The metabolic syndrome (MetS) is a clustering of cardiovascular risk factors including abdominal obesity, hypertension, abnormal glucose homeostasis and dyslipidaemia(Reference De Ferranti and Osganian1). This syndrome increases the risk of atherosclerosis, type 2 diabetes and stroke and enhances the mortality rate by 20–80 %(Reference Halpern, Mancini and Magalhaes2–Reference Isomac, Almgern and Tuani5). It seems that insulin resistance is the primary defect in the MetS(Reference Weiss6). Findings from the Third National Health and Nutrition Examination Survey have revealed that the MetS is prevalent among 24 % of men and 23 % of women in the USA(Reference Ford, Giles and Dietz7). In Iran, the MetS is highly prevalent in adults, with an estimated prevalence of more than 30 %(Reference Azizi, Salehi and Etemadi8, Reference Azadbakht, Kimiagar and Mehrabi9). In children, there is no general consensus on the cut-off values and components of the MetS(Reference Halpern and Mancini3, Reference Mancini10). Based on the modified Adult Treatment Panel III definition, 10 % of Iranian adolescents are affected by the syndrome(Reference Esmaillzadeh, Mirmiran and Azadbakht11). Since the process of atherosclerosis, which is linked to obesity and other components of the MetS, starts at an early age, the primary prevention and treatment of childhood MetS are of great importance(Reference Weiss6).
Lifestyle interventions, including diet therapy, have long been recommended for the management of the MetS(Reference Grundy, Cleeman and Daniels12). In order to prescribe a therapeutic diet, all abnormalities of the MetS should be taken into account(Reference Giugliano, Ceriello and Esposito4). Most individuals with the MetS are overweight or obese, and weight loss is the key recommendation to improve all aspects of the MetS(Reference Shenoy, Poston and Reeves13). Among children, energy restriction and weight loss can hinder growth and development(Reference Halpern, Mancini and Magalhaes2). Therefore, the appropriate diet for the management of childhood MetS is the one that meets the growth requirements of children besides modifying their metabolic abnormalities(Reference Halpern, Mancini and Magalhaes2). The Dietary Approaches to Stop Hypertension (DASH) eating plan might be beneficial for patients with the MetS(Reference Azadbakht, Mirmiran and Esmaillzadeh14). The DASH diet is rich in vegetables, fruits, whole grains, low-fat dairy products, Mg, K, Ca and fibre(15). The DASH diet also has a low Na content (2400 mg/d)(Reference Obarzanek, Sacks and Vollmer16). Adherence to the DASH diet has been reported to lower blood pressure(15) and lipid profiles(Reference Moore, Conlin and Ard17). The beneficial effect of the DASH diet on glycaemic control and liver enzymes in type 2 diabetic patients has also been documented(Reference Azadbakht, Surkan and Esmaillzadeh18). Limited data indicating the effects of the DASH diet on the MetS are available. We are aware of just one study that has assessed the effects of the DASH diet on the MetS among adults(Reference Azadbakht, Mirmiran and Esmaillzadeh14). To our knowledge, the effects of the DASH diet on the features of the MetS in children have not been examined yet. Furthermore, the DASH diet has been applicable and acceptable among the adult population(Reference Harnden, Frayn and Hodson19), but its acceptability in children remains unknown. The DASH diet contains high amounts of low-fat dairy products, Ca, K and Mg, all of which have been shown to affect the features of the MetS(Reference Azadbakht, Mirmiran and Esmaillzadeh20, Reference Al-Solaiman, Jesri and Mountford21). Moreover, the restricted Na content of the DASH diet leads to lower blood pressure levels(15). In addition, greater amounts of dietary fibre, folate, vitamin C, carotenoids, phytosterols, phytochemicals and antioxidants in the DASH diet(Reference Couch, Saelens and Levin22) make it suitable for treating the MetS and maintaining normal growth in children and adolescents. Therefore, the present study was conducted to assess the effects of recommendations to follow the DASH diet v. usual dietary advice (UDA) on the MetS and its features in adolescents. We hypothesised that recommendations to follow the DASH diet would be an appropriate approach for the treatment of children with the MetS. Since dietary adherence of females is higher than that of males(Reference Wilson and Ampey-Thornhill23), we conducted the present study among adolescent girls.
