Plant-based diets lower the risk of age-related diseases(Reference Benzie and Wachtel-Galor1–Reference Key, Appleby and Rosell3). These diseases are associated with oxidation-induced damage to protein, lipid and DNA(Reference Beckman and Ames4–Reference Rees, Kennett and Whitelock6). Consequently, benefits of plant-rich diets are suggested to be due to their high antioxidant content(Reference Benzie and Wachtel-Galor1, Reference Frei, England and Ames7–Reference Thomasset, Berry and Garcea10). Supplementation trials with ‘pure’ antioxidants, such as vitamin C or vitamin E, have not shown expected benefits, and it is becoming clear that mechanisms other than simple radical scavenging are important(Reference Bjelakovic, Nikolova and Gluud11, Reference Packer and Cadenas12). Nonetheless, direct and indirect antioxidant actions may still play a role. Furthermore, important interactions between food components are likely to play their role. Therefore, the study of whole foods is preferable to that of single components, extracts or supplements(Reference Dwyer9, Reference Packer and Cadenas12).
Tea (Camellia sinensis), especially green tea, is rich in polyphenolic antioxidants and has many reputed health benefits(Reference Khan and Mukhtar13–Reference Benzie and Szeto17). The bioavailability of polyphenols is poor, but some are absorbed and found in measurable, though low ( < 2 μmol/l), quantities in human plasma(Reference Yang, Lambert and Sang14, Reference Williamson and Manach15). It is obvious from epidemiological studies that the regular intake of tea is associated with improved health, and there is also some supporting experimental evidence from animal and cell culture studies(Reference Khan and Mukhtar13–Reference Wang, Gong and Yan16, Reference Erba, Riso and Bordoni18–Reference Kager, Ferk and Kundi20), though green tea polyphenols have been reported also to have a damaging pro-oxidant effect on cells in vitro (Reference Suh, Chon and Oh21, Reference Halliwell22). In previous studies, we reported that the total polyphenolic content of teas correlates strongly (r >0·95) with the total antioxidant content(Reference Benzie and Szeto17) and that the total antioxidant content of human plasma increases significantly within 30 min of ingestion of a single dose of green tea(Reference Benzie, Szeto and Strain23). Still, how and whether these findings relate to modulation of oxidative stress and to possible health effects of tea are not clear. In human intervention trials, the effects of green tea on biomarkers of lipid metabolism, inflammation and oxidative stress are conflicting, and further study is needed, especially in relation to oxidation-induced, potentially mutagenic damage to DNA, as this can have far-reaching effects on health(Reference Erba, Riso and Bordoni18, Reference Klaunig, Xu and Han19, Reference Henning, Niu and Liu24–Reference Collins26).
There are several ways to investigate DNA damage, but a well-validated method is the comet assay, which is most commonly performed on peripheral lymphocytes(Reference Collins26–Reference Hoelzl, Knasmuller and Mišík29). The comet assay is a versatile biomonitoring tool, which is widely used for in vitro testing of potential genoprotective or genotoxic agents, and is used to study intervention-related changes in basal levels of oxidation-induced DNA damage and resistance of DNA to oxidant challenge. Another biomarker of oxidation-induced DNA damage is 7,8-dihydro-2-deoxyguanosine (8-oxodG)(Reference Halliwell and Gutteridge5, Reference Gedik and Collins27). Measuring 8-oxodG in cells is problematical, but measurement of 8-oxodG in urine is becoming more common, with liquid chromatography with tandem MS being the method of choice, owing to its high sensitivity and specificity(Reference Cooke, Olinski and Loft30–Reference Lee, Chung and Benzie32). There are numerous published studies that have used urine 8-oxodG as a putative biomarker of DNA damage(Reference Møller and Loft25). However, the origin of 8-oxodG in urine is probably not from repair of oxidatively damaged guanine bases in DNA, but from removal of oxidised bases from the nucleotide pool, and it is now regarded as a biomarker of ‘whole-body’ oxidative stress, rather than a marker of DNA damage(Reference Cooke, Olinski and Loft30). Use of the comet assay in combination with urine 8-oxodG offers a way to study intervention-related effects on DNA damage and resistance to damage as well as on ‘global’ oxidative stress. This was the approach taken in the present work. We studied the effects on oxidation-induced DNA damage and urine 8-oxodG in a controlled human supplementation trial of multiple cross-over design using two types of green tea (‘Longjing’ and ‘screw-shaped’), both of which are commonly consumed locally. We hypothesised that if green tea protects DNA and/or ameliorates global oxidative stress, then such effects would be seen with both types of green tea. The in vitro effect of different concentrations of green tea on human lymphocytic DNA was investigated also to see if in vitro and in vivo (i.e. supplementation) effects were similar. Finally, the total antioxidant content of infusions of each of the two teas was measured using the ferric-reducing antioxidant power assay(Reference Benzie and Strain33).
