Feeding behaviour and other lifestyle habits play a major role in health optimisation, obesity and metabolic disorder control(Reference Roberts, Campbell and Troop1). The obesity crisis and obesity-associated diseases have heightened public health concerns in many societies(Reference Izadi, Radahmadi and Ghasemi2,Reference Patel3) . Among more than 1 billion adults who are currently overweight worldwide, at least 300 million individuals are clinically diagnosed as obese. Obesity and being overweight pose a major risk of other chronic diseases(Reference Xiao, Liu and Cline4).
Feeding behaviour refers to all habits related to receiving/consuming food and containing essential nutrients. Feeding behaviour is physiologically controlled through processes associated with energy and nutrient needs, such as appetitive, reward and fear behaviours as well as food consumption(Reference Douglass, Kucukdereli and Ponserre5,Reference Livneh and Andermann6) . Food consumption is a highly complex survival. Different factors may participate in feeding regulation, such as neural response in several brain nuclei, hormonal signalling (of leptin, ghrelin, insulin and cortisol) and environmental factors(Reference LeDoux7). Among these factors, the role of neural pathways and/or brain nuclei is more significant than other ones(Reference LeDoux7). Regulating the feeding behaviour via neural changes has a major role in the maintenance of body energy balance. Recent research studies have recognised various neural circuits involving in homoeostatic feeding control. These circuits act in cooperation with the regulation of feeding(Reference Douglass8). Understanding those brain nuclei that are involved in feeding behaviour and their connections could be effective in developing and maintaining the treatments concerning the feeding disorders. Hence, identifying the brain nuclei cell types and their specific functions in the regulation of feeding behaviour is very significant for consciously choosing between the available clinical approaches.
Some studies have emphasised the major role of the hypothalamus and mesolimbic dopaminergic systems as key mediators in regulating food intake behaviour(Reference Narayanan, Guarnieri and DiLeone9,Reference Sweeney and Yang10) . Nevertheless, the amygdala is seen as the main brain region with a critical role in feeding regulation(Reference Aschbacher, Kornfeld and Picard11). The central amygdala (CeA) is a significant extra-hypothalamic region that has a determining impact on different physiological aspects and nutrition behaviours(Reference Anesten, Dalmau Gasull and Richard12,Reference Qiao, Ren and Li13) . Several CeA inputs/outputs provide a vast connection surface for this core(Reference Lu, Chen and Wei14). The CeA is also involved in different brain functions, such as regulating various emotional states (e.g. stress, fear, anxiety, pain and motivation), cognition, energy balance, reward system, food consumption and digestive behaviour(Reference Bernard, Huang and Besson15–Reference Zhang, Li and Guo27). Because it is part of the amygdala–hypothalamic complex circuit, a significant role of the CeA in feeding behaviour is established, respectively, through direct and indirect projections from the brain stem and the paraventricular nucleus (PVN) of the hypothalamus(Reference Petrovich and Gallagher23,Reference Merali, McIntosh and Kent28,Reference Petrovich, Canteras and Swanson29) . Based on the results of previous studies, electrical lesions of the CeA could induce changes in feeding behaviour(Reference Petrovich, Ross and Mody24). Petrovich et al. (2009) designed an experiment concerning the roles of CeA and basolateral amygdala (BLA) in associative learning. The CeA and BLA were bilaterally neurotoxic-lesioned in rats under an aversive cue that induced feeding cessation (presentation of a tone paired with foot shocks). When BLA-lesioned rats were presented with this aversive cue, they showed inhibition of eating. In contrast, CeA lesions in rats under the same protocol did not lead to a similar effect. Hence, the CeA, but not BLA, is crucial to controlling feeding by an aversive cue. An evidence has shown that CeA is an important nucleus in regulating feeding behaviour in aversive conditioning(Reference Petrovich, Ross and Mody24).
The CeA has extensive connections with other important brain centres that are involved in nutritional and reward systems, as well as intrinsic features of the nucleus. Therefore, the role of CeA compared with other brain regions in feeding regulation is more critical(Reference Douglass8). Also, since various types of information come together in the CeA (from the cortical region to the subcortical area, reward system and limbic system), the functions of this core are more distinctive than other brain regions(Reference Fadok, Markovic and Tovote18). The CeA acts as the major output nucleus of the amygdala(Reference Ehrlich, Humeau and Grenier17). Finally, the significance of CeA relates to different functions of the neurotransmitters and hormones involving in feeding behaviour(Reference Anesten, Dalmau Gasull and Richard12,Reference Kim, Zhang and Muralidhar21,Reference Jin, Jiang and Luan30–Reference Zhang-Molina, Schmit and Cai32) . Several brain nuclei participate in the regulation of feeding behaviour, and many studies have focused on identifying the neural mechanisms of food intake regulation(Reference Izadi, Radahmadi and Ghasemi2,Reference Petrovich33) ; however, the CeA is largely understudied. Therefore, this review aims to highlight the significance of the CeA nucleus in food consumption by investigating its interaction with various physiological systems, such as the reward, digestive and emotional ones.
