Type 2 diabetes (T2D) has become a major contributor to the global disease burden. At least 425 million people worldwide have T2D, and an increase to 629 million is expected by the year 2045(1). India is at the forefront of the T2D epidemic, ranking second among countries with the greatest number of people living with the condition globally(1). A recent nationwide T2D survey found high prevalence rates among low socio-economic groups in urban as well as rural areas in India, where two-thirds of the population resides(Reference Anjana, Deepa and Pradeepa2). The costs related to treatment of T2D are of great concern to public health and the economy of India given the limited healthcare resources to manage the dramatic rise in prevalence alongside coexisting burdens of undernutrition and communicable diseases(Reference Malik, Willett and Hu3). Thus, effective, affordable and sustainable strategies for T2D prevention are needed to reduce early death and disabilities, contain climbing healthcare and societal costs, and improve overall quality of life.
In urban south India, nearly half of daily energy intake comes from refined grains, with white rice constituting >75 % of refined grain intake(Reference Radhika, Van Dam and Sudha4). Compared with brown rice, a whole grain, white rice contains little dietary fibre, Mg, and other phytochemicals, all of which may reduce risk for T2D. In epidemiological studies, consumption of white rice has been associated with increased risk for T2D(Reference Hu, Pan and Malik5) and in a pooled analysis of three large US cohorts substituting 50 g/d of brown rice for white rice was associated with a 16 % lower risk of T2D(Reference Sun, Spiegelman and van Dam6). In our previous short-term trial using continuous glucose monitoring, replacing white rice with brown rice significantly reduced the 24-h glycaemic response and fasting insulinaemic response among overweight South Indians(Reference Mohan, Spiegelman and Sudha7). These findings, combined with our previous qualitative studies in a similar South Indian population, which identified strategies to increase acceptability of brown rice and demonstrated willingness to participate in a brown rice intervention, provided the rationale for the present trial(Reference Sudha, Spiegelman and Hong8–Reference Mattei, Malik and Wedick11). Here we aimed to investigate the effect of substituting brown rice for white rice on metabolic markers of T2D risk among adults in urban South India.
Methods
Setting
This study was conducted at a large tertiary care centre for diabetes in Chennai, a major city in South India. The study protocol was approved by the Institutional Review Boards of both the Harvard T.H. Chan School of Public Health and Madras Diabetes Research Foundation and registered with ClinicalTrials.gov (NCT01814735).
Study population
Participants were recruited from MDRF as well as from surrounding offices through advertisements. Staff from these facilities and their relatives were invited to participate in this study. Eligibility criteria were age 25–65 years; BMI ≥ 23 kg/m2 and a habitual consumer of rice. Two additional eligibility criteria specifying a daily rice consumption of >200 g per d and having an elevated waist circumference were dropped prior to recruitment for feasibility and logistical reasons. It was also assumed that most South Asians with an elevated BMI would also have central adiposity. Exclusion criteria included having a fasting blood glucose ≥ 126 mg/dl (7·0 mmol/l) or postprandial blood glucose ≥ 200 mg/dl (11·1 mmol/l); a diagnosed chronic disease that may affect the study outcomes or that would make participation potentially harmful, including diabetes, severe kidney disease, CVD (coronary artery disease, peripheral vascular disease), history of stroke, cancer, severe psychological disorders (schizophrenia, dementia), or hypothyroidism; being pregnant or lactating; and plans to relocate in the next year. At the screening visit, height and weight were measured, and a questionnaire was administered to assess habitual rice intake, medical history, demographics and lifestyle factors. A total of 352 participants were screened of which 171 were eligible and willing to participate (Fig. 1). These participants underwent an oral glucose tolerance test to determine existing and newly diagnosed T2D. Five newly diagnosed cases were identified and these participants were subsequently excluded, leaving 166 participants for inclusion in the study. All participants were given full details about the trial, after which each gave informed written consent.