Subjects and methods
Participants
A total of sixty female post-pubescent adolescent girls aged 11–18 years with the MetS participated in the present study. The MetS was defined as having three or more of the following criteria, according to the Adult Treatment Panel III criteria modified for children and adolescents(Reference Kelishadi, Ardalan and Gheiratmand24): (1) abdominal adiposity (waist circumference >75th percentile for age); (2) low levels of serum HDL-cholesterol ( < 500 mg/l); (3) hypertriacylglycerolaemia (TAG levels ≥ 1000 mg/l); (4) elevated blood pressure (systolic blood pressure (SBP) or diastolic blood pressure (DBP) >90th percentile for age and height from the National Heart, Lung and Blood Institute's recommended cut-off points); (5) impaired glucose homeostasis (fasting plasma glucose levels ≥ 1000 mg/l). Subjects were not included in the study if they had CVD or kidney or liver diseases or were taking any medications affecting nutrient metabolism, blood lipids and blood pressure or any vitamin and mineral supplements. The required sample size for the present study was determined using serum TAG levels(Reference Kelishadi, Ardalan and Gheiratmand24) as a key dependent variable. We used the standard formula suggested for two-period, two-treatment cross-over studies. Given the 80 % power and type I error of 5 %, we required a sample size of thirty-two participants to detect significant differences between the two groups. Considering the high dropouts in cross-over trials, we recruited sixty female adolescents based on the inclusion criteria. Parental written consent and adolescent assent were obtained. During the study, eleven subjects were dropped out. The reasons for dropping out were as follows: fear of blood sampling (n 6); educationally busy (n 2); surgery (n 1); personal reasons (n 1); mother's demise (n 1). Finally, forty-nine adolescent girls completed the whole trial. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki. The study was ethically approved by the Food Security Research Center of Isfahan University of Medical Sciences and has been registered at the Iranian registry of clinical trials (http://www.irct.ir; identifier: IRCT201110191485N6).
Study design
In this cross-over randomised clinical trial, after obtaining detailed information about their lifestyle characteristics in a run-in period of 2 weeks, the participants were randomly allocated to follow the DASH diet or UDA for 6 weeks. Random assignment was done by the use of computer-generated random numbers. A 4-week washout period separated the two phases of the study. During this period, the participants were requested to go back to their old eating habits. After the washout period, the participants were crossed over to the alternate arm: those consuming the DASH diet in the first phase were asked to follow UDA and vice versa. Except for the participants and the study dietitian who provided dietary advice, all study personnel (including those working in the laboratories) and other researchers were blinded to the dietary assignment. Each participant completed a 3 d dietary record (two week days and one weekend day) during the run-in period as well as throughout each phase of the intervention. Food intake was recorded by the participant at the time the foods were consumed to minimise reliance on memory. To assess compliance to the DASH diet, the average of the 3 d dietary records obtained from the participants in the DASH phase was used. Food diaries were analysed for their nutrient content using the Nutritionist-IV software (First Databank), which was modified for Iranian foods. Compliance to the DASH diet was also assessed using serum vitamin C levels as well as dietary records. The participants were asked to not change their habitual physical activity levels throughout the study. They were asked to record their physical activities for 2 d in the run-in period and throughout each phase of the intervention.