The amygdala
The amygdala is a collection of cells located near the brain stem. As part of the brain’s limbic system, it is located bilaterally in the medial temporal lobes of the brain(Reference Janak and Tye34). Also, it is involved in different functions, including emotional processing, and has a pivotal role in memory-related, feeding and appetitive behaviours(Reference Petrovich and Gallagher23,Reference Zhang, Li and Guo27,Reference Janak and Tye34) . Even though at least thirteen different sub-nuclei compose this nucleus, the most clearly defined amygdala sub-nuclei are, respectively, the lateral (LA), BLA and central (CeA) nuclei of the amygdala(Reference Ehrlich, Humeau and Grenier17,Reference Gilpin, Herman and Roberto35) . The LA is the major site that receives inputs from visual, auditory and somatosensory (including pain) systems(Reference LeDoux7). The BLA is critical for acquisition and expression of fear conditioning(Reference LeDoux7) as well as the control of emotional behaviours(Reference Murray36). The neural circuits of BLA to CeA are important for appetitive behaviours(Reference Petrovich, Ross and Mody24) because different BLA neurons project to CeA neurons that mediate and/or suppress appetitive behaviours(Reference Kim, Zhang and Muralidhar21). Accordingly, the CeA influences feeding mechanisms via multiple routes. Therefore, among all amygdala subdivisions, this core has the most significant role in regulating the feeding behaviour(Reference De Francesco, Valdivia and Cabral16,Reference Hu, Bashir and Li19–Reference Kim, Zhang and Muralidhar21,Reference Petrovich and Gallagher23,Reference Petrovich, Ross and Mody24,Reference Zhang, Li and Guo27) .
The central amygdala
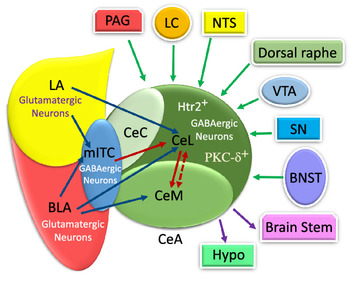
The CeA is a striatal-like structure(Reference Fadok, Markovic and Tovote18,Reference Gilpin, Herman and Roberto35) and is involved in different functions relevant to food consumption, appetitive behaviours, energy balance and digestive behaviour(Reference De Francesco, Valdivia and Cabral16,Reference Hu, Bashir and Li19–Reference Kim, Zhang and Muralidhar21,Reference Petrovich and Gallagher23,Reference Petrovich, Ross and Mody24,Reference Zhang, Li and Guo27) . Moreover, it also participates in non-feeding functions, such as fear, anxiety, pain, cognition, motivation and stress(Reference Bernard, Huang and Besson15,Reference Ehrlich, Humeau and Grenier17–Reference Ip, Zhang and Farzi20,Reference McCullough, Morrison and Hartmann22,Reference Petrovich and Gallagher23,Reference Wang, Shen and Jiang25,Reference Ye and Veinante26) . This nucleus includes lateral (CeL), capsular (CeC) and medial (CeM) subdivisions(Reference Fadok, Markovic and Tovote18,Reference Kim, Zhang and Muralidhar21,Reference Gilpin, Herman and Roberto35,Reference Pape and Pare37,Reference van den Burg and Stoop38) . In Figure 1, several CeA subdivisions and neurons that are associated with food intake behaviour are illustrated(Reference Douglass, Kucukdereli and Ponserre5,Reference Ip, Zhang and Farzi20,Reference Petrovich, Ross and Mody24,Reference Zhang-Molina, Schmit and Cai32,Reference Anderberg, Anefors and Bergquist39–Reference Kim, Yoon and Nakajima42) .