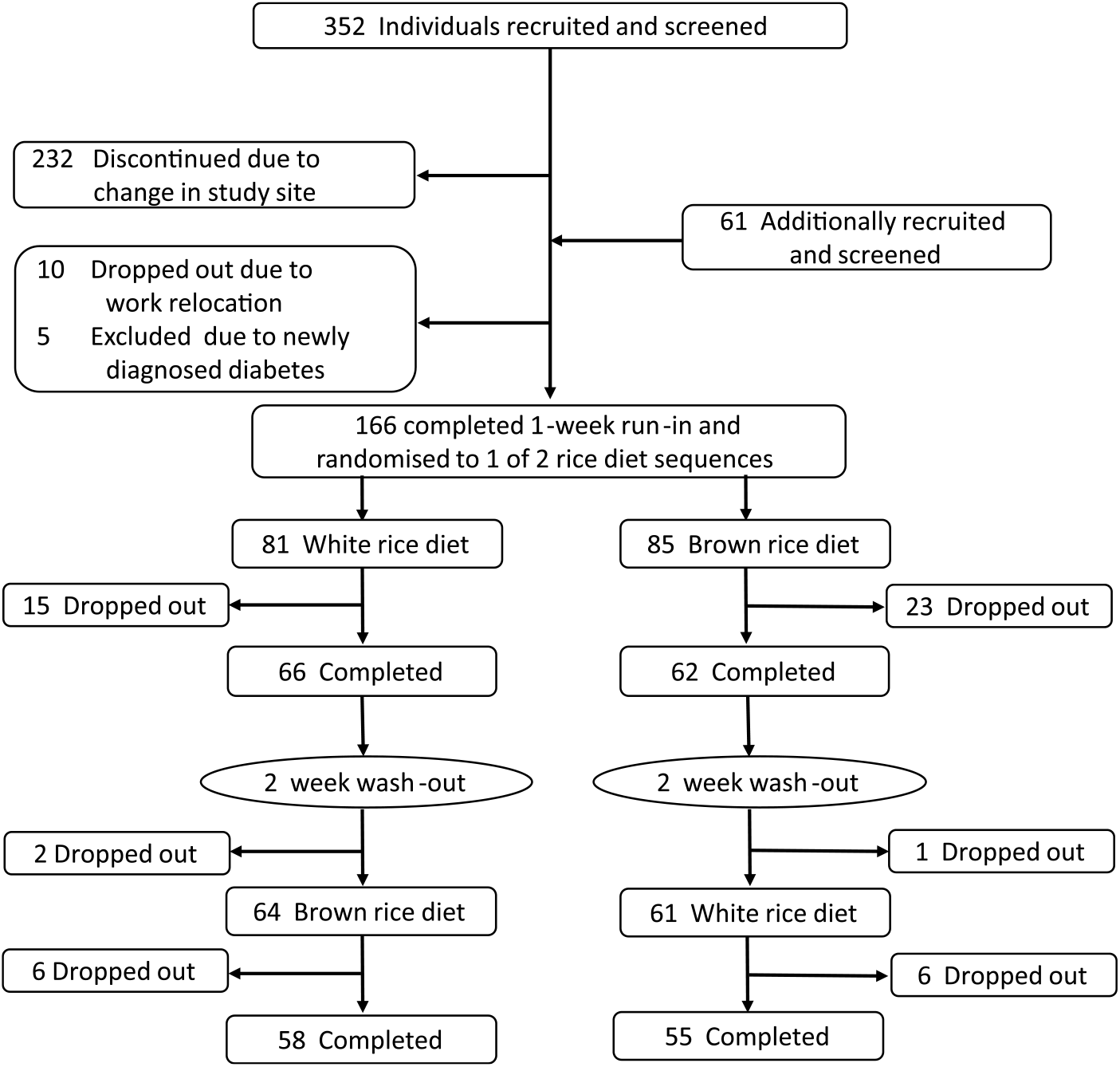
Fig. 1. Participant screening, enrollment and follow-up. Inclusion criteria: age 25–65 years; BMI ≥23 kg/m2 and a habitual consumer of rice. Exclusion criteria: fasting blood glucose ≥126 mg/dl (7·0 mmol/l) or postprandial blood glucose ≥200 mg/dl (11·1 mmol/l); diagnosed chronic disease that may affect study outcomes or would make participation potentially harmful, including diabetes, severe kidney disease, CVD, history of stroke, cancer, severe psychological disorders (schizophrenia, dementia), or hypothyroidism; being pregnant or lactating; and plans to relocate in the next year. At the end of the study, 121 participants had completed the white rice diet and 120 participants had completed the brown rice diet.
Study design
This study was a non-blinded randomised cross-over dietary intervention trial, consisting of a parboiled brown rice or a white rice regimen, each providing two ad libitum meals/d (breakfast and lunch), 6 d/week for 3 months with a 2-week washout period. Prior to randomisation, a 1-week run-in phase was conducted. Randomisation was performed using computer-generated random numbers with an equal treatment allocation ratio. A stratification factor for sex was included in the randomisation scheme. Intervention meals were administered and consumed at the on-site dining facility. To monitor whether participants received the correct test foods according to randomisation, they were given photo identification badges with colour-coded ribbons when they entered the dining area. Participants were also requested to sign a register kept at the dining area before picking up their meal, which was used to count their number of participatory days in the intervention. Study staff had direct contact with participants during daily visits to the centre, and this helped to provide motivation to continue in the study. Participants who dropped-out were contacted to ascertain their reason for discontinuation. Compliance was assessed by direct observation of daily participation, number of meals consumed, and serving size of foods consumed as well as monthly 24-h diet recalls.