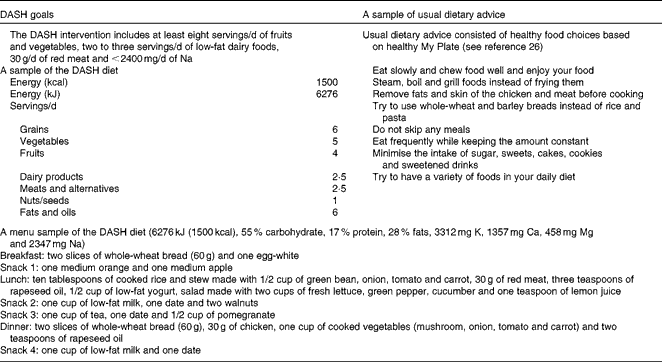
The Dietary Approaches to Stop Hypertension diet
The DASH diet used in this intervention was slightly modified from the original version of the DASH diet for adults to more closely conform to the unique nutritional needs of adolescents. Energy requirements of each participant were calculated individually based on equations suggested by the American Dietetic Association, Pediatric Weight Management Guidelines for weight maintenance(25). We did not prescribe weight loss diets for adolescents. All diets were designed to maintain the current weight. Diets were prescribed according to the energy requirements of the adolescents. The macronutrient composition of the DASH diet was as follows: carbohydrates, 53–58 %; proteins, 15–18 %; total fats, 26–30 % of total energy. The dietary goals of the DASH group as well as a sample of the 1 d menu of the DASH diet are summarised in Table 1. The DASH diet was planed to contain high amounts of whole grains, fruits, vegetables and low-fat dairy products as well as low amounts of saturated fats, cholesterol, refined grains, sweets and red meat. Ca, K and Mg contents of the DASH diet were higher than those recommended during UDA. The DASH diet contained < 2400 mg Na/d. Both groups receiving the DASH recommendations and UDA were visited monthly; each session for a patient took about 30–45 min. In the sessions, the study nutritionist explained the benefits of the diet to the participants. The participants also received the required information to use the exchange list of foods. They were taught as to how to record their dietary intakes. A 7 d menu cycle based on the energy requirements of each participant was planned. Moreover, the participants were in touch with the nutritionist by phone every day. The dietitian made calls to the participants to remind them to record their food intakes and physical activities and to ask them to follow the prescribed diet. The dietitian also asked the participants if there was any problem with the diet. She also responded to the questions that the participants had about the diet. The participants were also free to make calls to the dietitian whenever they had questions regarding their diet.
Table 1 Dietary goals of the Dietary Approaches to Stop Hypertension (DASH) intervention v. usual dietary advice and a sample menu of the DASH diet

Usual dietary advice
The UDA group was given general oral and written information about healthy food choices based on healthy MyPlate(26). The eating patterns of the group were similar to that of a typical Iranian diet (carbohydrates, 50–60 %; proteins, 15–20 %; total fats, < 30 %; dietary fibre, 14 g/d; SFA, 24 g/d(Reference Azadbakht and Esmaillzadeh27)). Ca, dairy product, nut and legume contents of this diet were lower than those of the DASH diet. No dietary menus were provided for this group.
Measurements
Body weight was measured while the participants were minimally clothed and without shoes, using digital scales, and it was recorded to the nearest 0·1 kg. Height was measured in a standing position and without shoes, using a tape meter while the shoulders were in a normal position. BMI was calculated as weight (in kg) divided by height (in m2). Waist circumference was measured to the nearest 0·1 cm at the narrowest level over light clothing, using an unstretched tape meter, without any pressure to the body surface. Data on physical activities were collected through physical activity records. The participants were taught at the run-in period (before the start of the intervention) as to how to record these data. They were requested to record the type of physical activity and its intensity as well as the duration of that activity. The sum of the durations of activities in the physical activity records had to be 24 h. In case of any problem in recording, the dietitian contacted the participants to further review the type, intensity and duration of the activities recorded in the records. Data from the physical activity records are expressed as metabolic equivalents. Blood pressure was measured twice after the participants sat for 15 min using a standard digital sphygmomanometer (OMRON, M2; HEM.7117-E) with an appropriate cuff size. Overall, two blood pressure measurements were made at a 5 min interval, and the mean of the two measurements was used for the analysis. To avoid subjective errors, all measurements were made by the same nutritionist.
Fasting blood samples for the measurement of glucose and lipid concentrations were drawn after an overnight fast of 12 h. Fasting blood glucose concentration was measured on the day of blood collection using the enzymatic colorimetric method with glucose oxidase. Serum concentrations of total cholesterol and TAG were measured using commercially available enzymatic reagents (Pars Azmoon). Serum HDL-cholesterol levels were measured after precipitation of the apoB-containing lipoproteins with phosphotungstic acid. LDL-cholesterol levels were calculated according to the Friedewald equation. Inter-assay and intra-assay CV were 0·9 and 1·1 % for total cholesterol and 1·6 and 1·2 % for TAG, respectively. Serum insulin concentrations were measured using ELISA kits and an ELISA reader (Diagnostic Biochem Canada Inc.). Insulin resistance was estimated on the basis of fasting glucose and insulin concentrations using the homeostasis model assessment for insulin resistance (HOMA-IR) method. Plasma vitamin C levels were measured using commercially available kits (Glory Science Company) by the ELISA method.