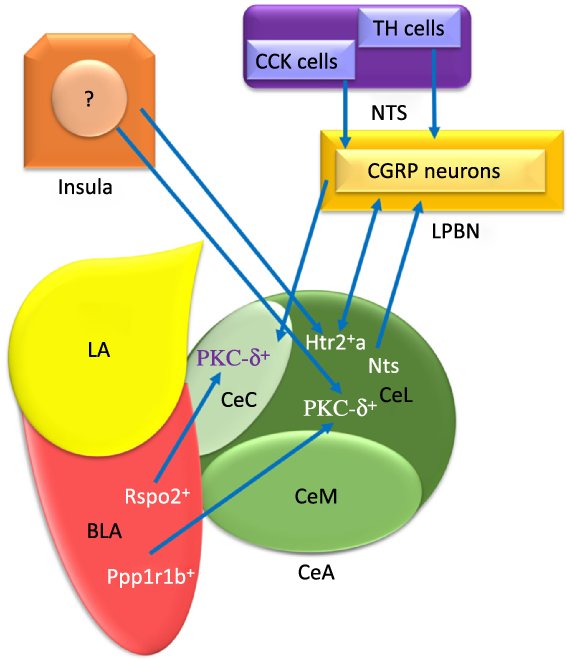
Fig. 1. The schematic organisation of information flow in the central amygdala (CeA) regarding feeding. Basolateral amygdala (BLA) affects the CeA with its protein phosphatase 1 regulatory subunit 1B+ (Ppp1r1b+) and R-spondin2+ (Rspo2+) neurons that may provoke and inhibit appetite, respectively. Insula modulates feeding behaviour via non-selective connections with both protein kinase C-delta (PKC-δ+) and Serotonin receptor 2a+ (Htr2a+) neurons. The nucleus tractus solitarius (NTS) terminates food intake with glutamatergic input projections from the tyrosine hydroxylase (TH) and cholecystokinin (CCK) cells to PKC-δ+ neurons of both lateral part of the CeA (CeL) and capsular part of the CeA (CeC) via activating the calcitonin-gene-related peptide (CGRP) lateral parabrachial nucleus (LPBN) neurons. Also, the BLA Ppp1r1b+ neurons project into PKC-δ+ neurons in CeL. In addition, the BLA Rspo2+ and CGRP neurons of LPBN modulate appetite behaviour via projecting to PKC-δ+ neurons in the CeC. LA, lateral amygdala; CeM, medial part of the CeA; Htr2a+, Serotonin receptor 2a+; Nts, Neurotensin. ![]() , Excitatory
, Excitatory
The input/outputs of the central amygdala and their modulatory effects on food intake
The conductance-based model with a dynamical system for the CeA neural network represents the biophysical interpretation of an excitable cell in regulating the feeding behaviour(Reference Zhang-Molina, Schmit and Cai32). The intra-connections and projections modulate feeding behaviour in the CeA(Reference Kim, Zhang and Muralidhar21) where there are direct and indirect connections with different hypothalamic nuclei(Reference Petrovich and Gallagher23,Reference Zhang, Li and Guo27,Reference Petrovich, Canteras and Swanson29) . Information is transferred to the CeA nucleus from multiple regions, such as the brain stem, diencephalon nuclei and limbic system(Reference Wilson, Grillo and Fadel43). Therefore, the CeA would connect to the brain stem areas that control the expression of innate behaviours and their associated physiological responses(Reference Hasanein, Mirazi and Javanmardi44).
Inputs into the central amygdala
The numerous intra- and inter-connections between different amygdala nuclei provide inputs to the CeA(Reference Gilpin, Herman and Roberto35). For instance, the BLA affects the CeA through its protein phosphatase 1 regulatory subunit 1B+ (Ppp1r1b+) parvocellular neurons and R-spondin2+ (Rspo2+) magnocellular neurons(Reference Kim, Zhang and Muralidhar21,Reference Ye and Veinante26) . As represented in Fig. 1, the Ppp1r1b+ and Rspo2+ neurons provoke and inhibit appetitive behaviours which are normally regulated in the CeA(Reference Kim, Zhang and Muralidhar21). However, the glutamatergic neurons in the LA and BLA have synapses with GABAergic neurons in the medial intercalated cells that are located between the BLA and CeA. It can be seen in Fig. 2 that these GABAergic medial intercalated cells connect the LA, BLA and CeA areas(Reference Ehrlich, Humeau and Grenier17,Reference Gilpin, Herman and Roberto35,Reference Pape and Pare37) . Also, the BLA and LA send glutamatergic outputs, respectively, to the CeL/M and CeL subdivisions of the amygdala(Reference Gilpin, Herman and Roberto35,Reference Pape and Pare37) . In Fig. 2, the CeL and CeM make local inhibitory circuits together due to sending γ-aminobutyric acid (GABA) interneurons(Reference Gilpin, Herman and Roberto35,Reference Pape and Pare37) . Hence, a distinct population of neurons in the CeA perform an opposing role in feeding(Reference Zhang-Molina, Schmit and Cai32). The GABAergic protein kinase C-delta (PKC-δ +) neurons in the CeL suppress feeding(Reference Wang, Shen and Jiang25,Reference Zhang-Molina, Schmit and Cai32) . By contrast, the serotonin receptor 2a (Htr2a+/PKC-δ –) neurons in the CeL stimulate food intake(Reference Douglass8,Reference Zhang-Molina, Schmit and Cai32,Reference Wang, Kim and Schmit45) . The activation of PKC-δ + neurons in both CeC and CeL leads to the inhibition of appetitive behaviours. These two types of appetite suppression happened via threat stimuli and aversive tastes in the CeC, as well as through satiety in the CeL(Reference Kim, Zhang and Muralidhar21). Also, BLA Rspo2+ and calcitonin-gene-related peptide (CGRP) of the lateral parabrachial nucleus (LPBN) neurons would mediate food consumption via projecting to PKC-δ + neurons in the CeC; however, Kim et al. (2017) demonstrated that BLA Ppp1r1b+ modulates appetitive behaviour via projecting to PKC-δ + neurons in the CeL(Reference Kim, Zhang and Muralidhar21). As represented in Fig. 1, both CeL and CeM seem to promote reward-related behaviours by receiving inputs from the BLA Ppp1r1b+(Reference Kim, Zhang and Muralidhar21).