Materials and methods
The present study was divided into two parts: (a) in vitro testing of green tea; (b) a human supplementation trial.
In part (a), 1 % (w/v) infusions of each tea were prepared in PBS at 90°C (infusion time 10 min), and then filtered. Freshly prepared infusions were diluted in PBS to give 0·1, 0·05, 0·01 and 0·005 % (w/v) tea solutions. The total antioxidant content (as the ferric-reducing antioxidant power value) was measured (in triplicate), as described previously(Reference Benzie and Szeto17, Reference Benzie and Strain33).
For in vitro testing of tea, pooled lymphocytes from five healthy volunteers were used. Lymphocytes were harvested from venous blood, aliquoted into Eppendorf tubes and cryopreserved following an established protocol(Reference Collins26, Reference Szeto and Benzie28). The cell count of lymphocyte suspensions to be cryopreserved is typically approximately 3 × 106/ml. On the days of testing, cryopreserved lymphocytes were thawed, washed in cold PBS and pelleted by centrifugation (10 min at 1900 rpm). The PBS was discarded, and the cells were immediately exposed to controlled oxidant challenge induced by H2O2. In this challenge step, 1 ml of H2O2 (at 30 and 60 μmol/l in PBS, with PBS as control) was added to each cell pellet, and cells were dispersed by gently tapping the tubes. The tubes were kept on ice for 5 min, then centrifuged as above and the supernatant was discarded. Each cell pellet was washed with 1 ml cold PBS, re-centrifuged and the PBS was removed. Eppendorf tubes were tapped gently to disperse the cells in the remaining PBS. The cells were immediately mixed with warm 1 % (w/v) low melting point agarose in PBS and embedded on microscope slides for comet assay testing, as described below.
For the comet assay, all reagents were of highest purity available and were from Sigma (St Louis, MO, USA) unless otherwise specified. Highly purified MilliQ water was used. Clean glass microscope slides were pre-coated with 1 % (w/v) standard agarose solution in PBS, and stored at room temperature. On testing days, 85 μl of warm 1 % (w/v) standard agarose in PBS was pipetted onto each slide required and covered with a cover slip (18 × 18 mm). The slides were placed at 4°C for about 5 min for the agarose to solidify. To the lymphocytes in Eppendorf tubes from the above in vitro tea pre-treatment and subsequent oxidant challenge steps, 1 % (w/v) low melting point agarose in PBS (at 37°C) was added. The tube was tapped gently to mix and 85 μl of the low melting point agarose/cell mixture was transferred onto the solidified agarose layer on a microscope slide. Cell numbers in 85 μl of each low melting point agarose/cell suspension are estimated to be approximately 2500. The cover slip was replaced and the slide was chilled for about 5 min. A duplicate set of slides was made for each treatment for each sample. Cover slips were removed, the slides were placed in lysis solution in a staining jar (with no contact between slides) and left at 4°C for 1 h. Lysis solution was 37·22 g of Na2EDTA, 146·1 g NaCl and 1·211 g of Tris dissolved in MilliQ water and the volume made up to 1 litre with MilliQ water; pH was adjusted to 10, if necessary, and 0·4 ml Triton X-100/40 ml was added to the solution immediately before use. After lysis, the slides were placed into 