Intervention diets
A total of nine white rice-based and nine corresponding brown rice-based recipes were developed and standardised for the trial, based on our previous work(Reference Wedick, Sudha and Spiegelman12,Reference Shanmugam, Nagarajan and Mookambika13) . A description of the intervention menu and individual rice-based dishes have been previously reported(Reference Wedick, Sudha and Spiegelman12). We used the same strain of rice (BPT 5204) which we used in our previous proof-of-concept continuous glucose monitoring study(Reference Mohan, Spiegelman and Sudha7). Non-parboiled white rice is the most commonly consumed type of rice across India, thus we selected it as the control diet in this trial. Because brown rice was not available in the market, our team worked with a local rice miller to produce brown rice from the same rice strain (BPT 5204) that was parboiled for the intervention. Milling was coordinated to produce batches of brown and white rice from a single strain to minimise intra-varietal variations due to season and other environmental factors. Brown rice can remain shelf stable for a longer time when parboiled since parboiling arrests lipolytic enzyme activity reducing the chance of rancidity due to the high fat content in rice bran. The glycaemic index (GI) values of individual intervention dishes have been estimated and reported previously(Reference Wedick, Sudha and Spiegelman12). The estimated GI of the white rice-based dishes ranged from 69·4 to 84. For the brown rice-based dishes, GI values ranged from 50·2 to 59·7. Based on these values, there was an approximate difference in GI of 25–30 % between intervention foods. Meals were prepared in accordance with Hazard Analysis and Critical Control Point (HACCP) regulations and dietitians regularly monitored cooking practices, facilities, storage and dispatch of food.
Measurements
A detailed description of study measurements has been previously reported(Reference Wedick, Sudha and Spiegelman12). The primary outcomes for this intervention were fasting plasma glucose, insulin, glycosylated Hb (HbA1c) and lipids (total cholesterol, TAG, LDL-cholesterol and HDL-cholesterol). High-sensitivity C-reactive protein (hs-CRP) was assessed as a secondary endpoint as the intervention may impact inflammation in addition to biomarkers of glycaemic control and lipids. Blood samples were obtained at baseline and at the end of each intervention and analysed at the National Accreditation Board for Testing and Calibration Laboratories and the College of American Pathologists accredited central laboratory at Dr. Mohan’s Diabetes Specialities Centre in Chennai, India. Fasting blood (after 10–12 h fast) was obtained by an experienced phlebotomist. Samples were immediately centrifuged, aliquoted, transported on dry ice and stored at −80°C. All assays were performed at the end of the study to control for assay variation and laboratory technicians were blinded to participant study group assignment. Plasma glucose (glucose oxidase peroxidase method), serum cholesterol (cholesterol oxidase-peroxidase-4-aminophenazone method), serum TAG (glycerol phosphate oxidase-peroxidase-4-aminophenazone method) and HDL-cholesterol (direct method with polyethylene glycol pretreated enzymes) were measured using a Hitachi 912 Autoanalyzer (Roche Diagnostics, GmbH) and utilising kits supplied by Boehringer Mannheim. LDL-cholesterol was calculated using the Friedewald formula in participants with TAG ≤400 mg/dl (4·5 mmol/l). Serum insulin concentration was estimated using Dako kits; HbA1c was measured using a Variant machine (Biorad). Plasma concentrations of hs-CRP were measured by a highly sensitive nephelometric assay using a monoclonal antibody to CRP coated on polystyrene beads (Dade Behring). Insulin resistance was estimated from the homeostasis model assessment of insulin resistance (HOMA-IR) by the following formula: (fasting insulin (mU/ml)) × (fasting glucose (mmol/l)/22·5)(Reference Matthews, Hosker and Rudenski14).