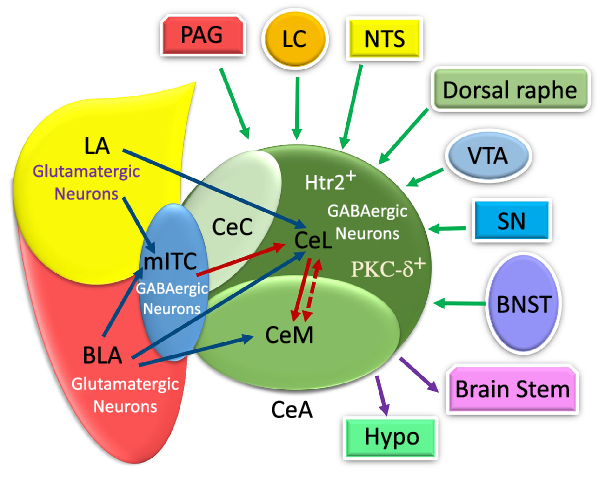
Fig. 2. The schematic illustration of internal and external input/outputs of the central amygdala (CeA). The lateral amygdala (LA) and basolateral amygdala (BLA) affect synapse on medial intercalated cells (mITC) (which is connected to the CeA) via their glutamatergic neurons. The CeA is composed of medial part of the CeA (CeM), capsular part of the CeA (CeC) and lateral part of the CeA (CeL) mostly with GABAergic neurons. The LA and BLA excite CeL and CeL/M, respectively, via glutamatergic projections. The CeA receives internal LA and BLA inputs in the amygdala circuits. Additionally, it receives information from multiple regions, such as the periaqueductal grey (PAG), locus coeruleus (LC), nucleus tracts solitaries (NTS), dorsal raphe, ventral tegmental area (VTA), substantia nigra (SN) and bed nucleus of the stria terminalis (BNST). Also, the CeA and mostly the CeM exert outputs to the brains tem and hypothalamus. PKC-δ+, protein kinase C-delta+; Htr2a+, serotonin receptor 2a+; PSTN, parasubthalamic nucleus; DMC, dorsal motor complex; Hypo: hypothalamus. ![]() , Input;
, Input; ![]() , Output;
, Output; ![]() , Excitatory;
, Excitatory; ![]() , Inhibitory;
, Inhibitory; ![]() , Inhibitory Interneurons.
, Inhibitory Interneurons.
Furthermore, the CeA receives a huge amount of information from multiple regions of the brain(Reference Douglass8,Reference Gilpin, Herman and Roberto35,Reference Verma, Wood and Lach46) . The sensory and cortical inputs come from the ventral tegmental area, locus coeruleus, nucleus tractus solitarius (NTS), periaqueductal grey, bed nucleus of the stria terminalis, parasubthalamic nucleus, substantia nigra and, finally, dorsal raphe nucleus(Reference Douglass, Kucukdereli and Ponserre5,Reference Douglass8,Reference Gilpin, Herman and Roberto35,Reference Pape and Pare37) (Fig. 2).
The NTS terminates food intake with glutamatergic input projections from the tyrosine hydroxylase and cholecystokinin cells to the PKC-δ + neurons of both CeL and CeC; also, the NTS activates CGRP of the LPBN neurons(Reference Douglass8,Reference Lu, Chen and Wei14,Reference Ye and Veinante26,Reference Hardaway, Halladay and Mazzone41,Reference Andermann and Lowell47–Reference Carter, Soden and Zweifel49) . Moreover, the CGRP receptors in PKC-δ + neurons are probably the aim of CGRP neurons in the LPBN(Reference Campos, Bowen and Schwartz48,Reference Han, Soleiman and Soden50) . However, Wang et al. (2019) indicated that the CGRP neurons in the LPBN suppress food consumption via excitatory projection to Htr2a+ neurons in the CeA(Reference Wang, Kim and Schmit45).
Satiety is the feeling of fullness and suppression of appetite and hunger. The satiety-related signals from LPBN projections excite PKC-δ + neurons in the CeA and mediate appetite by suppressing food intake(Reference Douglass8,Reference Fadok, Markovic and Tovote18,Reference Ye and Veinante26,Reference Andermann and Lowell47) . Therefore, these signals mainly suppress appetite drive and create the feeling of satiety by releasing GABA from LPBN to PKC-δ + neurons in the CeA(Reference Fadok, Markovic and Tovote18,Reference Hardaway, Halladay and Mazzone41) .
Another pathway for transporting food intake information is the insula-CeA connection(Reference Zhang-Molina, Schmit and Cai32). The insula activates two opposite ways in modulating the feeding behaviour via non-selective connections with both PKC-δ + and Htr2a+ neurons in the CeA(Reference Zhang-Molina, Schmit and Cai32). Despite similar synaptic strength of the insula-CeA pathways in both PKC-δ + and Htr2a+ neurons, food consumption behaviour will be decreased(Reference Zhang-Molina, Schmit and Cai32).