Statistical analysis
To ensure the normal distribution of variables, we used the Kolmogorov–Smirnov test. Log transformation was applied for non-normally distributed variables. The analyses were carried out based on the intention-to-treat approach. Missing values were treated based on the last-observation-carried-forward method. Descriptive statistics (means, sem or sd and range) for general characteristics of the study participants are reported. Data on dietary intakes and physical activities were compared by a paired t test. For each dependent variable, we computed the changes from baseline by subtracting the baseline value from the end-of-trial value. Within-group and between-group changes in anthropometric measures as well as biochemical indicators were compared using a paired-samples t test. Differences in the prevalence of the MetS and its components were examined using the χ2 test. We also assessed whether the carry-over effect was significant. To assess the carry-over effect, we computed the average of end-of-trial values for each variable for two treatments separately and compared the means of the two treatment orders using a t test. P values < 0·05 were considered as significant.
Results
General characteristics of the study population are given in Table 2. The mean age and weight of the participants were 14·2 years and 69 kg, respectively. Their mean BMI and waist circumference were 27·3 kg/m2 and 85·6 cm, respectively. At baseline, mean SBP and DBP of the participants were 120·6 and 73·3 mmHg.
Table 2 Baseline characteristics of the study participants (Mean values and standard deviations; minimum and maximum values; ranges)

The average energy intake of the participants during the run-in period, based on 3 d dietary records, was 7510 kJ/d (1795 kcal/d). The mean dietary intakes of grains, fruits and vegetables were 7·6, 2·4 and 2·2 servings/d, respectively. The mean intakes of dairy products, meat and alternatives, and fats were 1, 4·2 and 10·2 servings/d, respectively. Dietary energy density was 4·6 kJ/g (1·1 kcal/g). The participants obtained 13 % of their energy from proteins, 50 % from carbohydrates and 37 % from fats. Mean Na and K intakes were 2218 and 2481 mg/d, respectively.
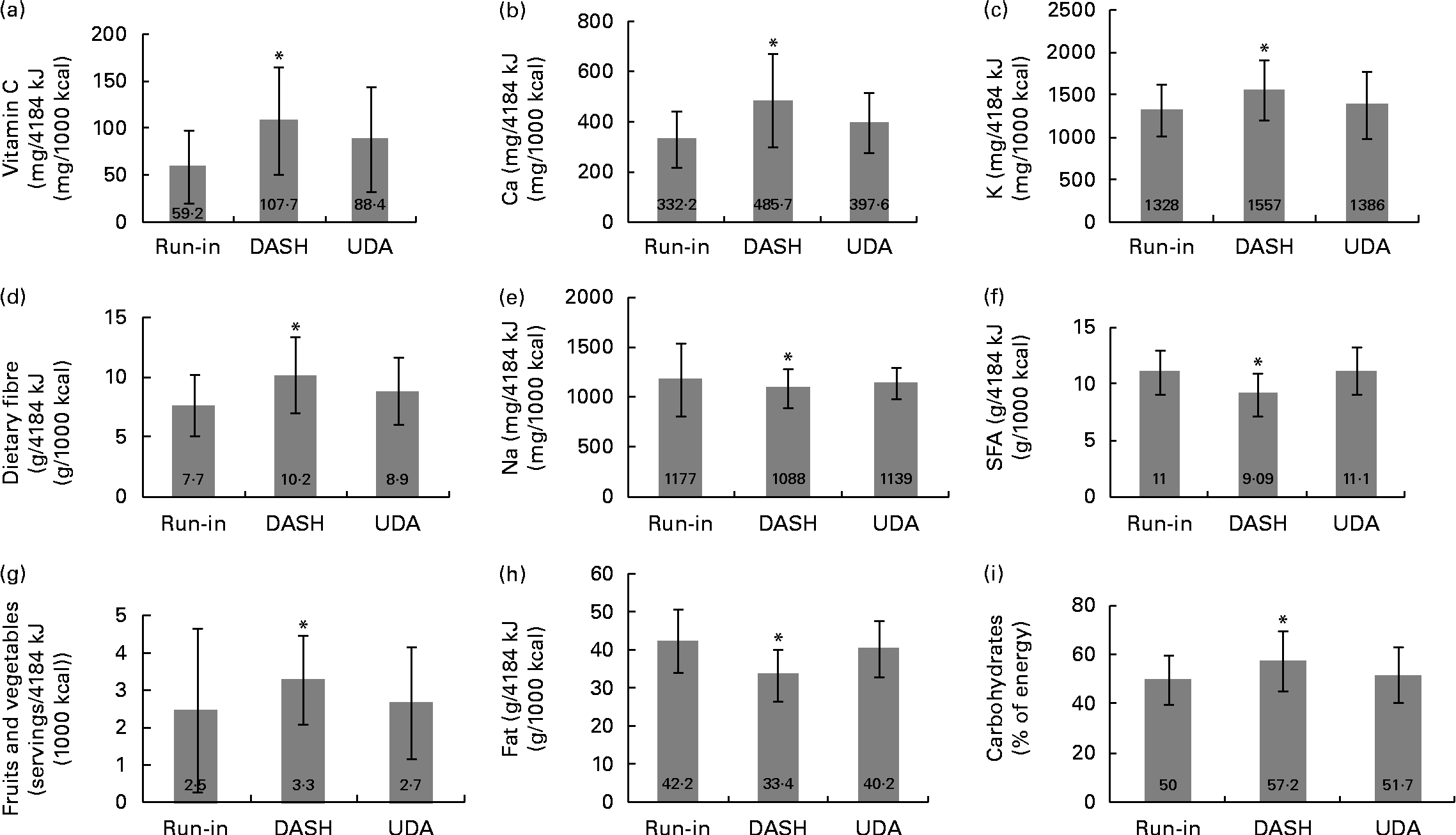