Anthropometric measurements were collected at baseline and monthly at clinic visits through the end of follow-up. Body weight (kg), height (cm) and waist circumference (cm, measurement taken at the end of expiration) were measured using standardised methods. BMI was calculated as weight (kg) divided by height (m2). Overweight (≥23 kg/m2) and obesity (≥25 kg/m2) were defined according to the Asia Pacific Classification (WHO, 2000)(15). Percentage body fat was measured using a body composition monitor based on bioelectrical impedance (Salter-Omron, HBF 212). Blood pressure was measured at baseline only using a blood pressure monitor (Omron, Hem7101). Diet was assessed at baseline using a validated interviewer-administered food frequency questionnaire evaluating usual consumption habits over the past year, and with monthly 24-h diet recalls during follow-up. Satiety (assessed with a seven-point Likert scale) and adverse effects of intervention meals were also ascertained monthly. Physical activity was assessed at baseline via the MDRF – Physical Activity questionnaire (MPAC), a valid and reliable interviewer-administered tool(Reference Anjana, Sudha and Lakshmipriya16) and smoking and other lifestyle and sociodemographic factors were assessed on the screening questionnaire.
Statistical methods
A sample of 166 participants was randomised for the present study to provide a power of 80 % for the cross-over design to detect a percentage change between 2 (fasting glucose) to 14 (fasting insulin and HOMA) in the intervention group, allowing for 15 % drop-out, with a type 1 error rate of 0·05, an assumed intra-class correlation of 0·85 and assuming no change in the intervention group. Intra-class correlations for baseline biochemical and clinical characteristics were calculated to assess consistency across study periods. To calculate point and interval estimates of effect, mean and standard deviation baseline and end of follow-up values of each biomarker were calculated for each intervention group, along with their respective change (and standard deviation) from baseline. To test the statistical differences in changes from baseline between the brown and white rice intervention arms in biomarkers, body measurements, and dietary intake, mixed models for repeated measurements were used with the robust variance and an exchangeable working correlation matrix(Reference Fitzmaurice, Laird and Ware17). Given sufficient sample size, this approach provides confidence intervals and P values that are guaranteed to be valid regardless of the distribution of the dependent variables(Reference Fitzmaurice, Laird and Ware17). An advantage of this approach is that the results can be reported in the measured scale of interest. Sub-group analysis by sex, age, physical activity, baseline anthropometric and biochemical measurements and opinion about whether brown rice was considered healthy were conducted along with corresponding tests for heterogeneity using the robust score test. To test the robustness of our results, various sensitivity analyses were conducted including adjusting the analysis for change in BMI and testing for a potential carry-over effect by creating an interaction term between study period and intervention group. We also evaluated between-group differences using end of follow-up measurements only as a potential strategy to improve statistical efficiency and a multiple outcome 1 df test that simultaneously evaluated primary and secondary outcomes as one composite variable(Reference Pocock, Geller and Tsiatis18). Between-group differences in satiety of intervention foods were evaluated using the Wilcoxon signed-rank test. Statistical tests were two-sided and performed using SAS version 9.2 for UNIX.
Results
During the first period of the study, fifteen participants dropped out of the white rice group and twenty-five dropped out of the brown rice group (Fig. 1). Prior to crossing over to the other diet period, an additional two participants discontinued from the white rice group and one participant discontinued from the brown rice group. During the second diet period, six participants discontinued from each group leaving 121 and 120 participants available for analysis from the white rice and brown rice periods, respectively, with an overall dropout rate of 33 %. The majority of dropouts were due to job change (30 %) or personal reasons (34 %).
Baseline characteristics of study population
Men comprised 55 % of the study population and participants had a mean age of 37 years and BMI of 28·1 kg/m2 at baseline (Table 1). Of the participants, 68 % had pre-diabetes with HbA1 c between 5·7 and 6·4 %, and 40 % of participants had the metabolic syndrome, defined as having at least three of the five following components: elevated waist circumference (≥90 cm for men, ≥80 cm for women); low HDL-cholesterol (<40 mg/dl (1·0 mmol/l) for men, <50 mg/dl (1·3 mmol/l) for women); high TAG (≥150 mg/dl; 1·7 mmol/l); elevated blood pressure (systolic ≥ 130 or diastolic ≥ 85 mmHg) and impaired fasting glucose (≥100 mg/dl; 5·6 mmol/l). Participants consumed 59 % of total energy from carbohydrate, 11 % of energy from protein and 29 % of energy from fat. Estimated intake of white and brown rice at baseline was 204 (sd 85·3) and 1·3 (sd 2·8) g per d, respectively. Of the participants, 74 % considered brown rice to be healthy. Intra-class correlations ranged from 0·40 for HOMA-IR to 0·98 for body weight.
