripheral blood mononuclear cells at 3 and 6 h after the consumption of three test meals enriched with coconut fat (n 12 for IL-8, IL-1β and PPARγ and n 7 for IL-6 and CPT1A), linseed oil (n 10 (3 h) and n 11 (6 h) for IL-8, IL-1β and PPARγ and n 6 (3 h) and n 7 (6 h) for IL-6 and CPT1A) or cod liver oil (n 10 for IL-8, IL-1β and PPARγ and n 7 for IL-6 and CPT1A). The mRNA data are presented relative to the fasting mRNA levels. Values are medians, with interquartile ranges represented by vertical bars. * Median value was significantly different from that at 0 h (P < 0·05). † Median value was significantly different from that at 3 h (P < 0·05).
Gene expression and release of inflammatory markers in ex vivo stimulated peripheral blood mononuclear cells
To further investigate the difference in inflammatory response elicited by the two major n-3 fatty acids (ALA and EPA) used in the postprandial study, we measured the effect on cytokine release (n 8) and mRNA expression (n 7) levels in ex vivo experiments. As shown in Fig. 2, we found that EPA significantly increased the release of IL-8 and IL-6 compared with unstimulated cells (Fig. 2(b) and (d)). ALA increased the release of IL-8 while reducing the release of IL-6 compared with unstimulated cells (Fig. 2(b) and (d)). Concomitant EPA significantly increased the IL-8 mRNA level compared with unstimulated cells (Fig. 2(a)), significantly increased the IL-6 mRNA level compared with ALA-stimulated cells (Fig. 2(c)), while no significant effect on the mRNA expression of IL-1β was observed (Fig. 2(e)). In contrast, ALA had no effect on the mRNA level of any of these cytokines compared with unstimulated cells.