Compliance to the DASH diet was assessed through dietary records as well as quantification of serum vitamin C levels. The energy-adjusted dietary intakes of the participants throughout the run-in period and the intervention phases are shown in Fig. 1. Mean dietary energy intakes were not significantly different between the two groups during the intervention (DASH: 6788·1 (se 269·4) kJ/d (1622·4 (se 64·4) kcal/d) v. UDA: 7078·1 (se 297·5) kJ/d (1691·7 (se 71·1) kcal/d), P= 0·31). During the DASH phase, the participants consumed higher amounts of carbohydrates, Ca, K and dietary fibre and lower amounts of fats, saturated fats and Na compared with the UDA phase (all P values < 0·05). The participants also tended to consume higher amounts of fruits and vegetables (P= 0·06) and vitamin C (P= 0·08) in the DASH phase than in the UDA phase. Dietary energy density was significantly lower during the DASH phase than during the UDA phase (4·1 (se 0·0) v. 5·0 (se 0·0) kJ/g (1·0 (se 0·0) v. 1·2 (se 0·0) kcal/g), respectively, P= 0·01). Serum vitamin C levels also tended to be higher in the DASH phase than in the UDA phase (860 (se 104) v. 663 (se 76) ng/l, respectively, P= 0·06). The change in vitamin C levels in the DASH phase was 168 (se 129) and it was − 138 (se 97) ng/l in the UDA phase. Comparison of the changes between the two groups revealed a borderline significant difference (P= 0·06). All these data indicate a relatively good compliance of the participants to the DASH eating plan.

Fig. 1 Energy-adjusted dietary intakes of the participants throughout the run-in period and the 6-week intervention. The Dietary Approaches to Stop Hypertension (DASH) diet was high in fruits, vegetables, whole grains and low-fat dairy products as well as low in saturated fats, total fats, cholesterol, refined grains and sweets ((a) vitamin C, (b) calcium, (c) potassium, (d) dietary fibre, (e) sodium, (f) SFA, (g) fruits and vegetables, (h) fats and (i) carbohydrates). The usual dietary advice (UDA) group received general oral and written information about healthy food choices, and the intakes of the group were as those for the usual Iranian diet (carbohydrates, 50–60 %; proteins, 15–20 %; total fats, < 30 %). Values are means, with standard errors (given within each bar) represented by vertical bars. * Mean values were significantly different from those of the run-in and UDA phases: (a) P= 0·08, (b) P= 0·003, (c) P= 0·02, (d) P= 0·03, (e) P= 0·12, (f) P< 0·001, (g) P= 0·06, (h) P< 0·001 and (i) P= 0·01 (paired t test).