Table 1. Baseline characteristics of participants (n 166)
(Mean values and standard deviations; percentages)
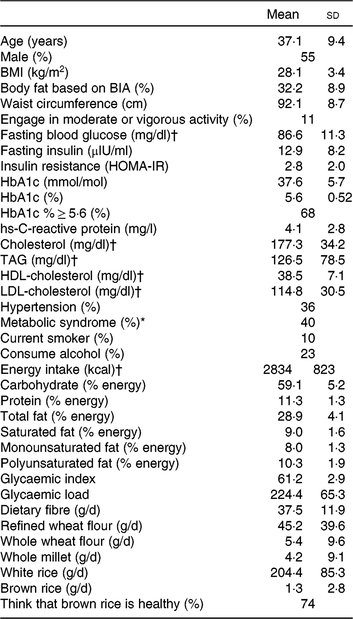
BIA, bioelectrical impedance analysis; HOMA-IR, homeostasis model assessment of insulin resistance; HbA1c, glycosylated Hb.
* The metabolic syndrome is defined as having at least three of the following: elevated waist circumference (≥90 cm in men, ≥80 cm in women); low HDL-cholesterol (<40 mg/dl (1·0 mmol/l) in men, <50 mg/dl (1·3 mmol/l) in women); high TAG (≥150 mg/dl; 1·7 mmol/l); elevated blood pressure (systolic ≥ 130 or diastolic ≥ 85 mmHg) and impaired fasting glucose (≥100 mg/dl; 5·6 mmol/l).
† To convert glucose in mg/dl to mmol/l, multiply by 0·0555. To convert cholesterol in mg/dl to mmol/l, multiply by 0·0259. To convert TAG in mg/dl to mmol/l, multiply by 0·0113. To convert energy in kcal to kJ, multiply by 4·184.
Intervention effect
During the intervention, no significant between-group differences were observed for markers of glycaemic control or lipids (Table 2). However, non-significant trends in decreased levels of fasting blood glucose, insulin, HbA1c, HOMA-IR, TAG, total cholesterol and LDL-cholesterol were observed for the brown rice diet compared with the white rice diet. Our secondary outcome, hs-CRP increased less in the brown rice diet (0·03 (sd 2·12) mg/l) compared with the white rice diet (0·63 (sd 2·35) mg/l) (P = 0·04). No significant between-group differences were observed for anthropometric measures, although non-significant trends in increased body weight, BMI, and percentage body fat were observed for the brown rice diet compared with the white rice diet. Among participants with the metabolic syndrome at baseline, there was a reduction in HbA1c during the brown rice diet relative to the white rice diet (−0·18 (se 0·08), P = 0·03) while no difference in HbA1c was observed between diets among participants who did not have the metabolic syndrome (0·05 (se 0·05), P = 0·26) (P-for-heterogeneity = 0·02) (Table 3). No other statistically significant between-group differences were observed by metabolic syndrome status in study outcomes. The difference between the brown rice and white rice groups was not statistically significant based on the multiple outcome of 1 df test including primary and secondary outcomes as one composite outcome (P = 0·09).
Table 2. Changes in intervention outcomes from baseline between white rice (WR) and brown rice (BR) diets
(Mean values and standard deviations; mean values with their standard errors)
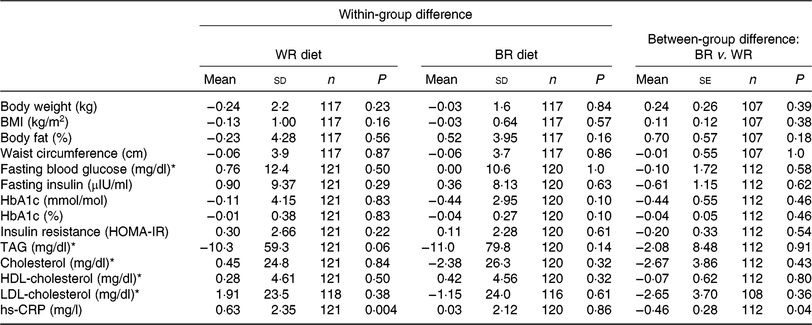
HbA1c, glycated Hb; HOMA-IR, homeostasis model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein.
* To convert glucose in mg/dl to mmol/l, multiply by 0·0555. To convert TAG in mg/dl to mmol/l, multiply by 0·0113. To convert cholesterol in mg/dl to mmol/l, multiply by 0·0259.
Table 3. Between-group difference in changes in intervention outcomes between white rice (WR) and brown rice (BR) diets by baseline metabolic syndrome status*
(Mean values with their standard errors)
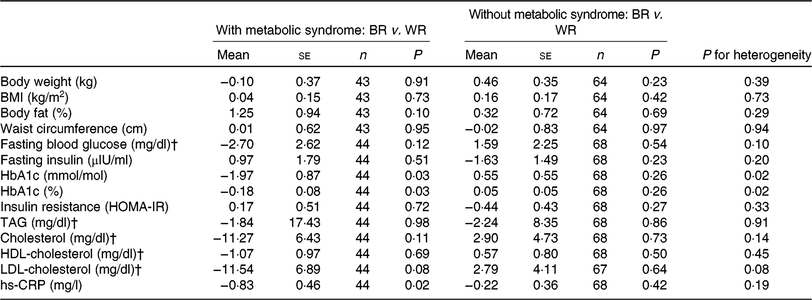
HbA1c, glycated Hb; HOMA-IR, homeostasis model assessment of insulin resistance; hs-CRP, high-sensitivity C-reactive protein.