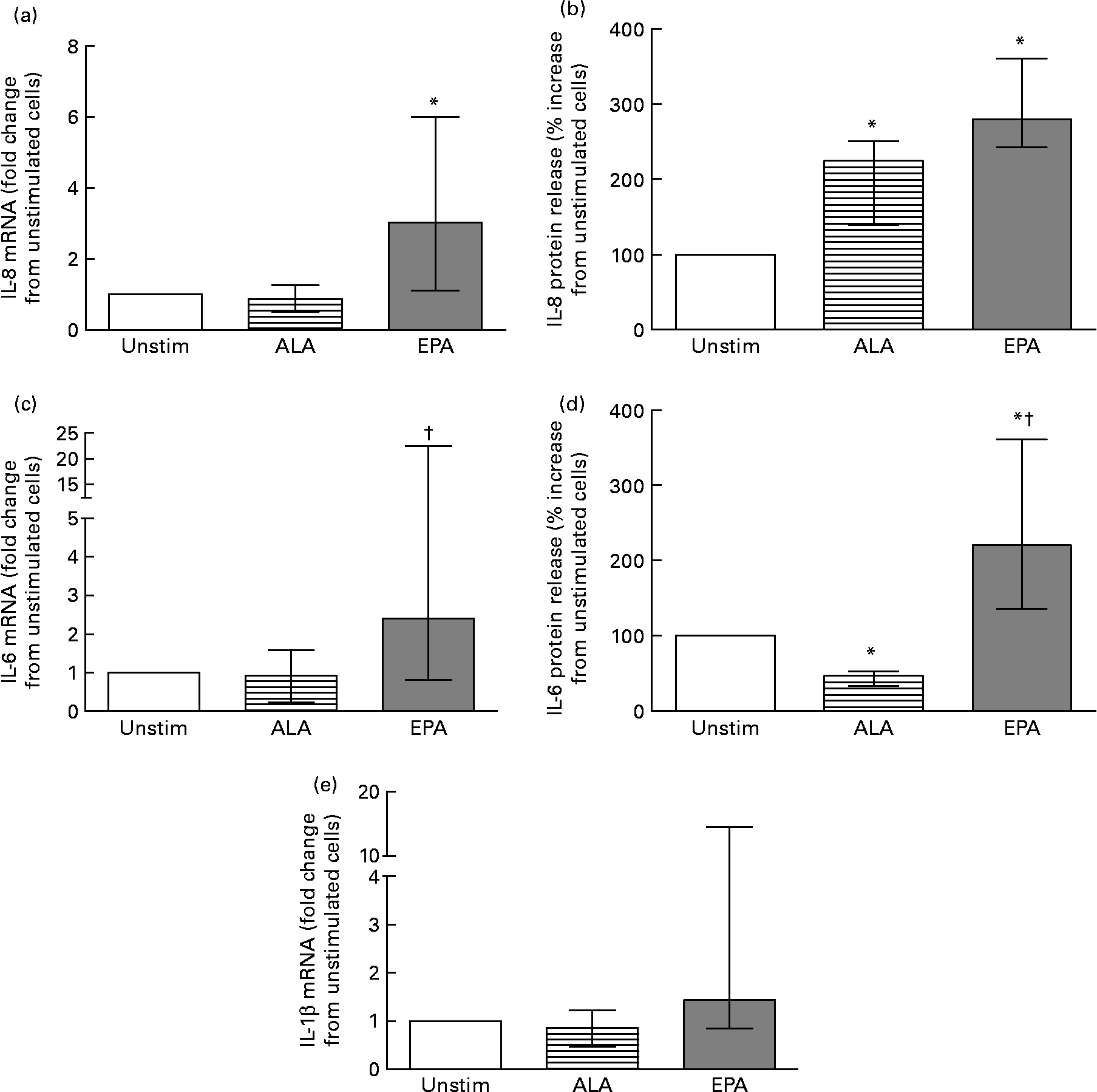
Fig. 2 mRNA level (a, c, e) (n 7) and protein release (b, d) (n 8) of IL-8 (a, b), IL-6 (c, d) and IL-1β (e) in ex vivo isolated peripheral blood mononuclear cells after 24 h without (Unstim) or with 60 μm-α-linolenic acid (ALA; 18 : 3n-3) or -EPA (20 : 5n-3). mRNA levels are presented relative to unstimulated cells. Protein release data are presented as percentage increase compared with unstimulated cells. Values are medians, with interquartile ranges represented by vertical bars. * Median value was significantly different from that for unstimulated cells (P < 0·05). † Median value was significantly different from that for ALA-stimulated cells (P < 0·05).
Gene expression of protein involved in lipid metabolism in ex vivo stimulated peripheral blood mononuclear cells
Since PUFA are natural ligands for PPAR we also analysed the mRNA levels of the three PPAR isoforms and CPT1A. We found no change in PPARα mRNA and minor changes in PPARδ mRNA level after incubation with either EPA or ALA (data not shown). PPARγ mRNA was significantly increased in cells stimulated with EPA compared with ALA (Fig. 3(a)). We also found that both ALA and EPA significantly increased CPT1A mRNA compared with unstimulated cells (Fig. 3(b)), with EPA having the most prominent effect compared with ALA.