Findings from the physical activity records revealed that the activity levels of the participants were not significantly different when comparing the two intervention phases (mean physical activity in the DASH phase: 32·8 (se 0·5) metabolic equivalents-h/d and that in the UDA phase: 32·7 (se 0·4) metabolic equivalents-h; P= 0·85)
The effects of recommendations to follow the DASH diet v. UDA on anthropometric measures and blood pressure (BP) are summarised in Table 3. Recommendations to follow the DASH diet resulted in a significant reduction in BMI and waist circumference, while UDA led to a significant decrease in waist circumference only. However, changes in weight, waist circumference and BMI were not significantly different between the two phases. Recommendations to follow the DASH diet did not considerably influence SBP (P= 0·82) and DBP (P= 0·21), whereas during the UDA phase, the participants experienced a significant increase in SBP (P= 0·04) and DBP (P= 0·01). Although changes in SBP between the two phases were not statistically significant (P= 0·13), the alterations in DBP were significant (P= 0·01); that is, recommendations to follow the DASH diet prevented the increase in DBP compared with the usual recommendations.
Table 3 Effects of recommendations to follow the Dietary Approaches to Stop Hypertension (DASH) diet v. usual dietary advice on anthropometric measures and blood pressure of children with the metabolic syndrome (Mean values with their standard errors)

* The DASH diet was high in fruits, vegetables, whole grains and low-fat dairy products and low in saturated fats, total fats, cholesterol, refined grains and sweets. The amount of Na intake was 2400 mg/d.
† The usual dietary advice group received general oral and written information about healthy food choices, and the intakes of the group were as those for the usual Iranian diet (carbohydrates, 50–60 %; proteins, 15–20 %; total fats, < 30 %).
‡ Calculated by subtracting the values at the 6th week from the values at baseline.
§ For comparison of within-group differences by a paired t test.
∥ For comparison of between-group differences by a paired t test.
The effects of recommendations to follow the DASH diet on the features of the MetS are given in Table 4. Plasma concentrations of fasting blood glucose and serum lipid profiles were not affected by the recommendations to follow the DASH diet. However, these recommendations resulted in a significant decrease in serum insulin levels (P= 0·04) and a non-significant reduction in the HOMA-IR score (P= 0·12). However, when we compared the changes in fasting blood glucose, serum lipid and insulin levels and HOMA-IR score between the two phases, we failed to find a significant difference.
Table 4 Effects of recommendations to follow the Dietary Approaches to Stop Hypertension (DASH) diet v. usual dietary advice on metabolic profiles in the participants (Mean values with their standard errors)

HDL-C, HDL-cholesterol; LDL-C, LDL-cholesterol; HOMA-IR, homeostasis model assessment for insulin resistance.
* The DASH diet was high in fruits, vegetables, whole grains and low-fat dairy products and low in saturated fats, total fats, cholesterol, refined grains and sweets. The amount of Na intake was 2400 mg/d.
† The usual dietary advice group received general oral and written information about healthy food choices, and the intakes of the group were as those for the usual Iranian diet (carbohydrates, 50–60 %; proteins, 15–20 %; total fats, < 30 %).
‡ Calculated by subtracting the values at the 6th week from the values at baseline.
§ For comparison of within-group differences by a paired t test.
∥ For comparison of between-group differences by a paired t test.
The effects of recommendations to follow the DASH diet on the prevalence of the MetS and its components are presented in Table 5. Despite a slight decline in the prevalence of the MetS and its features, we failed to find a significant effect of recommendations to follow the DASH diet on most of its features. Compared with UDA, recommendations to follow the DASH diet resulted in a significant reduction in the prevalence of the MetS and high blood pressure.
Table 5 Effects of recommendations to follow the Dietary Approaches to Stop Hypertension (DASH) diet v. usual dietary advice on the prevalence of the metabolic syndrome and its components in the participants

High WC, waist circumference >75th percentile for age; high FPG, fasting plasma glucose levels ≥ 1000 mg/l; high TAG, serum TAG levels ≥ 1000 mg/l; low HDL-C, serum HDL-cholesterol levels < 500 mg/l; high BP, systolic or diastolic blood pressure >90th percentile for age and height from the National Heart, Lung and Blood Institute's recommended cut-off point; MetS: metabolic syndrome was defined as having three or more of the above-mentioned criteria, according to the Adult Treatment Panel III criteria modified for children and adolescents.
* The DASH diet was high in fruits, vegetables, whole grains and low-fat dairy products and low in saturated fats, total fats, cholesterol, refined grains and sweets. The amount of Na intake was 2400 mg/d.