40 ml electrophoresis solution at 4°C for 20 min × 2 changes for DNA unwinding, after which slides were placed into an electrophoresis tank with gels just covered by pre-cooled electrophoresis solution. Electrophoresis solution was 12 g NaOH and 0·372 g of Na2EDTA dissolved in MilliQ water and the volume made up to 1 litre with MilliQ water. Electrophoresis was run in a cold room (4°C environment) for 30 min at 25 V (constant voltage setting). The current was set to 0·30 A by adjusting the level of electrophoresis solution. After electrophoresis, slides were removed from the tank and placed in a neutralising buffer at 4°C for 5 min with two changes. The neutralising buffer (pH 7·5) was 48·44 g Tris dissolved in MilliQ water and the volume made up to 1 litre with MilliQ water. The slides were then air-dried at room temperature and stained just before reading with 40 μl of 2 mg/l aqueous solution of ethidium bromide. The %DNA content in the comet tail of fifty cells on each slide (i.e. 100 cells for each treatment of each sample) was scored using a fluorescent microscope (Nikon Eclipse E600; Nikon, Tokyo, Japan) and Komet 5·5 computerised image capture and analysis system (Kinetic Imaging Limited, Liverpool, Merseyside, UK).
In part (b), a single-blinded, multiple cross-over intervention trial with eighteen healthy subjects (nine males and nine females), aged 35–50 years (mean 42·6 (sd 3·6) years) was performed. Subjects took 2 × 150 ml 1 % (w/v) freshly prepared green tea (or hot water as control) daily for 4 weeks. Subjects made their own tea following a set of standardised instructions and using the teabags and mugs provided. Subjects were non-smokers, without hypertension, not on regular medication and with no previous history of stroke, myocardial infarction, angina, diabetes mellitus, cancer or any illness that had required hospitalisation during the previous 12 months. Female volunteers were excluded if pregnant, lactating or on hormone replacement therapy. At entry, six subjects were allocated to each treatment (Longjing tea, screw-shaped tea or water). The study is described as single blinded because the analyst did not know the nature of each treatment, but it was unavoidable that subjects knew if they were taking tea or water. After a 6-week wash-out period, the procedure was repeated with subjects crossed-over onto one of the other two treatments. The procedure was repeated after a second 6-week washout. By the end of the study, all the subjects had taken all the three treatments. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the Human Subjects Ethics Sub-committee of The Hong Kong Polytechnic University. A written informed consent was obtained from all the subjects. Compliance was assessed by inquiry and by counting returned tea bags at the end of each intervention: return of < 15 % of the number of tea bags that should have been consumed was regarded as satisfactory compliance (i.e. at least 85 % compliance was needed). Fasting venous blood (heparinised) and urine samples (without preservative) were collected before and after each supplementation phase. The study protocol is outlined in Fig. 1.

Fig. 1 Overview of the supplementation study protocol. Fpg, formamidopyrimidine glycosylase; LC-MS/MS, liquid chromatography with tandem MS; 8-oxodG, 7,8-dihydro-2-deoxyguanosine.
