Obesity is characterised by chronic low-grade inflammation(Reference Monteiro and Azevedo1). Its aetiology is multifactorial, and the current epidemic is partially due to the increased availability and consumption of highly palatable diets and reduced energy expenditure(Reference Astrup, Buemann and Western2), which leads to increased adipose tissue. It is recognised as one of the major risk factors for the development of chronic and disabling diseases(3).
White adipose tissue is a dynamic endocrine organ that releases several adipokines and pro-inflammatory factors(Reference Henry, Bensley and Wood-Bradley4). It has been shown that high levels of pro-inflammatory adipokines in obesity may contribute to the reduction in lipid oxidation in insulin-sensitive organs, leading to lipotoxicity and insulin resistance(Reference Zhang and Zhang5). IL-6 is an important acute-phase mediator with both pro- and anti-inflammatory properties(Reference Tilg, Trehu and Atkins6), and exhibits many biological functions. In addition to its role as the main acute-phase protein synthesis regulator, it is induced with other cytokines, such as TNF-α(Reference Eklund7). TNF-α is a key modulator of adipocyte metabolism, with a direct role in several insulin-mediated processes, including glucose homeostasis and lipid metabolism. High levels of TNF-α are a major contributor to the development of adipose tissue insulin resistance(Reference Arner8). Also, it is associated with significant tissue damage from reactive oxygen species and the promotion of angiogenesis(Reference Khera, Dick and Nicholson9). Moreover, elevated TNF-α concentrations and IL-6 have been linked to insulin resistance in obesity(Reference Illán-Gómez, González-Ortega and Orea-Soler10). In addition, IL-6 and TNF-α promote leptin production by the adipose tissue, but leptin enhances inflammatory cytokine production as well(Reference Zhang and Zhang5). Hyperleptinaemia is correlated with pro-inflammatory responses and with the chronic sub-inflammatory state observed in obesity(Reference Fantuzzi11). Moreover, leptin induces cholesterol uptake by macrophages, angiogenesis and platelet aggregation, and stimulates oxidative stress in endothelial cells, inhibiting vasorelaxation and increasing the risk of atherosclerosis(Reference Rasouli and Kern12). Resistin, another pro-inflammatory adipokine, appears to act through binding to Toll-like receptor 4, a cell-surface receptor and a key component of the inflammatory response to bacterial lipopolysaccharide(Reference Tarkowski, Bjersing and Shestakov13). Located at the site of inflammation, resistin is a molecule that shows a strong correlation with other inflammatory markers, such as IL-6 and TNF-α(Reference Bokarewa, Nagaev and Dahlberg14). Also, resistin is associated with decreased insulin sensitivity(Reference Tarkowski, Bjersing and Shestakov13) and seems to be correlated with the development of obesity-related diseases, such as non-alcoholic fatty liver disease(Reference Pagano, Soardo and Pilon15, Reference Singhal, Patel and Qi16). Studies have suggested that circulating concentrations of pro-inflammatory molecules reflect excess body fat and predispose an individual to a higher risk of developing metabolic diseases(Reference Indulekha, Anjana and Surendar17, Reference Stirnadel, Lin and Ling18). In addition, the adipose tissue hypersecretion of pro-inflammatory adipokines, such as IL-6, TNF-α, leptin and resistin, may play an important role in the pathophysiology of obesity-related complications(Reference Hauner19).
Lycopene is a lipophilic carotenoid which is responsible for the red colour in various fruits and vegetables(Reference Khachik, Carvalho and Bernstein20) and is commonly found in tomatoes(Reference Mangels, Holden and Beecher21). This carotenoid is well known for its antioxidant properties(Reference Di Mascio, Kaiser and Sies22–Reference Ferreira, Russell and Rocha24), and has been reported to display anti-inflammatory effects in adipocytes(Reference Marcotorchino, Romier and Gouranton25) and liver(Reference Bignotto, Rocha and Sepodes26), along with preventing CVD(Reference Hung, Huang and Chen27). Evidence is increasing that lycopene or tomato preparations can decrease inflammatory markers(Reference Marcotorchino, Romier and Gouranton25–Reference Gouranton, Thabuis and Riollet28), and may improve diseases with chronic inflammatory backgrounds such as obesity(Reference Ghavipour, Saedisomeolia and Djalali29). However, the effect of lycopene on pro-inflammatory adipokines, especially leptin and resistin, in obesity has not yet been evaluated.