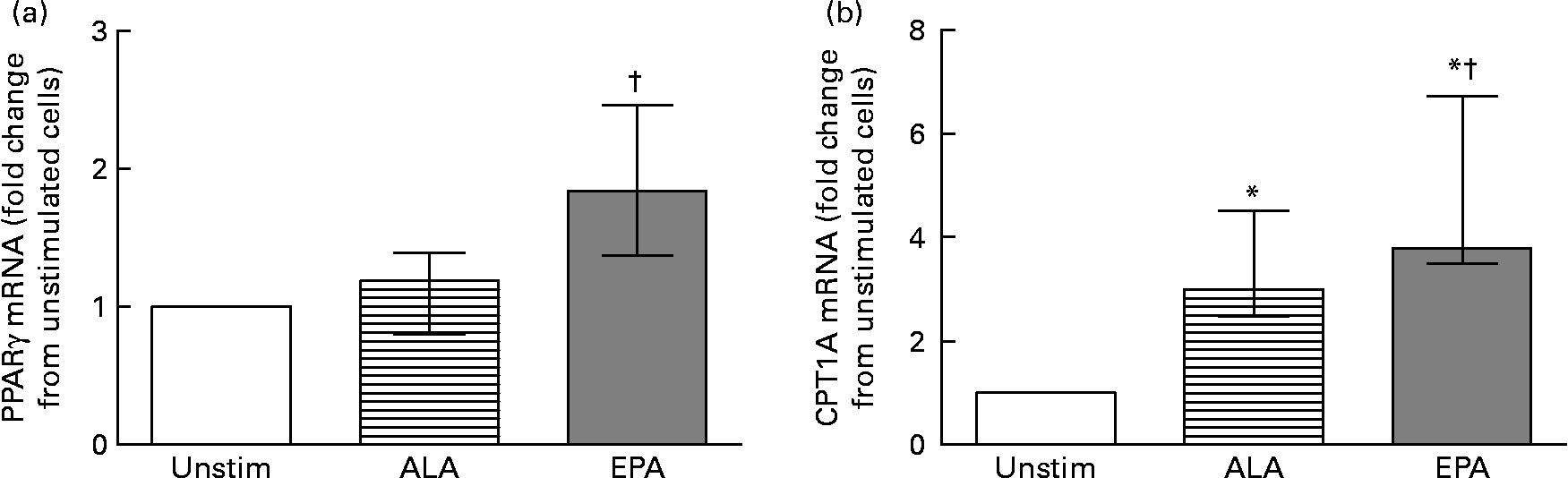
Fig. 3 mRNA level of PPARγ (a) and carnitine palmitoyltransferase-1A (CPT1A) (b) in ex vivo isolated peripheral blood mononuclear cells after 24 h without (Unstim) or with 60 μm-α-linolenic acid (ALA; 18 : 3n-3) or -EPA (20 : 5n-3) (n 7). Data are presented relative to unstimulated cells. Values are medians, with interquartile ranges represented by vertical bars. * Median value was significantly different from that for unstimulated cells (P < 0·05). † Median value was significantly different from that for ALA-stimulated cells (P < 0·05).
Discussion
The present study shows that intake of meals containing n-3 fatty acids of plant v. marine origin leads to different postprandial inflammatory responses measured as gene expression in PBMC, whereas smaller differences were found in the postprandial responses of inflammatory markers in serum and plasma. This was further supported by ex vivo PBMC experiments, where we observed that the plant n-3 fatty acid ALA had less pro-inflammatory potential at a given time and dose compared with EPA, further supporting the notion that n-3 fatty acids with different chain lengths and degree of saturation may have different inflammatory potential in PBMC.
Humans are mainly in a postprandial state during the day. However, only a few studies have investigated the inflammatory postprandial response after a high-fat meal(Reference Poppitt, Keogh and Lithander18, Reference Payette, Blackburn and Lamarche19). We observed a significant increase in the mRNA expression level of IL-8 after the intake of cod liver oil cake compared with linseed cake. In contrast, we found no postprandial effect on the circulating levels of IL-8 and CRP after intake of any of the cakes. We detected, however, an increase in the plasma level of IL-6 after intake of the linseed cake, while no changes in circulating levels of IL-6 were observed after intake of the coconut and the cod liver oil cakes. The finding of moderate or no effects on circulating inflammatory markers after intake of a single meal with different fat quality is in accordance with previous publications(Reference Poppitt, Keogh and Lithander18–Reference Jimenez-Gomez, Lopez-Miranda and Blanco-Colio21). Inflammatory markers measured in the circulation may reflect an overall systemic effect; however, important local beneficial or detrimental effects on circulating cells such as monocytes will not necessary be detected since these cells only contribute to a small percentage of the total cells in plasma. Changes in plasma levels of inflammatory markers after intake of dietary fat can therefore be caused by changes in the secretion of inflammatory markers from peripheral tissue such as adipose tissue and muscle rather than directly by circulating cells. Furthermore, since the plasma levels of inflammatory markers are generally low in healthy subjects, any effect in circulating cells would be difficult to detect compared with effects in peripheral tissue. PBMC are exposed to the same environmental factors as the arterial wall which makes them suitable for studying gene expression and changes in mRNA expression levels. PBMC may therefore be a more suitable model system to study postprandial inflammatory responses. Gene expression profiling of PBMC from single-meal studies has demonstrated that different fat quality influences inflammatory responses(Reference Jimenez-Gomez, Lopez-Miranda and Blanco-Colio21, Reference Camargo, Ruano and Fernandez23). Jimenez-Gomez et al. observed a higher postprandial response in the mRNA expression of IL-6 after intake of butter and olive oil breakfasts than after a walnut-based