* The metabolic syndrome is defined as having at least three of the following: elevated waist circumference (≥90 cm in men, ≥80 cm in women); low HDL-cholesterol (<40 mg/dl (1·0 mmol/l) in men, <50 mg/dl (1·3 mmol/l) in women); high TAG (≥150 mg/dl; 1·7 mmol/l); elevated blood pressure (systolic ≥ 130 or diastolic ≥ 85 mmHg) and impaired fasting glucose (≥100 mg/dl; 5·6 mmol/l).
† To convert glucose in mg/dl to mmol/l, multiply by 0·0555. To convert TAG in mg/dl to mmol/l, multiply by 0·0113. To convert cholesterol in mg/dl to mmol/l, multiply by 0·0259.
Between-group changes in study outcomes did not differ significantly by sex, age or baseline physical activity level (P-for-heterogeneity ≥ 0·05) (Supplementary Table S1). For participants who had a BMI ≥ 25 kg/m2 at baseline, there was a suggestion of a benefit of brown rice relative to white rice on HbA1c, total cholesterol and LDL-cholesterol compared with participants with a BMI < 25 kg/m2 (P-for-heterogeneity < 0·05). For participants without prediabetes, there was a suggestion of a benefit of brown rice relative to white rice on HDL-cholesterol compared with participants with prediabetes (P-for-heterogeneity = 0·04); however, this could be due to chance. Among participants who thought brown rice was healthy, the brown rice group gained more weight than the white rice group, while among participants who did not think brown rice was healthy, there was no difference in weight change between groups (P-for-heterogeneity = 0·002). Changes in HbA1c were more favourable in the brown rice group among those who did not think brown rice was healthy while there was no difference among those who thought brown rice was healthy (P-for-heterogeneity = 0·01) (Supplementary Fig. S1). There was no evidence of a carry-over effect between rice periods for the primary outcomes and adjusting the analysis for change in BMI did not alter results (data not shown). Results were also unchanged when end of follow-up measurements only were used to assess between-group differences (data not shown).
Differences in dietary intake and satiety
Based on data from repeated 24-h dietary recalls throughout the intervention, the brown rice group had a lower GI and a significant decrease in intake of refined grains and percentage of energy from carbohydrate and significant increase in intake of whole grains, fibre and percentage of energy from total and saturated fat compared with the white rice group (Table 4). The brown rice group also showed a trend towards a suggestive increase in intake of percentage of energy from protein, which, may be due to the higher protein content of brown rice compared with white rice. Based on mean ratings from a Likert scale administered monthly, the brown rice group reported feeling more satiated from intervention meals than the white rice group throughout the study; however, the difference in satiety was statistically significant only during the second month of the trial (Supplementary Table S2).
Table 4. Differences in dietary intake between white rice (WR) and brown rice (BR) diets from baseline to end of follow-up
(Mean values and standard deviations; mean values and 95 % confidence intervals)
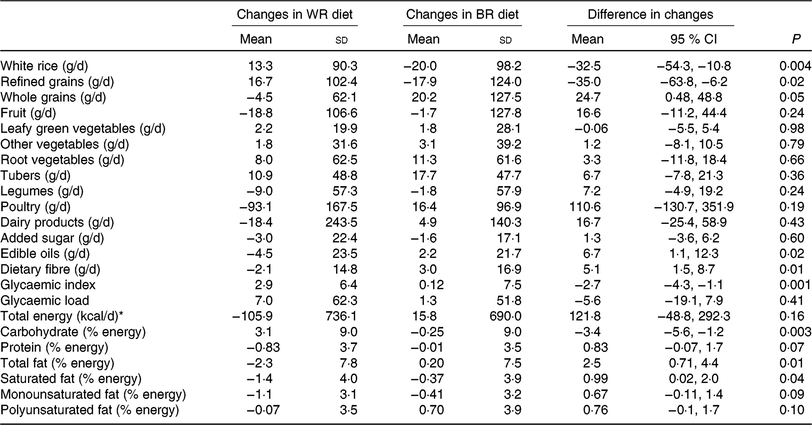
* To convert energy in kcal to kJ, multiply by 4·184.