Since pro-inflammatory adipokines, such as IL-6, TNF-α, leptin and resistin, have been linked to adiposity, and lycopene presents anti-inflammatory effects, we hypothesise that lycopene supplementation can modulate epididymal adipose tissue in vivo, reducing the expression of pro-inflammatory cytokines in obesity. The decreased production of these adipokines by lycopene could have a major impact on obesity and the prevalence of obesity-related diseases.
Methods
Animals and experimental protocol
Male Wistar rats (10 weeks old, weighing approximately 350 g), from the Animal Center of Botucatu Medical School, São Paulo State University, UNESP (Botucatu, SP, Brazil), were initially divided to receive either a commercial chow diet (C, n 6; 12 % energy from fat) or a high-fat diet (49·7 % energy from fat) and sugar in the drinking water (300 g/l) (DIO, n 12), for 6 weeks. The high-fat diet was designed in our laboratory to contain a powdered commercial chow diet – NUVILAB CR-1 (Nuvital®; Sogorb Indústria e Comércia Ltda), a wafer biscuit, condensed milk, palm oil, vitamins and minerals. The diet-induced obesity model was adapted from our previous study(Reference Nascimento, Sugizaki and Leopoldo30), which was used to mimic obesity from Western occidental dietary habits. The nutritional composition of the diets is presented in Table 1. After 6 weeks under a nutritional overload, DIO rats were randomly assigned into two groups: DIO (n 6) and DIO supplemented with lycopene-rich tomato oleoresin (DIO+L, n 6). Tomato oleoresin was mixed with maize oil equivalent to 10 mg lycopene/kg body weight (BW) per d(Reference Ibrahim, Ahmed and El-din31, Reference Ali and Agha32) and given orally every morning for a 6-week period(Reference Bahcecioglu, Kuzu and Metin33, Reference Wang, Ausman and Greenberg34). To avoid differences in the energy provided, all groups received the same maize oil volume (approximately 2 ml/kg BW per d). Rats were housed in individual cages in an animal facility at the Internal Medicine Experimental Laboratory, Botucatu Medical School, UNESP, under a controlled ambient temperature (22–26°C) and lighting (12 h light–12 h dark cycle) condition. Dietary consumption was measured daily, and BW was assessed weekly. The animals were killed by decapitation under deep sodium pentobarbital anaesthesia (50 mg/kg, intraperitoneal injection). Plasma and epididymal adipose tissues were collected at 12 weeks and stored at − 80°C until ready for analysis. Epididymal adipose tissue was selected because of its similar inflammation patterns in visceral fat(Reference Gea-Sorlí, Bonjoch and Closa35). The experiment was conducted in accordance with the Guidelines for the Care and Use of Experimental Animals and the diets followed the specifications from Nutrient Requirements of the Laboratory Rats. The protocol was approved by the local Ethical Committee for Animal Research (protocol no. 920-2012).
Table 1 Nutritional composition of the diets
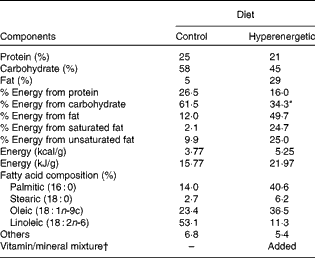
* Energy from sugar in the drinking water (300 g/l) was not included.
† Based on the vitamin/mineral amounts of the chow diet, for each kg of the hyperenergetic diet, the following nutrients were added: Fe, 25·2 mg; K, 104·8 mg; Se, 73·1 μg; molybdenum sulphate, 150·0 μg; vitamin B12, 34·5 μg; vitamin B6, 6 mg; biotin, 0·12 mg; vitamin E, 32·6 mg; vitamin D, 61·2 μg; vitamin A, 4·6 mg.
Lycopene preparation
Tomato oleoresin (Lyc-O-Mato 6 % dewaxed; LycoRed Natural Products Industries) was mixed with maize oil and stored at 4°C in the dark until used as described previously(Reference Ferreira, Russell and Rocha24). The tomato oleoresin–maize oil mixture was stirred for 20 min in a water-bath at 54°C before being fed to the animals. Each millilitre of the solution contained 5 mg of total lycopene. Stability of lycopene was monitored at 450 nm, and confirmed by diode-array spectra, as described previously(Reference Yeum, Booth and Sadowski36). Lycopene was stable in the tomato oleoresin–maize oil mixture for 9 weeks at − 20°C.