All the samples were kept at 4°C until centrifuged and processed, which was within 2 h of collection. Urine samples were aliquoted and stored at − 80°C for batch analysis of 8-oxodG by liquid chromatography with tandem MS, which was performed as previously described in detail(Reference Lee, Chung and Benzie32). Urine creatinine was measured on the day of sample collection using a commercial Jaffe reaction test kit method. Lymphocytes were harvested from venous blood, aliquoted and cryopreserved following an established protocol(Reference Collins26, Reference Szeto and Benzie28). Cryopreserved lymphocytes in Eppendorf tubes were thawed at 37°C, washed in cold PBS, centrifuged briefly and the supernatant discarded. The cells were then subjected to a standard oxidant challenge (induced by H2O2 and following the same procedure as described above for the in vitro tea-treated lymphocytes). In addition, oxidation-induced damage in unchallenged cells was also measured, using the formamidopyrimidine glycosylase (Fpg) enzyme-assisted comet assay. Fpg creates breaks at oxidation-induced DNA lesions, increasing the specificity and sensitivity of the comet assay(Reference Collins and Dušinská34). In the Fpg-assisted comet assay testing, freshly thawed cells were not challenged with H2O2, but were washed, embedded and lysed as described above. After lysis, slides were transferred into a staining jar containing 40 ml Fpg enzyme buffer solution (pH 8·0) and left for 5 min. The buffer solution contains 0·04 g HEPES, 7·45 g KCl, 0·186 g Na2EDTA, 1·58 g Tris and 0·2 g bovine serum albumin dissolved in MilliQ water and the volume was made up to 1 litre. The buffer was drained and this washing procedure was repeated two more times. The slides were removed from the jar and 50 μl of Fpg enzyme solution in buffer (or 50 μl Fpg buffer as control) was pipetted onto each gel. The protein concentration of the Fpg enzyme solution was 0·72 mg/l, which was measured by Bradford's method(Reference Bradford35). Gels were covered with cover slips, and the slides were kept in a moist box (to prevent desiccation) at 37°C for 30 min. The cover slips were removed and the slides were transferred into electrophoresis solution at 4°C for 20 min with two changes of solution for DNA unwinding. Slides were placed into the electrophoresis tank with gels just covered by pre-cooled electrophoresis solution, and electrophoresis, neutralisation, air drying, staining and %DNA in comet tail scoring were performed as above.
Statistical analysis
Graphpad software (version 4.0; San Diego, CA, USA) was used. DNA damage (as %DNA in comet tail) results are expressed as means and standard deviations. Statistical analyses of in vitro effects of pre-treatment with green tea and of the supplementation-related changes in DNA damage were performed using Repeated-measures ANOVA with Dunnet's post-test. For urine 8-oxodG responses, the Kruskal–Wallis test was used. Spearman's correlation was used to investigate the relationship of urine 8-oxodG with Fpg-linked comet data. A P value of < 0·05 was considered statistically significant.
Power calculation
The key variables were %DNA in comet tail after: (i) oxidant challenge; (ii) Fpg treatment of unchallenged cells. Our previous work shows that for cells stressed with 30 μm-H2O2, the sd is 4·3 % DNA in the comet tail. Therefore, to detect a difference of ≥ 3·5% in the mean with 90 % power and at the 5 % significance level, it is estimated (by PS-Power and sample size calculation v. 3.0.4; http://www.power-analysis.com/home.htm) that eighteen subjects in a cross-over trial are needed. For Fpg-linked comet data, the sd is 3·5 % (our data). Therefore, to detect a difference of ≥ 3·0% in the mean with power of 90 % and at the 5 % significance level, sixteen subjects in a cross-over trial are needed. We selected 90 % power in initial calculations to allow for some missing data due to non-compliance or due to technical problems.
Results
The ferric-reducing antioxidant power values of freshly prepared 1 % (w/v) infusions of the two types of teas were similar: mean of triplicate measurements for Longjing tea was 6670 μmol/l, and for screw-shaped tea was 7220 μmol/l. The results of the genoprotective effects of Longjing and screw-shaped green teas at the various concentrations tested in vitro are shown in Figs. 2 and 3, respectively. No detectable DNA damage was caused by either tea at any of the concentrations tested. Oxidant challenge clearly induced marked increase in damage; however, there was significantly (P < 0·05) less DNA damage in challenged cells that had been pre-treated with low doses of green tea than the cells with no tea pre-treatment. In cells stressed with 30 μmol/l H2O2, there was 20–30 % less DNA damage in tea-treated cells compared with control cells, while in cells stressed with 60 μmol/l H2O2, there was 30–40 % less DNA damage in tea-treated cells compared with control cells. The two teas gave a similar level of protection against induced challenge. Interestingly, no dose–response effect was observed. Indeed, the highest protection was seen at the lowest dose (0·005 %, w/v) of each tea, although there was no statistically significant difference in effects across the different tea concentrations (Figs. 1 and 2).