Outputs of the central amygdala
As illustrated in Fig. 2, the CeA has connections with almost all brain regions, such as the bed nucleus of the stria terminalis, lateral hypothalamus, PVN, ventral tegmental area, PBN, NTS, dorsal vagal complex and substantia nigra(Reference Douglass8,Reference Qiao, Ren and Li13,Reference Pape and Pare37,Reference Holt, Pomeranz and Beier51–Reference Veening, Swanson and Sawchenko54) . There are numerous projections from the CeA to different brain regions(Reference Douglass8,Reference Qiao, Ren and Li13,Reference Pang, Chen and Xue31,Reference Pape and Pare37,Reference Takayama, Johno and Hayashi52) . However, the CeM, unlike the CeL, seems to be more responsible for sending output projections to brain areas, such as the brain stem and hypothalamus(Reference Ehrlich, Humeau and Grenier17,Reference Pape and Pare37) . Similarly, the CeL sends projection to different brain regions, including the preproglucagon neurons in the NTS(Reference Holt, Pomeranz and Beier51), periaqueductal grey and PVN of the thalamus(Reference Penzo, Robert and Li55). The terminal projections from CeC to the periaqueductal grey, PBN, NTS and ventromedial medulla have been observed too(Reference Lu, Chen and Wei14).
Factors affecting the responsiveness of the central amygdala to food intake
Three main factors affect the responsiveness of the CeA to environmental parameters regarding the feeding behaviour; these factors could be categorised as receptors, neurotransmitters/neuropeptides and gene expression in the CeA(Reference Patel3,Reference Kim, Zhang and Muralidhar21,Reference McCullough, Morrison and Hartmann22,Reference Jin, Jiang and Luan30,Reference Pang, Chen and Xue31,Reference Giraudo, Billington and Levine56) . These factors could increase and/or decrease food consumption (respectively, orexigenic and anorexigenic factors)(Reference Kim, Zhang and Muralidhar21).
The central amygdala and different receptors as well as neurotransmitters/neuropeptides
The CeA contains receptors that are related to food consumption(Reference van den Burg and Stoop38). There is evidence of opioid receptors containing kappa (κ) and mu (μ) receptors that mediated food consumption in the CeA(Reference Giraudo, Billington and Levine56,Reference Kim, Shi and Olszewski57) . Moreover, a unilateral opioid–opioid pathway from the CeA to PVN increased food intake via μ receptors(Reference Giraudo, Billington and Levine56). The CeA contains GABA and glutamate receptors that act differently(Reference Patel3). For instance, the direct injection of glutamate receptor agonist N-methyl-D-aspartate in the CeA increased food consumption(Reference Patel3).
Similar to various biochemical factors, different receptors could also exhibit different effects on food consumption by activating different CeA neurons(Reference Jin, Jiang and Luan30,Reference Pang, Chen and Xue31) . A study has shown that direct injection of GABAA receptor agonist in the CeA decreased food consumption(Reference Patel3). According to Anesten et al. (2019), the stimulation of glucagon-like peptide-1 receptors in the CeA nucleus reduces food intake(Reference Anesten, Dalmau Gasull and Richard12). Food consumption may be decreased by the microinjection of IL-6 into the CeA via the enhancement of glucagon-like peptide-1 receptors(Reference Anesten, Dalmau Gasull and Richard12). Moreover, Anderberg et al. (2014) demonstrated the anorectic effects of dopamine which are caused by the activation of the D2 receptor in the CeA(Reference Anderberg, Anefors and Bergquist39). Furthermore, Pang et al. (2015) reported that secretin administration excited its receptors in the CeA. Also, the inhibitory effects of these receptors were mediated on food intake through the spontaneous electrical activity of GABAergic neurons and cAMP-protein kinase in the CeA(Reference Pang, Chen and Xue31). According to some studies, approximately all neural populations of the CeA are GABAergic neurons(Reference Douglass8,Reference Zhang-Molina, Schmit and Cai32,Reference Pape and Pare37,Reference Butler, Oliver and Sharko58) . The pituitary adenylate cyclase-activating peptide, which is a very important regulatory peptide in food consumption as well as its PAC1 receptors, all was highly expressed in the CeA(Reference Iemolo, Ferragud and Cottone59). Also, pituitary adenylate cyclase-activating peptide-induced anorexia and weight loss activate the local melanocortin and tropomyosin-related kinase receptor type B(Reference Iemolo, Ferragud and Cottone59) (Fig. 3). Notably, orexin-A (a prominent neuropeptide in modulating food consumption) could mediate the orexigenic effects on the CeA via the OX1 receptors(Reference Jin, Jiang and Luan30).