Total body fat
Total body fat was measured as the sum of epididymal, retroperitoneal and visceral fat deposits, and was used to calculate the adiposity index(Reference Nascimento, Luvizotto and Leopoldo37) to confirm obesity in the animals.
Plasma lycopene analysis
A 400 μl aliquot of plasma was used for lycopene analysis, as described previously(Reference Ferreira, Russell and Rocha24). Briefly, plasma samples were extracted with 3 ml chloroform–methanol (2:1) followed by 3 ml hexane. The samples were dried under N2 and resuspended in 100 μl ethanol, of which 25 μl were injected into the HPLC. The results were adjusted by an internal standard containing echinenone. The inter- (n 3) and intra-assay (n 8) CV was 9 %. The recovery of the added internal standard was consistently >90 %. All sample processing and analyses were performed under red light.
Biochemical measurements
Glucose concentration was assayed from the tip of the tail and determined by using a glucometer (Accu-Chek Go Kit; Roche Diagnostic Brazil Limited). Hormonal concentrations of insulin, leptin, adiponectin (Millipore), resistin (Immuno-Biological Laboratories, Inc.), TNF-α and IL-6 (R&D Systems, Inc.) were measured by an immunoassay, using a microplate reader (Spectra Max 190; Molecular Devices). The glucose:insulin ratio was used for insulin sensitivity assessment(Reference Coatmellec-Taglioni, Dausse and Ribière38).
Gene expression
Total RNA was extracted from epididymal adipose tissue using the reagent TRIzol (Invitrogen). The SuperScript II First-Strand Synthesis System for RT-PCR (Invitrogen) kit was utilised for the synthesis of 20 μl of complementary DNA from 1000 ng of total RNA. The mRNA levels of leptin (assay Rn 00565158_m1; Applied Biosystems), resistin (assay Rn 00595224 m1; Applied Biosystems), TNF-α (assay Rn 00562055_m1; Applied Biosystems), IL-6 (assay Rn 01410330_m1; Applied Biosystems) and monocyte chemoattractant protein-1 (MCP-1, assay Rn 00580555_m1; Applied Biosystems) were determined by real-time PCR. Quantitative measurements were made with a commercial kit (TaqMan qPCR; Applied Biosystems) in a detection system (StepOne Plus; Applied Biosystems). Cycling conditions were as follows: enzyme activation at 50°C for 2 min, denaturation at 95°C for 10 min, complementary DNA products were amplified for forty cycles of denaturation at 95°C for 15 s and annealing/extension at 60°C for 1 min. Gene expression was quantified in relation to the values of the C group after normalisation by an internal control (cyclophilin: assay Rn 00690933_m1; Applied Biosystems) by the method 2-ΔΔC T, as described previously(Reference Livak and Schmittgen39).
Statistical analysis
Results are expressed as means and standard deviations, and significance of differences were calculated by one-way ANOVA followed by Tukey's post hoc test, using SigmaStat version 3.5 for Windows (Systat Software, Inc.). Differences were considered significant at P< 0·05. Power calculations for the main outcome variables were above 80 %.
Results
Body weight and body fat
Food intake was reduced in the DIO groups; however, energy intake was similar among the groups. The animals showed the same BW at baseline. At the end of the experiment, the hyperenergetic-fed animals showed a significant BW and adiposity index when compared with control rats. In comparison with the DIO group (Table 2), consumption of the lycopene-containing maize oil mixture (DIO+L) did not interfere with BW and the adiposity index (Table 2).
Table 2 Body weight (BW), dietary intake and adiposity index (Mean values and standard deviations, n 6)
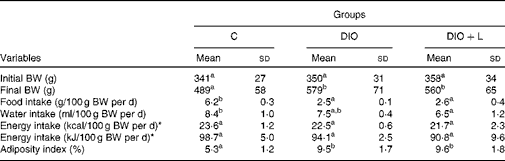
C, control; DIO, animals subjected to diet-induced obesity; DIO+L, DIO supplemented with lycopene for 6 weeks.
a,bMean values with unlike superscript letters were significantly different (P< 0·05; one-way ANOVA with Tukey's post hoc test).