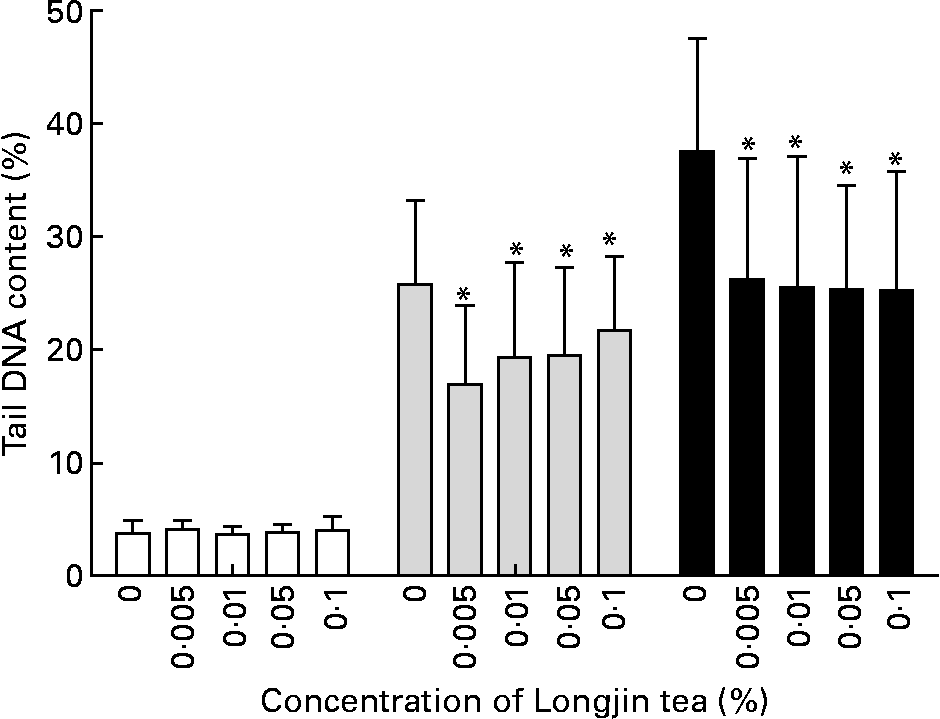
Fig. 2 Longjing tea in vitro treatment: %DNA in comet tail in lymphocytes pre-incubated with different concentrations (% w/v) of green tea followed by PBS treatment (no challenge (□)) or challenge with 30 μm-H2O2 (![]() ) or 60 μm-H2O2 (■). Results (mean values and standard deviations) on two gels/treatment (fifty cells scored in each gel). * Mean values were significantly different when compared with cells with same level of challenge but without pre-incubation with tea (P < 0·05).
) or 60 μm-H2O2 (■). Results (mean values and standard deviations) on two gels/treatment (fifty cells scored in each gel). * Mean values were significantly different when compared with cells with same level of challenge but without pre-incubation with tea (P < 0·05).

Fig. 3 Screw-shaped tea in vitro treatment: %DNA in comet tail in lymphocytes pre-incubated with different concentrations (% w/v) of green tea followed by PBS treatment (no challenge (□)), or challenge with 30 μm-H2O2 (![]() ) or 60 μm-H2O2 (■). Results (mean values and standard deviations) on two gels/treatment (fifty cells scored in each gel): * Mean values were significantly different when compared to cells with same level of challenge but without pre-incubation with tea (P < 0·05).