† The usual dietary advice group received general oral and written information about healthy food choices, and the intakes of the group were as those for the usual Iranian diet (carbohydrates, 50–60 %; proteins, 15–20 %; total fats, < 30 %).
‡ For comparison of within-group differences by the χ2 test.
§ For comparison of between-group differences by the χ2 test.
For most MetS-related variables, we found no evidence of a carry-over effect, except for DBP, for which we observed a significant carry-over effect from period 1 to period 2 (P= 0·03).
Discussion
In the present randomised cross-over clinical trial, we found that recommendations to follow the DASH diet among children, compared with UDA, resulted in improved diet quality and reduced the prevalence of the MetS and high blood pressure. No other significant effects were observed for the recommendations to follow the DASH diet compared with UDA. To our knowledge, the present study is the first one to examine the effects of recommendations to follow the DASH diet on the MetS and its features in adolescents.
Along with increasing prevalence of childhood obesity, the prevalence of the MetS is rising among children and adolescents. This syndrome would in turn lead to increased rates of type 2 diabetes mellitus and CVD among youths. Therefore, the management of childhood MetS is an important priority for health care systems at individual and population levels. Weight reduction is the cornerstone in the management of the MetS; however, weight loss is generally not recommended for children since childhood is a period of rapid growth and development. The suitable dietary approach for childhood MetS is still a matter of discussion mainly due to scarcity of data. We hypothesised the DASH dietary pattern to be a suitable choice for improving the features of childhood MetS that could maintain the weight of children and provide adequate nutrients for their growth and development. In the present study, compared with UDA for childhood MetS, recommendations to follow the DASH diet resulted in significant reductions in dietary energy density and intakes of saturated fats and Na along with increased intakes of K, Ca, dietary fibre, fruits, vegetables and vitamin C.
In the present study, we observed a significant favourable effect of recommendations to follow the DASH diet on the prevalence of the MetS and high BP. This finding is in line with those of earlier studies on adolescents that have reported the beneficial effects of the components of the DASH diet on the MetS. The results of the National Health and Nutrition Examination Survey 1999–2002 revealed that the Healthy Eating Index and fruit scores were associated with a lower prevalence of the MetS in US adolescents(Reference Pan and Pratt28). Fibre-rich, nutrient-dense, plant-based foods have also been inversely associated with the prevalence of childhood MetS(Reference Carlson, Eisenmann and Norman29). In a cross-sectional study among 2130 youths with type 1 diabetes, Liese et al. (Reference Liese, Bortsov and Günther30) found that the greater adherence to the DASH diet was inversely associated with the LDL:HDL ratio and glycated Hb. However, they failed to find significant associations with serum TAG and LDL-cholesterol levels.
In the present study, recommendations to follow the DASH diet, compared with UDA, prevented the rise in DBP, but did not affect SBP. This finding is in contrast to those of the previous studies that reported the significant effect of the DASH eating pattern on SBP, not on DBP, among children(Reference Couch, Saelens and Levin22, Reference Moore, Singer and Bradlee31). This difference in findings might be explained by study methodologies. Both the mentioned studies used a parallel design and their participants were pre-hypertensive, hypertensive or healthy children. In a parallel study among adults with the MetS, Azadbakht et al. (Reference Azadbakht, Mirmiran and Esmaillzadeh14) demonstrated a significant improvement in all the features of the MetS after a 6-month intervention with the DASH diet. The major difference between the present study and that of Azadbakht et al. (Reference Azadbakht, Mirmiran and Esmaillzadeh14) is that we did not prescribe weight loss diets for adolescents, while they prescribed a weight-reducing DASH diet. In another study, adherence to the DASH diet by adult patients with type 2 diabetes led to a significant reduction in body weight, waist circumference, fasting blood glucose, glycated Hb, LDL-cholesterol levels, and both SBP and DBP after 8 weeks. Although this study had a cross-over design, significant weight reduction of diabetic patients might be responsible for metabolic improvements(Reference Azadbakht, Fard and Karimi32). Furthermore, we found a reasonable acceptability of the DASH diet by adolescents in the present study; however, due to the age group of the study participants, the dietary adherence might not be perfect. Better adherence might have resulted in significant reductions in glucose and lipid profiles in earlier studies among adults. As the adherence to the DASH diet was not completely achieved in the present study, one might not attribute the effects to the changes in nutrient intakes. There is the possibility that the observed effects on blood pressure and the overall MetS may have been due to the within-group significant reduction in BMI and waist circumference in the DASH diet group. It must be noted that the two groups were not significantly different in terms of dependent variables at study baseline. Although the participants met the criteria for the MetS, they were not on the upper end of abnormal cut-points for the features of the MetS, which might reduce the chance of achieving significant changes in these features following the intervention. For example, mean baseline fasting plasma glucose values were less than 900 mg/l and almost 90 % of the participants had normal values. Moreover, dietary intakes of the participants in the run-in period revealed that they were eating a relatively nutrient-dense diet with fairly high amounts of fruits and vegetables. Their diet had a high content of K as well as a low content of Na, which might contribute to the non-significant changes in the dependent variables after the intervention.