breakfast (rich in ALA)(Reference Jimenez-Gomez, Lopez-Miranda and Blanco-Colio21). In the present study, we observed that both intake of cakes with linseed oil and coconut fat seemed to be less pro-inflammatory than intake of cake with cod liver oil. Interestingly, when we incubated PBMC ex vivo with the fatty acids ALA or EPA, the same pattern with respect to the inflammatory response was observed, with EPA having the most prominent inflammatory potential compared with ALA. Anti-inflammatory effects on circulating markers have been observed in studies among healthy individuals after intake of fish oil supplements even though many studies also show no effect(Reference Myhrstad, Retterstol and Telle-Hansen27, Reference Basu, Devaraj and Jialal28). However, little is known about the postprandial or long-term effect of intake of fish oil on the expression of inflammatory genes in PBMC(Reference Bouwens, Grootte and Jansen22, Reference Bouwens, van de Rest and Dellschaft29). It has previously been shown that the n-3 fatty acid ALA has anti-inflammatory activity(Reference Basu, Devaraj and Jialal28) and inhibits expression and secretion of IL-6 from cultured human monocytes(Reference Zhao, Etherton and Martin30). The present study shows that n-3 PUFA act differently on inflammatory cytokines in PBMC ex vivo. EPA increases the mRNA level and release of IL-8 and IL-6 from PBMC, whereas ALA seems to have a more neutral effect on the mRNA expression and more inconsistent effect on secretion of inflammatory markers. It has previously been shown that the mRNA level of cytokines including IL-8, IL-1β and IL-6 in adipose tissue of rats supplemented with marine n-3 fatty acids was increased(Reference Rokling-Andersen, Rustan and Wensaas31). This is in accordance with the present results, thus supporting the notion that these fatty acids are able to induce an inflammatory response also in other tissues. The mechanisms behind the different responses of fatty acids are not known. However, butter and walnuts, but not olive oil, have been shown to elicit postprandial activation of nuclear transcription factor κB in PBMC isolated from healthy men(Reference Bellido, Lopez-Miranda and Blanco-Colio32). PBMC express PPAR(Reference Bouwens, Afman and Muller33) and Toll-like receptors(Reference Riordan, Skinner and Nagree34) which can mediate the fatty acid responses by regulating gene expression(Reference Hong and Tontonoz17). Based on our ex vivo findings, it is tempting to speculate that fatty acids with a higher number of double bonds and/or a longer chain length are stronger activators of both PPAR and Toll-like receptor signalling than fatty acids with fewer double bonds and shorter chain length even though this is in contrast to other reports(Reference Erridge and Samani15, Reference Lee, Plakidas and Lee35, Reference Krey, Braissant and L'Horset36). In the present study we used a single dose of 60 μm of the different fatty acids. The dose of 60 μm has been shown by others to be well tolerated in different human cell lines and within the dose–response effect on cytokine production(Reference Zhao, Etherton and Martin30, Reference Toborek, Lee and Garrido37, Reference Hennig, Meerarani and Ramadass38) and in PBMC ex vivo (Reference Dooper, van and Graus39). In addition, we have recently shown that the plasma concentrations of ALA and EPA are 65 and 35 μmol/l, respectively, in a healthy Norwegian adult population(Reference Ulven, Kirkhus and Lamglait40). After 7 weeks of intervention with fish oil the plasma concentration of EPA increased to 75 μmol/l. Thus the dose used in the ex vivo experiment is comparable with physiological levels.