Adherence
According to direct observation by study staff during intervention meals, participant attendance, which was recorded in a register in the dining area, in both groups was moderate with an average participation rate of 61·2 % during the brown rice period and 64·5 % during the white rice period. Mean consumption of rice from intervention meals as measured by number of spoons served per participant was 182 g during the brown rice period and 175 g during the white rice period. This was supported by data from 24-h diet recalls, which showed that the brown rice group had an increased intake of fibre compared with the white rice group over the course of the study (Table 4).
Discussion
In this 3-month cross-over trial, which enrolled healthy overweight and obese adults in urban South India, replacing parboiled brown rice for white rice did not significantly improve markers of glycaemic control or lipid parameters. However, beneficial trends were observed for brown rice on most markers and a slight benefit on hs-CRP was observed in the brown rice compared with the white rice group. Of note, a benefit of brown rice was observed on HbA1c among participants with the metabolic syndrome and among those with a BMI ≥ 25 kg/m2 at baseline, which may be clinically significant depending on baseline HbA1c level. These sub-group findings support our previous short-term trial, which found that replacing white rice with brown rice for 5 d in a similar South Indian population significantly reduced the 24-h glycaemic response and fasting insulinaemic response(Reference Mohan, Spiegelman and Sudha7). Findings from two small trials in Japan(Reference Hsu, Kise and Wang19) and Vietnam(Reference Bui, Le and Nguyen do20) also provide evidence for beneficial effects of replacing white rice with pregerminated brown rice on blood glucose and lipid concentrations. In addition, a recent 8-week clinical study of glutinous brown rice showed a significant reduction in HbA1c among patients with T2D(Reference Nakayama, Nagai and Uehara21). These findings are consistent with prospective cohort studies in Western and Asian populations that have reported positive associations between intake of white rice and risk of T2D(Reference Hu, Pan and Malik5) and our analysis that showed an inverse association between substituting brown rice for white rice on T2D risk(Reference Sun, Spiegelman and van Dam6).
The observed benefit of brown rice among those with the metabolic syndrome and who were overweight is where we would expect to see greatest benefit, since these groups have more room for improvement and may be more susceptible to the adverse effects of white rice. For a healthy weight population without the metabolic syndrome, the benefit of replacing white rice with brown rice may only be seen in a longer trial to assess prevention of weight gain or the metabolic syndrome, which would be logistically difficult to implement. We did not observe benefits of brown rice among participants with prediabetes, but some trends were noted, which may have reached statistical significance with a larger sample size or longer duration. Given the design of the present study, individuals with the metabolic syndrome would, in theory, be the right target and benefits of brown rice in this group may be helpful in decreasing the progression to T2D.
During the trial, the white rice group unexpectantly experienced slight reductions in body weight, BMI, percentage body fat and TAG, although differences were not statistically significant. This suggests the possibility of diminished statistical power to detect between-group differences owing to favourable changes in these risk factors in the control group. However, it is not clear what may have motivated these changes. Based on 24-h recall data collected throughout the trial, the white rice group decreased their intake of energy while the brown rice group increased their energy intake, although the between-group difference was not significant. The brown rice group also increased intake of total and saturated fat and edible oils relative to the white rice group, which could adversely impact body weight and cardiometabolic risk. However, the magnitude of this risk is unclear given that the brown rice group also improved healthful aspects of the diet such as increased intake of fibre. We also observed that among participants who thought brown rice was healthy, the brown rice group gained more weight and percentage body fat than the white rice group. Based on this finding, we can speculate that the brown rice group may have rationalised their intake of more energy. Since awareness and availability of brown rice was low at the time of the study, we needed to impart knowledge about brown rice to encourage volunteers to participate. The knowledge about the health benefits of brown rice in the setting of an open label, ad libitum trial to better reflect real life may have influenced eating behaviours. Since blinding was not possible, we assessed the effect of the intervention under a ‘real world’ clinical setting and evaluated intervention delivery rather than efficacy(Reference Satija, Yu and Willett22). In studies where blinding is not possible, design strategies such as adjustments in sample size calculations should be considered to reduce the potential effects of unintended behaviours.