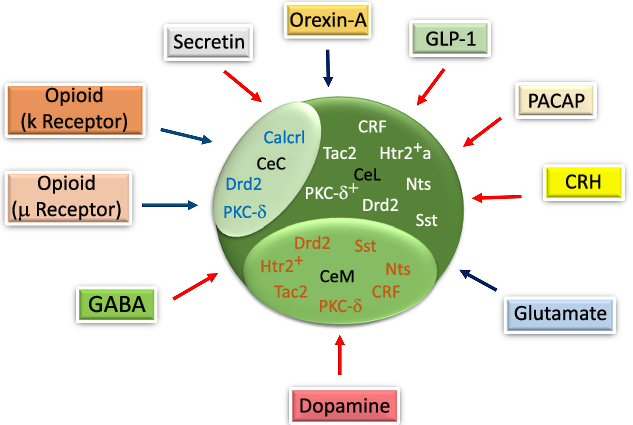
Fig. 3. The schematic diagram of gene expression in different parts of the central amygdala (CeA) and the effects of neurotransmitters/neuropeptides on food intake in the CeA. Corticotropin-releasing hormone (CRH), Tachykinin 2 (Tac2), serotonin receptor 2a+ (Htr2a+), somatostatin (Sst), Protein kinase C-delta+ (PKC-δ+) and neurotensin (Nts) are expressed in the lateral part of the CeA (CeL). The calcitonin receptor-like (Calcrl) and PKC-δ are expressed in the capsular part of the CeA (CeC). Htr2+, Sst and Nts are expressed in the medial part of the CeA (CeM). Glucagon-like peptide-1 (GLP-1), pituitary adenylate cyclase-activating peptide (PACAP), CRH, dopamine, gamma-aminobutyric acid (GABA) and secretin decrease. Orexin-A, glutamate, μ and κ increase the food intake through their specific receptors in the CeA. Drd2, dopamine receptor 2. ![]() , Increase;
, Increase; ![]() , Decrease.
, Decrease.
The central amygdala and gene expression
In previous studies on gene expression, it was reported that various genes were expressed in different CeA subdivisions that could have affected CeA functions in mediating feeding behaviour. The corticotropin-releasing hormone (CRH), Htr2a, neurotensin (Nts), dopamine receptor 2, PKC-δ, somatostatin and tachykinin 2 were expressed in the CeL, while calcitonin receptor-like, dopamine receptor 2 and PKC-δ were all expressed in the CeC(Reference Kim, Zhang and Muralidhar21,Reference McCullough, Morrison and Hartmann22) . However, electrophysiological studies have mostly documented PKC-δ + and Htr2a+ neurons under a category of neurons with delayed firing patterns(Reference Douglass, Kucukdereli and Ponserre5,Reference Zhang-Molina, Schmit and Cai32) , yet, their roles were seen to be different in determining food intake behaviour(Reference Zhang-Molina, Schmit and Cai32) (Fig. 3). Also, it was indicated that Htr2a, Nts, somatostatin, CRF, PKC-δ, dopamine receptor 2 and tachykinin 2 were expressed in the CeM(Reference Kim, Zhang and Muralidhar21,Reference McCullough, Morrison and Hartmann22) . Finally, similar to other neurons, the electrophysiological characterisation of GABAergic neurons in the CeA depends on their specific gene expression(Reference Ehrlich, Humeau and Grenier17,Reference Zhang-Molina, Schmit and Cai32) . Among these genes, CeL somatostatin, CRH, Nts, tachykinin 2, CeM Nts, somatostatin and tachykinin 2 provide an appetitive drive basis, while CeL PKC-δ would modulate appetitive behaviour negatively(Reference Kim, Zhang and Muralidhar21).
The central amygdala and different food intake modulation systems
The CeA is a critical brain region with significant roles in a variety of physiological and behavioural responses that are essential for survival, such as reward processing, digestion(Reference Jin, Jiang and Luan30,Reference Hardaway, Halladay and Mazzone41,Reference Takayama, Johno and Hayashi52) and different emotional states like fear and stress conditions(Reference Douglass8,Reference Ip, Zhang and Farzi20,Reference Zhang, Li and Guo27) . In the following sections, this review will elaborate on the role of the CeA in these responses.
The central amygdala and reward system
The brain’s reward circuitry is one of the systems that are related to feeding behaviour(Reference Douglass8). This system includes various nuclei, such as the CeA, nucleus accumbens, midbrain dopaminergic system, cholinergic basal forebrain system, orbitofrontal cortex and anterior cingulate cortex(Reference Zhang, Li and Guo27). Among these cores, the CeA not only has anatomical and electrical connections to different nuclei in the reward system but also reacts to motivational and emotional stimuli(Reference Merali, McIntosh and Kent28). Hence, it plays an important role in regulating food intake behaviour(Reference Fadok, Markovic and Tovote18) and seems to be involved in food-seeking and appetite responses of the reward circuit as well. This might be due to its connection to the reward system, as well as the brain’s gustatory and feeding centres(Reference Fadok, Markovic and Tovote18).
Similarly, some studies have reported that the CeA circuits were activated with food-predictive cues (eating for hedonistic purposes or reward-associated reasons) via a population of the prepronociceptin-expressing cells in the CeA, which reinforce the rewarding properties of palatable food(Reference Hardaway, Halladay and Mazzone41,Reference Herzog60) . The prepronociceptin-expressing cells make a network of connections and projections to the ventral bed nucleus of the stria terminalis, PBN and NTS to process reward behaviours(Reference Hardaway, Halladay and Mazzone41) and promote high-fat diet consumption that leads to obesity(Reference Hardaway, Halladay and Mazzone41). Even though they promote reward behaviour, their effects are not significant for the metabolism and energy homoeostasis(Reference Hardaway, Halladay and Mazzone41). Moreover, Torruella-Suárez et al. (2020) have reported the subset of Nts neurons in the CeA that lead to a hedonic consumption paradigm(Reference Torruella-Suarez, Vandenberg and Cogan61). The Nts and Htr2a+ make fundamental pathways neurons by projecting from the CeA to the PBN, respectively, in palatable drinking and eating patterns(Reference Torruella-Suarez, Vandenberg and Cogan61).