* Energy intake includes energy from sugar in the drinking water.
Lycopene uptake and absorption
In the present study, lycopene was analysed as the total of both cis and all-trans isomers in plasma. Due to the lack of lycopene in the fed diets(Reference Ferreira, Russell and Rocha24), there was no detectable lycopene in the plasma of the C or DIO groups. However, after 6 weeks of carotenoid supplementation, lycopene plasma concentrations were evident in the DIO+L group (Table 3).
Table 3 Plasma measurements of lycopene, adipokines, glucose, insulin and the glucose:insulin ratio (Mean values and standard deviations, n 6)
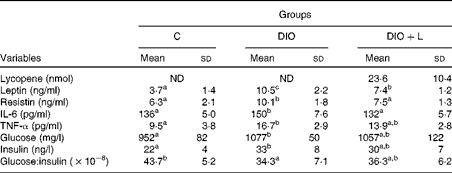
C, control; DIO, animals subjected to diet-induced obesity; DIO+L, DIO supplemented with lycopene for 6 weeks; ND, not detected.
a,b,cMean values with unlike superscript letters were significantly different (P< 0·05; one-way ANOVA with Tukey's post hoc test).
Insulin sensitivity
The hyperenergetic diet was associated with a significant increase in glucose and insulin levels when compared with the C animals, showing reduced insulin sensitivity. Glucose and insulin levels were not modulated by lycopene supplementation. Also, lycopene showed no effect on insulin sensitivity (Table 3).
Adipokine levels
The consumption of the hyperenergetic diet was associated with a significant increase in inflammatory marker expression, such as IL-6, MCP-1 and leptin. However, resistin and TNF-α expression in epididymal adipose tissue showed no difference between the C and DIO groups. Lycopene supplementation restored the gene expressions of IL-6, MCP-1 and leptin to the C levels, while a decreased resistin gene expression in epididymal adipose tissue (Table 4). The plasma levels of TNF-α, IL-6, leptin and resistin were found to be significantly elevated in the DIO group. Lycopene supplementation significantly decreased leptin levels, and restored the plasma concentrations of IL-6 and resistin to the C levels. There was no difference between the DIO and DIO+L groups in plasma TNF-α concentrations (Table 3). As a recent inflammatory biomarker(Reference Jang, Kim and Park40), the leptin:adiponectin ratio was calculated. The DIO group showed an increase in the leptin:adiponectin ratio (C: 0·21 (sd 0·07) v. DIO: 1·11 (sd 0·26), P< 0·001), while the lycopene-supplemented group presented a lower ratio (DIO: 1·11 (sd 0·26) v. DIO+L: 0·56 (sd 0·11), P< 0·001).
Table 4 Adipokine mRNA levels in epididymal adipose tissue (Mean values and standard deviations, n 6)
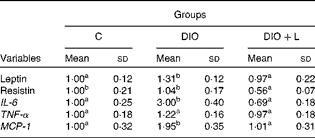
C, control; DIO, animals subjected to diet-induced obesity; DIO+L, DIO supplemented with lycopene for 6 weeks; MCP-1, monocyte chemoattractant protein-1.
a,bMean values with unlike superscript letters were significantly different (P< 0·05; one-way ANOVA with Tukey's post hoc test).
Discussion
Obesity is usually associated with the consumption of hyperenergetic diets and a decrease in energy expenditure(Reference Astrup, Buemann and Western2), resulting in the expansion of the adipose tissue mass and inducing a chronic inflammatory state(Reference Hauner19). A high SFA intake from a hyperenergetic diet has been associated with obesity-linked inflammation, and induces inflammation-related gene expression in adipose tissue(Reference van Dijk, Feskens and Bos41). In the present study, lycopene displayed anti-inflammatory effects(Reference Marcotorchino, Romier and Gouranton25, Reference Gouranton, Thabuis and Riollet28) in plasma (0·01–0·04 μm) after 6 weeks of supplementation in concentrations below the range that can be normally seen in human subjects (0·2–0·9 μm)(Reference Boileau, Boileau and Erdman42). This can be related mainly to low lycopene bioavailability(Reference Boileau, Boileau and Erdman42), and also in part to the animals fasting overnight. A previous study has found that a peak accumulation of lycopene in rat plasma occurs between 4 and 8 h after a single oral administration(Reference Mathews-Roth, Welankiwar and Sehgal43).