) or 60 μm-H2O2 (■). Results (mean values and standard deviations) on two gels/treatment (fifty cells scored in each gel): * Mean values were significantly different when compared to cells with same level of challenge but without pre-incubation with tea (P < 0·05).
In the supplementation study, one subject did not comply, and there were technical problems with some samples, leaving data from only thirteen complete sample sets (i.e. pre- and post-samples from each of the three treatments) for across-treatment analysis. In relation to protection against oxidant challenge with 30 and 60 μmol/l H2O2, there was approximately 22 % less damage in challenged cells collected from these thirteen subjects after 4 weeks' supplementation with Longjing tea: a similar decrease was observed after 4 weeks' supplementation with screw-shaped tea (Table 1). The response to each tea was statistically significant (P < 0·05) compared with placebo (water). The results of the Fpg-linked comet assay for pre- and post-supplementation samples are shown in Table 2. The DNA damage revealed was approximately 30 % less in cells collected post-supplementation with Longjing tea and approximately 35 % less after supplementation with screw-shaped tea. The response to each kind of tea was similar and statistically significant (P < 0·05) compared with the response to water.
Table 1 Results of oxidant challenge comet assay in lymphocytes collected pre- and post- 4 weeks' supplementation in thirteen human subjects in controlled intervention trial of multiple cross-over design†
(Mean values and standard deviations)

* Mean values were significantly different compared with response to placebo (water) treatment (P < 0·05).
† %DNA in comet tail.
Table 2 Results of formamidopyrimidine glycosylase (Fpg)-assisted comet assay in lymphocytes collected pre- and post- 4 weeks' supplementation in thirteen human subjects in intervention trial of multiple cross-over design†
(Mean values and standard deviations)

* Mean values were significantly different compared with response to placebo (water) treatment (P < 0·05).
† %DNA in comet tail.
Urine 8-oxodG results on pre- and post-supplementation samples are shown in Fig. 4. The levels varied widely and no significant changes in urine 8-oxodG were observed. No significant correlation was found between urine 8-oxodG and Fpg comet assay results in the subjects for whom time-matched data were available (Spearman's ρ = 0·19, P = 0·47, n 17).

Fig. 4 Creatinine-standardised urine 7,8-dihydro-2-deoxyguanosine (8-oxodG) concentrations before (![]() ) and after (□) 4 weeks’ green tea or water supplementation; results are median with interquartile ranges and lowest and highest values (n 14).
) and after (□) 4 weeks’ green tea or water supplementation; results are median with interquartile ranges and lowest and highest values (n 14).
Discussion
Oxidation-induced DNA damage can have profound effects in terms of mutation, altered homeostasis, apoptosis and malignant change in cells(Reference Beckman and Ames4, Reference Halliwell and Gutteridge5, Reference Møller and Loft25–Reference Hoelzl, Knasmuller and Mišík29). Therefore, the search for genoprotective ‘functional foods’ is important and of potentially high impact to human health. Diet is known to influence health, and in particular antioxidant-rich foods are beneficial, though the mechanisms are not clear(Reference Benzie and Wachtel-Galor1–Reference Key, Appleby and Rosell3). Green tea is one of the richest dietary sources of polyphenolic antioxidants and has many reputed health benefits, but human studies of genoprotection by green tea are few. Some studies have investigated the effects of green tea or green tea extracts using urine 8-oxodG(Reference Hakim, Harris and Brown36, Reference Luo, Tang and Tang37). However, urine 8-oxodG is not a biomarker of DNA damage per se. Rather, 8-oxodG in urine is thought to be from sanitation of the nucleotide pool and to reflect oxidative stress at the ‘whole-body’ level(Reference Cooke, Olinski and Loft30. To assess the DNA damage, the most sensitive and specific methods presently available are measurement of 8-oxodG within DNA and the comet assay for single-strand breaks(Reference Collins26–Reference Hoelzl, Knasmuller and Mišík29).