Overall, somatostatin and PKC-δ neurons in the CeL provide a basis for the correct response to the appropriate predictive cue (e.g. responding to the sweet water-predictive cue and not the bitterness-predictive one) via receiving excitatory inputs from the insular cortex(Reference Petrovich33,Reference Schiff, Bouhuis and Yu62) . However, in this continually changeable environment, predicting the consequent events is not an easy task(Reference Badrinarayan, Prater and Orsini63); the outcome of this cue will inevitably depend on the CeA interactions(Reference Badrinarayan, Prater and Orsini63).
The central amygdala and digestive system
In previous studies, the anatomical evidence has supported the hypothesis regarding the reciprocal connections of the CeA with both NTS and dorsal vagal complex (two regions with neuromodulatory roles in gastric motility). Hence, the connections to the NTS and dorsal vagal complex make the CeA to operate as a gateway between them and the digestive system(Reference Jin, Jiang and Luan30,Reference Liubashina, Jolkkonen and Pitkänen64,Reference Zhang, Cui and Tan65) .
It is suggested that administration of orexin-A in the CeA could increase the gastrointestinal motility (for both contraction and emptying) through signals from the CeA to the dorsal motor nuclear complex and efferent activation of the vagus nerve (CeA–dorsal motor nucleus of the vagus–gastrointestinal tract axis)(Reference Jin, Jiang and Luan30).
Following the intravenous injection of ghrelin, an increase of gastric acid secretion may happen in response to ghrelin via the excitation of the neurons in the CeA(Reference Takayama, Johno and Hayashi52). In this way, the projection of excited neurons from the CeA to the LPBN acts as a mediator that first excites the NTS and dorsal motor nucleus of the vagus (with direct innervation) and subsequently the neural system of the stomach(Reference Takayama, Johno and Hayashi52). The increase of gastric acid secretion was shown to have followed the gastrin injection and subsequently activated the expression of c-Fos protein in the CeA neurons(Reference Yakabi, Iwabuchi and Nakamura66). Hence, CeA seems to act as a mediator between the nervous and endocrine systems in association with different brain areas from the brain stem to the cortex(Reference Petrovich and Gallagher23). The relationship between CeA and other systems (e.g. the endocrine system) shows the importance of CeA in determining feeding behaviour. Therefore, this core facilitates the extensive connections between the brain, gastrointestinal tract and digestive hormones(Reference Jin, Jiang and Luan30). It is suggested to further investigate this perspective in future studies.
The central amygdala and different emotional states
Feeding behaviour and other lifestyle habits may considerably influence health and optimise obesity control in today’s societies enduring various inconvenient emotional states(Reference Hurt, Kulisek and Buchanan67,Reference Izadi, Radahmadi and Ghasemi68) . Apart from eating essential nutrients for metabolic functions and energy requirements, feelings are key factors that may affect food consumption(Reference Zhang, Li and Guo27,Reference Herzog60,Reference la Fleur69) . For example, stress can lead to overeating to relieve stress and relax, while severe stress can cause undereating(Reference la Fleur69). Hence, the relevant CeA pathways, involved in feeding behaviour, would additionally be affected by different factors, such as emotional states like fear and stress(Reference Iemolo, Ferragud and Cottone59).
Role of the central amygdala on food intake through fear
Fear experience may affect behavioural responses like food intake(Reference Isosaka, Matsuo and Yamaguchi70). According to some studies, danger aversion and fear confrontation may suppress food intake(Reference Petrovich33,Reference Li and Kirouac71) . The relationship observed between the CeA and the PVN of the thalamus mediates the appropriate behaviour in facing danger cue or food intake(Reference Petrovich33,Reference Li and Kirouac71) . The LPBN neurons transfer the sense of danger to the CeA and activate PKC-δ + neurons that would suppress food intake(Reference Zhang-Molina, Schmit and Cai32,Reference Cai, Haubensak and Anthony40) . The neural circuits through which PKC-δ + neurons exert their inhibitory influence on feeding should be further investigated(Reference Cai, Haubensak and Anthony40). Also, in other studies, encountering innate fear conditioning has inhibited the activation of the Htr2a+ neurons in the CeA(Reference Douglass8,Reference Isosaka, Matsuo and Yamaguchi70) . Although the notion of reduced food consumption by fear through the neural activation of PKC-δ + and inhibition of Htr2a+ has previously been explored(Reference Douglass8,Reference Isosaka, Matsuo and Yamaguchi70) , the number of neurons participating in food intake reduction (through acute fear) remains unexplained so far(Reference Isosaka, Matsuo and Yamaguchi70).