Experimental high-fat diet models are considered appropriate to study obesity and its consequences(Reference Hariri and Thibault44). In addition to BW gain, the present experimental model induced an increase in body adiposity from the DIO animals (DIO and DIO+L)(Reference Ainslie, Proietto and Fam45, Reference Roberts, Barnard and Liang46). As with our recent study(Reference Nascimento, Luvizotto and Leopoldo37), animals from the DIO groups consumed smaller dietary amounts and similar energy intakes than those without the treatment (C group; Table 2). Even though the protocol devised for the present study was able to induce obesity, it showed that body adiposity is the best indicator of obesity(Reference Nascimento, Sugizaki and Leopoldo47). The expansion in weight, and especially body fat, occurs because the augmentation of fat consumption is not accompanied by a proportional increase of fat oxidation. This leads to the deposit of fat as TAG(Reference Tentolouris, Pavlatos and Kokkinos48, Reference Schrauwen and Westerterp49) in adipose tissue.
Many studies have demonstrated that white adipose tissue represents an important site of inflammation(Reference Monteiro and Azevedo1), showing insulin resistance and direct associations between adipose tissue and concentrations of TNF-α, IL-6 and C-reactive protein(Reference Indulekha, Anjana and Surendar17). Here, we demonstrate, in vivo, that the DIO-treated group showed an increase in the epididymal adipose tissue gene expression of IL-6 and MCP-1 (Table 4) and in the plasma concentrations of IL-6 and TNF-α (Table 3). Given that mRNA levels do not always represent the protein content, which exerts functional activity, plasma concentration is more relevant than mRNA levels. The divergent data between mRNA levels and plasma concentrations of TNF-α could be explained in part by the stability and translational efficiency of mRNA(Reference Khera, Dick and Nicholson9). Similarly, there was a decrease in insulin sensitivity accompanied by an increase in glucose and insulin levels (Table 3). Plasma IL-6 concentrations (Table 3) and the gene expression of MCP-1 and IL-6 (Table 4) were restored by lycopene supplementation in vivo, which is in agreement with the data of ex vivo and adipocyte models(Reference Gouranton, Thabuis and Riollet28). However, plasma TNF-α, glucose and insulin levels and insulin sensitivity (Table 3) were not modulated by the 6 weeks of lycopene supplementation in the present study. The probable mechanism that clarifies this lack can in part be explained by increased TNF-α. In fact, TNF-α seems to act locally at the site of adipose tissue through autocrine or paracrine pathways, affecting insulin resistance(Reference Arner8) by insulin receptor substrate proteins(Reference Hotamisligil50). The processes affecting insulin receptor substrates involve proteasome-mediated degradation, phosphatase-mediated dephosphorylation and Ser phosphorylation of insulin receptor substrate-1, which converts insulin receptor substrate-1 into an inhibitor of the insulin receptor Tyr kinase activity(Reference White51, Reference Pirola, Johnston and Van Obberghen52).
Leptin is a pleiotropic adipocytokine produced and secreted by adipose tissue(Reference Fantuzzi11). Experimental studies have suggested that leptin sensitivity may be under hormonal and nutritional control(Reference Bennet, Lindell and Karlsson53). As reported in the studies of diet-induced obesity(Reference López, Marti and Milagro54, Reference Wang, Orci and Ravazzola55), an increase of leptin in epididymal adipose tissue gene expression (Table 4) and plasma levels (Table 3) was found in DIO animals in the present experiment. Previous reports have shown a positive correlation between adipose tissue amounts and the expression of leptin(Reference Frederich, Löllmann and Hamann56, Reference Luvizotto, Nascimento and Síbio57), indicating that increased levels of leptin result from increased body fat(Reference Schrauwen and Westerterp49). Although there was no change in the adiposity index by lycopene supplementation (Table 2), the present data show that leptin plasma levels were lower in the DIO+L group, possibly by the down-regulation of its gene expression level in epididymal adipose tissue. High levels of leptin can stimulate pro-inflammatory cytokines and play an important role in obesity(Reference Fantuzzi11, Reference Sierra-Honigmann, Nath and Murakami58). In addition, hyperleptinaemia has been correlated with inflammation levels, since its serum concentration is elevated during active disease (rheumatoid arthritis), and is decreased when the disease is controlled(Reference Lee, Park and Park59, Reference Targonska-Stepniak, Majdan and Dryglewska60). Also, a recent study has reported that the leptin:adiponectin ratio is correlated with BMI and may be a useful biomarker for inflammation(Reference Jang, Kim and Park40). Here, we demonstrated that the increased leptin:adiponectin ratio in the DIO group was significantly decreased by lycopene supplementation. This suggests inflammatory attenuation associated with lycopene treatment in obese rats. Given that obesity is recognised as a chronic and systemic inflammatory disease(Reference Gregor and Hotamisligil61), the present data suggest that lycopene supplementation may attenuate the inflammatory response in obesity, at least in part, by minimising hyperleptinaemia and improving the leptin:adiponectin ratio.