In regard to previously published reports on green tea and cellular 8-oxodG, no effect was seen in a controlled study of twenty subjects who took a single dose of green tea extract(Reference Henning, Niu and Liu24), but a placebo-controlled supplementation trial with 300 ml/d (the same dose used in the present study) of green tea for 7 d reported a significant decrease in lymphocytic 8-oxodG in forty-seven smokers and twenty-seven non-smokers(Reference Klaunig, Xu and Han19). In a comet assay study, lymphocytes from ten subjects collected after a single dose (540 ml) of green tea showed increased resistance to damage induced in vitro by exposure to UVA radiation, though this could have been due to direct UVA absorption by the tea(Reference Morley, Clifford and Salter38). An animal study(Reference Kager, Ferk and Kundi20) showed a marked decrease (46 %) in Fpg-sensitive lesions in rat colonocytes collected after 5 d of high-dose green tea (equivalent to a human dose of 500 ml green tea/d), but no effect was seen with a low dose (100 ml/d equivalent).
We have found only one published report that used the comet assay to investigate the genoprotective effects of green tea in a human supplementation study(Reference Erba, Riso and Bordoni18). A controlled study of parallel design was performed in which twelve women took an extract of green tea for 6 weeks. The results on the ten women who completed the trial showed approximately 30 % less DNA damage in lymphocytes after in vitro iron-induced oxidant challenge(Reference Erba, Riso and Bordoni18). In the present study, the two types of tea tested (Longjing tea and screw-shaped tea) showed significant genoprotective effects of similar magnitude in both the in vitro and supplementation studies. In in vitro experiments, there was approximately 30 % less DNA damage in H2O2-challenged cells that had been pre-incubated for 30 min in low concentration (0·01 % or less, w/v) green tea. More importantly, this increase in resistance to oxidant challenge was also seen in lymphocytes collected after 4 weeks' supplementation with green tea. Furthermore, pre-existing oxidation-induced DNA damage was approximately 30 % lower after 4 weeks' supplementation with green tea. The effect of each tea was similar, as was their antioxidant content.
The finding that very similar effects were observed with both types of green tea suggests that genoprotective effects might be generalised to green tea, though further study is needed to confirm this. The mechanism(s) of protection remains to be established, but could be due to direct antioxidant effects of absorbed polyphenols, to tea-induced up-regulation of endogenous cellular defences, to increased DNA repair or to a combination of both. We did not measure the polyphenolic content of the two teas, though we (and others) have found that this correlates very strongly with the antioxidant content(Reference Benzie and Szeto17). The two green teas tested (Longjing and screw-shaped) were found to have very similar antioxidant content, but they were not selected for the study on this basis. The teas were selected because they are both popular and commonly consumed in our region. Follow-up study looking at the effects of different doses of the same tea and the effects of teas of different antioxidant content would be of interest. It is also noted that we did not measure the plasma polyphenolic concentrations. Future study is needed to determine if the genoprotective effects relate to plasma polyphenolic concentrations. It is worth noting that the in vitro protective effects of the green teas were observed at very low concentrations, but there was no apparent dose–response. In usual dietary practice, tea is generally taken at approximately 1 % (w/v), but bioavailability of tea polyphenols is poor(Reference Khan and Mukhtar13, Reference Williamson and Manach15, Reference Suh, Chon and Oh21). The low concentrations (0·005–0·1 %, w/v) in the in vitro experiments were used to emulate the in vivo concentrations reached after drinking tea. Interestingly, polyphenol-rich compounds, including green tea, generate H2O2 in solution and can damage DNA in vitro (Reference Suh, Chon and Oh21, Reference Halliwell22, Reference Wachtel-Galor, Choi and Benzie39). Whether this occurs in vivo is not known, but H2O2 is found in various biological fluids and its concentration in urine increases after the ingestion of polyphenolic-rich beverages(Reference Halliwell22, Reference Yuen and Benzie40). Indeed, it