Role of the central amygdala on food intake under stress and anxiety
Stress has become a common aspect of today’s lifestyle(Reference Ip, Zhang and Farzi20). As such, contradictory results have been observed regarding the stressors and food consumption level in various stress-related studies(Reference Ip, Zhang and Farzi20,Reference Zellner, Loaiza and Gonzalez72) . Stress-dependent eating often seems to happen under chronic stress situations(Reference Ip, Zhang and Farzi20). Contrarily, food intake reduction and weight loss have been observed under sub-chronic psychological stress at different levels of intensity(Reference Izadi, Radahmadi and Ghasemi68,Reference Glowa, Barrett and Russell73) . The sub-chronic administration of CRH into the PVN compared to its administration in the CeA increases food consumption earlier and more sharply(Reference Rayatpour, Radahmadi and Izadi74). As feeding-related CRH signalling in the CeA seems to act later than in the PVN, the CeA exhibits a stronger impact on the stress circuit and hypothalamic-pituitary-adrenal axis activation than on the food intake behaviour(Reference Rayatpour, Radahmadi and Izadi74,Reference Rani, Deep and Singh75) . Moreover, the acute CRH injection into the CeA has been reported to reduce food intake(Reference Diamanti-Kandarakis, Papalou and Kandaraki76). However, a complex relationship between stress and eating pathways(Reference Ip, Zhang and Farzi20) is such that both stress exposure and eating could increase the CRH release in the CeA(Reference De Francesco, Valdivia and Cabral16,Reference Merali, McIntosh and Kent28,Reference la Fleur69) . According to Petrovich et al. (2009), the CeA is an effective nucleus for the inhibition of food intake under chronic stress(Reference Petrovich, Ross and Mody24). Nevertheless, the impact of CeA nucleus on food intake seems to be independent of the hypothalamic-pituitary-adrenal axis function(Reference Izadi, Radahmadi and Ghasemi2).
In addition, the relation between stress and food consumption is mechanistically complex(Reference Ip, Zhang and Farzi20). Marlene et al. (2015) have shown that exposure to stress increased the glutamate level in the CeA(Reference Wilson, Grillo and Fadel43). Ip et al. (2019) demonstrated that stressful conditions enhanced food intake (especially, high-fat diet) by activating the neuropeptide Y (NPY) neurons in the CeA(Reference Ip, Zhang and Farzi20). In the aforementioned study, NPY neurons in the CeA expressed insulin receptors; therefore, the consumption of a high-fat diet reduced the insulin responsiveness of these neurons in the CeA(Reference Ip, Zhang and Farzi20). Furthermore, the NPY neurons in the CeA that were deprived of insulin receptors increased appetite and decreased energy expenditure under chronic stress(Reference Ip, Zhang and Farzi20,Reference Herzog60) . Stress decreased the expression of insulin (acting as a negative feeding regulator) in the CeA(Reference Ip, Zhang and Farzi20). Also, in the combination of stress and a high-fat diet, stress increased the appetite drive for overeating and overexpression of NPY(Reference Ip, Zhang and Farzi20). Thus, the NPY level and its orexigenic effects seem to have enhanced the coping level to stress conditions(Reference Ip, Zhang and Farzi20,Reference Herzog60) . It should be mentioned that an interaction between the NPY and Htr2a+ neurons in the CeA has been reported as well(Reference Ip, Zhang and Farzi20). According to this study, activation of these receptors might reinforce the effects of food intake with positive valence.
All in all, anxiety, often associated with or triggered by a high level of stress, is defined by persistent, excessive ongoing worries even without the presence of any stressor(Reference Roberts, Campbell and Troop1). In the state of anxiety, due to the anxiolytic impact of NPY in the amygdala, the intra-amygdala administration of this neuropeptide reduces the high-fat food intake without changing the total food intake(Reference Zhang, Li and Guo27,Reference Primeaux, York and Bray77) . Not only the presence of many NPY neurons and various NPY receptors in the amygdala but also the effects of NPY on other nutrition-related neuropeptides seem to be responsible for the occurrence of such behaviours(Reference Primeaux, York and Bray77). Hence, exclusive research is necessary to better understand the influence of different emotional states over the roles of CeA regarding food consumption.
Conclusion
Food consumption consists of multiple behavioural sequences. The CeA plays a unique physiological role in various normal and abnormal emotional states, such as fear, anxiety and stress, the current review emphasised the importance of the CeA in understanding feeding behaviour. Notably, CeA is involved in various processes including the reward system and regulation of feeding behaviour. Finally, neuropsychological and behavioural studies have indicated the crucial role of the CeA in food consumption and modulating the behaviours related to the reward system.
Future directions
With the widespread prevalence of obesity and related metabolic disorders affecting the life quality and health system, the feeding circuits should be understood properly. Neuroscience may utilise the research studies regarding different brain nuclei for the development and maintenance of treatment methods for obesity and other related metabolic disorders. Hence, identifying the cell types and their specific functions in the CeA is critical for the regulation of feeding behaviour and emotional processing. Finally, understanding the proper feeding and its relevant disorders requires extensive research about the modulatory roles of the CeA on food intake mechanisms.
Acknowledgements
The authors would like to express their gratitude to Isfahan University of Medical Sciences, Isfahan, Iran.
This research has received no grant from any funding agency, charitable or non-charitable institutions.
All authors contributed to the literature search, analysis of the published data, writing and revision of the manuscript.
No conflict of interest is declared regarding this work.