In the present study, we found that neither intake of a high-fat meal with a high ratio of saturated to unsaturated fat (coconut cake) nor a high-fat meal with equal energy percentage from saturated and unsaturated fat (linseed cake) changed the serum level of CRP. This is in accordance with the study of Poppitt et al. (Reference Poppitt, Keogh and Lithander18) which showed that the plasma CRP concentration did not change after intake of two high-fat test meals containing a high or low ratio of saturated to unsaturated fatty acids. This suggests that the ratio between saturated and unsaturated fat is not so important as the quantity and the quality of the fat(Reference Poppitt, Keogh and Lithander18).
It is interesting that we observed a reduction in plasma glucose and no increase in total cholesterol after intake of the linseed cake. Flaxseed is the richest source of the lignan secoisolariciresinol diglucoside. Human studies have shown that secoisolariciresinol diglucoside metabolites can reduce lipid and glucose concentrations, lower blood pressure, and decrease oxidative stress and inflammation(Reference Adolphe, Whiting and Juurlink41). Lignans accumulate especially in the de-oiled DM of the linseed (the linseed coarse meal as by-product from linseed oil production); however, we cannot rule out that some of the effects that we observed were caused by the presence of lignans in the linseed oil.
A potential weakness of the study is the lack of randomisation and the small number of participants. However, the number of participants was chosen based on previous similar studies where the effect of fat on inflammatory responses and/or gene expression has been analysed(Reference Poppitt, Keogh and Lithander18, Reference Jimenez-Gomez, Lopez-Miranda and Blanco-Colio21, Reference Bouwens, Grootte and Jansen22, Reference Tulk and Robinson42).
In conclusion, intake of a cake enriched with cod liver oil led to a significant increase in the gene expression level of IL-8 which was significantly different from the response observed after intake of linseed cake. This was further supported by the finding that ALA and EPA elicit different effects on the release and mRNA expression levels of inflammatory markers in PBMC cultured ex vivo, with EPA having the most prominent pro-inflammatory potential. Since the level of circulating inflammatory markers is low in healthy subjects and PBMC are exposed to the same environmental factors as the arterial wall, we suggest that transcription analysis in PBMC may be a more suitable model system for future postprandial inflammatory studies.
Acknowledgements
The present study was supported by grants from the Norwegian Research Council, Akershus University College, Nordic Centre of Excellence (NCoE) ‘SYSDIET’ by Nordforsk (070014), Academy of Finland, The Throne-Holst Foundation, The Freia Chocolate Factory Medical Foundation and the University of Oslo, Norway. We are grateful to all the participants in the study. We thank Ellen Raael and Marit Sandvik for excellent technical assistance.
M. C. W. M. was responsible for designing and conducting the single-meal experiment, analysing and interpreting the data, and writing the manuscript; I. N. was responsible for designing and conducting the single-meal experiment, analysing and interpreting the data, and writing the manuscript; V. H. T.-H. was responsible for designing and conducting the ex vivo experiment, analysing and interpreting the data, and writing the manuscript; T. K. was responsible for analysing and interpreting the data, and writing the manuscript; D. B. L. was responsible for designing and conducting the single-meal experiment, interpreting the data and writing the manuscript; K.-H. H. was responsible for analysing and interpreting the data, and writing the manuscript; M. M. was responsible for analysing and interpreting the data, and writing the manuscript; B. H. was responsible for designing and conducting the ex vivo experiment, analysing and interpreting the data, and writing the manuscript; K. R. was responsible for designing the ex vivo experiment, analysing and interpreting the data, and writing the manuscript; B. K. was responsible for designing the ex vivo experiment, analysing and interpreting the data, and writing the manuscript; L. G. was responsible for designing the ex vivo experiment, analysing and interpreting the data, and writing the manuscript; K. B. H. was responsible for designing and conducting the single-meal and ex vivo experiments, analysing and interpreting the data, and writing the manuscript; S. M. U. was responsible for designing and conducting the single-meal and ex vivo experiments, analysing and interpreting the data, and writing the manuscript.
There are no conflicts of interest.