is possible to speculate that this may be the reason why we did not observe a dose–response relationship between green tea and in vitro protection. There may be a pro-oxidant/antioxidant ‘break even’ point where, at higher concentrations, the protective effect of tea polyphenols is opposed by the damaging effect of H2O2 generated by them. This requires further study, but of interest is the in vitro finding that epigallocatechin gallate, the main green tea polyphenol, can induce the redox-sensitive antioxidant response element, which has various downstream cytoprotective effects(Reference Surh, Kundu and Na41–Reference Soares and Bach43). This cellular adaptation to a pro-oxidant change in redox tone (oxidative stress) is mediated by the transcription factor NrF2 (nuclear factor erythroid 2-related factor 2), and antioxidant response element induction results in, among others, increased expression of haeme oxygenase-1, glutathione and antioxidant and DNA repair enzymes(Reference Eggler, Gay and Mesecar42–Reference Benzie and Wachtel-Galor45). Such cellular adaptations would lead to decreased basal DNA damage through a combination of greater resistance to oxidant challenge and enhanced repair. Given the short incubation time (30 min) used in the in vitro part, we suggest that cytoprotective adaptations were unlikely to be responsible for the protective effects observed. There were more likely to be due to direct antioxidant action against the in vitro oxidant challenge. However, an adaptive mechanism may have been involved in the human intervention study. This is a potentially rewarding area for further study. No evidence of tea-related effects on whole-body oxidative stress, assessed by urine 8-oxodG, was observed in the present study. Urine 8-oxodG might be expected to decrease if there is improved direct antioxidant protection of the nucleotide pool(Reference Cooke, Olinski and Loft30–Reference Lee, Chung and Benzie32). However, despite the two teas having high (and very similar) in vitro total antioxidant content, we saw no change in urine 8-oxodG levels with tea supplementation. It is noted that biological variation in urine 8-oxodG is wide(Reference Lee, Chung and Benzie32), and a larger study might be needed to reveal the effects on this biomarker. Alternatively, there could be a targeting of antioxidant action at the DNA level or, as noted above, genoprotection could be due to an effect that is not related to simple ‘antioxidant’ action, but due to the creation of a mild but ultimately cytoprotective pro-oxidant change(Reference Benzie and Wachtel-Galor45).
In conclusion, both the in vitro treatment of cells and the 4 weeks' supplementation with a dietary-relevant dose of green tea were associated with a significant decrease in oxidation-induced DNA damage and a significant increase in resistance of DNA to oxidant challenge. Two green teas of similar antioxidant content gave similar protective effects in vitro and in vivo. No change in a specific biomarker of whole-body oxidative stress (urine 8-oxodG) was observed. The mechanisms and clinical relevance of this genoprotective effect of green tea are not yet clear, but it is reasonable to speculate that less DNA damage decreases the likelihood of mutation and phenotypic changes that lead to deleterious effects on health. Therefore, these genoprotective effects of green tea lend support to its use as a functional food and provide scientific evidence for the more confident recommendation of regular intake of green tea for health promotion.
Acknowledgements
The authors are grateful to The Hong Kong Polytechnic University for financial support for the present study and to the Ying Kee Tea House, Hong Kong for supplying the green tea used. We also thank the volunteers who took part in the study, and Professor Andrew Collins of University of Oslo, Norway, for supplying the Fpg enzyme. The authors have no conflict of interest. The work was financially supported by the Hong Kong Polytechnic University. Ying Kee Tea House, Hong Kong, supplied the green tea used. K. C. H. performed the in vitro and in vivo comet assay testing and helped in subject recruitment and follow-up. W. C. W. supervised the sample collection and initial work-up, supervised comet assay testing and performed the urine 8-oxodG measurements. I. F. F. B. supervised all aspects of the design and execution of the study. All the authors were involved in the statistical analysis and preparation of this paper.