The mechanisms through which the DASH diet affects metabolic health are largely unknown and still a matter for research. Several possibilities have been suggested: the high contents of K, Mg and fibre in the DASH eating pattern may explain the beneficial effects on metabolic profiles(Reference He, Markandu and Coltart33, Reference Streppel, Arends and van't Veer34). The low Na content in this diet can also explain the improvements in BP(Reference Elliott, Stamler and Nichol35). While controversial(Reference Dickinson, Nicolson and Cook36), Ca might also have modest BP-lowering effects(Reference Bucher, Cook and Guyatt37). Higher intakes of legumes(Reference Azadbakht, Kimiagar and Mehrabi9) and dairy products(Reference Azadbakht, Mirmiran and Esmaillzadeh20) and lower intakes of saturated fats(Reference Esmaillzadeh and Azadbakht38) can account for the beneficial effects of the DASH diet on metabolic parameters. In the present trial, vitamin C intake also tended to be higher in the DASH group than in the UDA group. Dietary intake of vitamin C has been suggested to lower BP by enhancing NO synthase activity(Reference Ward, Hodgson and Croft39, Reference D'Uscio, Milstein and Richardson40). Estimated folate consumption was also greater in the DASH group than in the UDA group. Several studies have indicated the lipid- and BP-lowering effects of folate(Reference Van Etten, de Koning and Verhaar41, Reference McEneny, Couston and McKibben42). Furthermore, as the DASH diet contains lower amounts of refined sugar, this would in turn help to prevent metabolic abnormalities(Reference Shikany, Phadke and Redden43). Most probably, the DASH diet exerts its beneficial effects through a combination of these factors and not just by a single dietary factor(Reference Al-Solaiman, Jesri and Mountford21).
The present findings must be interpreted in the context of some limitations. We used serum vitamin C levels and dietary records to assess the dietary compliance of adolescents. These are not perfect tools for assessing the adherence to the DASH diet. Having the cycle menus available might influence the food records and the participants might record what they knew they were supposed to consume based on their cycle menus. Finding a suitable biomarker for adherence to dietary patterns, in particular, to the DASH diet, can be considered in future investigations. We confined the study participants to girls; therefore, the findings cannot be extrapolated to all adolescents. Furthermore, since the Iranian food composition database was not complete, we had to use the USDA food composition database, which encompasses some enriched foods and might overestimate vitamin C, Fe and dietary fibre contents for Iranian foods. In addition, in the present study, the participants were given recommendations to follow the DASH diet (rather than being fed the diet). This might result in imperfect adherence to the DASH diet. Non-achievement of all the DASH goals by our participants (such as 15–16 g of dietary fibre/4184 kJ (1000 kcal)) might contribute to the non-significant changes in dependent variables.
In conclusion, recommendations to follow the DASH diet for 6 weeks among adolescent girls with the MetS, compared with UDA, reduced the prevalence of high blood pressure and the MetS and improved diet quality. No significant changes were found in glucose and lipid profiles. This type of healthy diet can be considered as a treatment modality for the MetS and its components in children.
Acknowledgements
The authors appreciate the Research Council of the Food Security Research Center, Isfahan University of Medical Sciences, for financial support provided for the present study.
The authors' contributions are as follows: P. S. and A. E. contributed to conception, design, statistical analysis and manuscript drafting. P. S. and S. R. contributed to the data collection. M. H. and R. K. contributed to the data collection and manuscript drafting. A. E. supervised the study. All authors approved the final manuscript for submission. A. E. is the guarantor of the work. None of the authors has any personal or financial conflicts of interest.