The main difference between brown rice and white rice is in the method of processing, during which the majority of the bran and some of the germ are removed(Reference Slavin, Martini and Jacobs23). These parts of the grain contain numerous phytochemicals including polyphenols, phytosterols, and lignans, as well as vitamins, minerals, essential fatty acids and fibre, many of which may confer protection against T2D and CVD risk(Reference Slavin, Martini and Jacobs23). Brown rice contains nearly exclusively insoluble cereal fibre, which has been consistently associated with improved insulin sensitivity and reduced risk for T2D in experimental and observational studies(24,Reference Weickert and Pfeiffer25) . Insoluble fibre may also improve satiety(Reference Clark and Slavin26), which is supported by our finding of greater satiety ratings during the brown rice period compared with the white rice period. Of note, baseline intake of dietary fibre was already high in our study population (almost double that of most national averages), which may impact the generalisability of our findings. Those with lower intakes of fibre may benefit more from replacing refined grains with whole grains in the diet. In addition, compared with brown rice other whole grains have higher fibre content and different attributes, which when considered alongside the high baseline fibre intake of participants, our findings may not be representative of replacing refined grains with whole grains in general.
Diets high in cereal fibre have also been linked to a low GI and glycaemic load (GL), due to slower digestion and absorption of glucose into the circulation. Low-GI and -GL diets have been associated with reduced cardiometabolic risk in prospective cohort studies(Reference Atkinson, Foster-Powell and Brand-Miller27,Reference Bhupathiraju, Tobias and Malik28) . We found that any degree of polishing of parboiled brown rice leads to a higher glycaemic response for the same rice strain, suggesting a greater role of polishing than parboiling on GI(Reference Shanmugam, Nagarajan and Mookambika13). The adverse effects of high-GI and -GL diets tend to be more evident in individuals with excess adiposity, who are generally more insulin-resistant than lean individuals(Reference Ding and Malik29). This is consistent with our findings of benefits of brown rice on HbA1c among participants with the metabolic syndrome and improvements in HbA1c, cholesterol and LDL-cholesterol among participants with BMI ≥ 25 kg/m2. The benefits of brown rice on cardiometabolic risk are likely due to the combination of having a low GI, high amounts of cereal fibre and the accompanying natural package of nutrients found in the intact grain. Moreover, brown rice and other whole grains are important contributors to overall diet quality(30).
To the best of our knowledge, this is the first randomised intervention trial that has evaluated substituting a refined carbohydrate staple for a whole grain alternative on biomarkers of cardiometabolic risk in a population of adults at risk of developing T2D in urban South India. This study is of great relevance, given the high prevalence of T2D in India and expected rates of increase over the next decade. Other strengths include the variety of intervention foods, standardised preparation and cooking methods and assessment of compliance measured by direct observation of study staff during intervention meals and by 24-h diet recalls throughout follow-up. A limitation of our study was loss of statistical power to detect between-group differences in primary outcomes due to potential behaviour changes in the white rice group. There was also a relatively high drop-out rate (33 %), mostly due to job relocation and small sample sizes for sub-group analyses. However, despite the loss of statistical power, the majority of our primary outcomes showed trends towards favourable changes in the brown rice compared with the white rice group, which may have reached statistical significance with a longer duration or if our target population was individuals with the metabolic syndrome. The duration of our study, although typical for dietary interventions, may have been insufficient to detect meaningful changes in clinical indices among a healthy population or it is possible that improving rice quality alone is not sufficient to lead to clinically meaningful changes in metabolic biomarkers in an overall healthy population.
In conclusion, substituting parboiled brown rice for white rice for 3 months showed a potential benefit on glycaemia among participants with the metabolic syndrome and among those who had an elevated BMI. Effects of brown rice on changes in body weight and glycaemia also differed by perception of the health benefits of brown rice, highlighting the importance of health-related behaviours. Larger studies of longer duration that consider intervention effectiveness and overall diet quality are needed to examine the role of brown rice in the prevention of T2D in India, particularly among individuals with the metabolic syndrome.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S000711451900076X
Acknowledgements
We would like to thank our colleagues in the Global Nutrition and Epidemiologic Transition (GNET) Initiative at the Harvard T.H. Chan School of Public Health and collaborating institutions for their support on this project.
This work was funded by the Fogarty International Center at the National Institutes of Health (R03TW008726). On behalf of Dr. Mohan and colleagues, we disclose that brown rice and a new high-fibre white rice is currently being marketed at Dr. Mohan’s Diabetes Specialities Centre.
V. M., F. B. H. and D. S. designed the research. V. S., M. R. B., P. V., N. L., R. G., A. K. and K. K. conducted the research and coordinated the study centre. D. S., B. H. and R. L. analysed the data. V. S., N. M. W., R. M. A., W. C. W., D. S., C. J., F. B. H., K. K. and V. M. contributed to data interpretation and provided critical revisions on the manuscript. V. S. M. contributed to data interpretation, wrote the manuscript and constructed the tables and figures. F. B. H. and V. M. had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.