Resistin concentration has been reported to be increased in obesity(Reference Luvizotto, Nascimento and Síbio57, Reference Satapathy, Ochani and Dancho62), and to be a link to obesity and insulin resistance(Reference Steppan, Bailey and Bhat63). The DIO animals did not show an increase in resistin gene expression in epididymal adipose tissue when compared with the C group (Table 4). However, resistin plasma concentration was greater in the DIO group than in the C group (Table 3). Increased resistin expression has been correlated with inflammatory markers, coronary artery disease(Reference Ohmori, Momiyama and Kato64) and atherosclerosis in patients with the metabolic syndrome(Reference Reilly, Lehrke and Wolfe65). Furthermore, resistin itself has been found to induce the expression of cytokines and chemokines in human articular chondrocytes(Reference Zhang, Xing and Hensley66). Also, in patients with gestational diabetes, elevations in serum resistin were correlated with serum IL-6 levels, but not with insulin levels. This suggests that changes in insulin sensitivity in these patients were mediated by inflammatory pathways that may involve resistin(Reference Kuzmicki, Telejko and Szamatowicz67). Both gene expression and plasma resistin levels were decreased with lycopene supplementation, which has been shown to display anti-inflammatory effects(Reference Marcotorchino, Romier and Gouranton25, Reference Gouranton, Thabuis and Riollet28), suggesting less inflammation in adipose tissue. The exact mechanism of lycopene affecting leptin and resistin levels remains to be determined. To the best of our knowledge, this is the first study which shows that lycopene can modulate both leptin and resistin gene expression and plasma concentrations in obese rats.
In summary, it was observed that lycopene has the ability to down-regulate adipokine mRNA levels in epididymal adipose tissue, such as leptin, resistin, IL-6 and MCP-1, along with the ability to restore leptin, resistin and plasma IL-6 concentrations in diet-induced obese rats. Therefore, it is highly probable that lycopene supplementation attenuates inflammation levels in adipose tissue. This could evidence the health effects of this carotenoid. This is the first time that lycopene has been shown to modulate leptin and resistin levels. Therefore, dietary lycopene may be proposed as an effective strategy to reduce inflammation in diet-induced obesity. Although the adopted experimental design did mimic the clinical situation, it gives no information regarding as to whether these findings are applicable to human subjects or not. However, it addresses some important benefits by using additional non-pharmacological therapy that is based on natural compounds in the treatment of human obesity. Moreover, the present study represents a contribution to the role of lycopene on inflammation related to obesity.
Acknowledgements
We thank Mario B. Bruno, José Carlos Georgette and Renata Capela for their technical support. We appreciate Barbara B. Golner for correcting the English grammar. We are grateful to LycoRed Natural Products Industries, Beer-Sheva, Israel, for supplying the tomato oleoresin. We also thank FAPESP (no. 10/06100-9, 10/19746-4, 11/19847-8 and 11/22786-0) for financial support. The authors' contributions were as follows: A. L. A. F., R. A. M. L. and A. F. N. designed the research; R. A. M. L., A. F. N., E. I., D. T. P., S. J. C. and C. R. C. conducted the research; A. F. N. and R. A. M. L. analysed the data; R. A. M. L., K.-J. Y. and A. L. A. F. wrote the paper. All authors read and approved the final manuscript. None of the authors had a personal or financial conflict of interest.






