N,N-dimethylglycine (DMG), a methylated derivative of the amino acid glycine with the chemical formula (CH3)2NCH2COOH, has been used for various human and animal applications. The molecule was first reported in 1943, and is a naturally occurring intermediate metabolite in plant and animal cells(Reference Tonda and Hart1, Reference Currell, Ayed and Dziedzic2). The choline-to-glycine pathway starts with choline being oxidised into betaine. Next, DMG is formed within the mitochondria from betaine (N,N,N-trimethylglycine) by the removal of one methyl group and, after this, it is further demethylated to sarcosine (N-methylglycine) and finally to glycine(Reference Craig3, Reference Garrow, Carmel and Jacobsen4). DMG is claimed to enhance oxygen utilisation and diminish muscle acidification, and for this reason, it is currently used as an enhancer of athletic performance in human athletes as well as in racing dogs and horses. This application is, however, primarily based on anecdotal reports. To date, only a few randomised, controlled studies have been performed and these have failed to demonstrate the aforementioned claims related to athletic performance(Reference Cupp, Tracy, Cupp and Tracy5).
A new application of DMG is in its use as a dietary supplement in poultry diets. Dietary supplementation with 167 mg Na-DMG/kg has been demonstrated to improve apparent total tract digestibility corrected for uric acid (ATTDua) of crude protein and N-free extract in broiler chickens by 6·3 and 13·0 % relative to the control, respectively. ATTDua of crude fat was already high in the control group (92·7 %), and was not affected by dietary DMG(Reference Kalmar, Cools and Buyse6). This beneficial effect of dietary DMG has been attributed to an emulsifying action at the gut level through which nutrients become more available for digestion and absorption(Reference Kalmar, Cools and Buyse6, Reference Kalmar, Cools and Verstegen7). An enhanced or rather more proximal emulsification of dietary fat through the action of a surfactant probably diminishes fatty insulation of non-fat nutrients. In turn, proximal liberation of these non-fat nutrients from a fatty coating renders them available sooner for enzymatic digestion and absorption through the intestinal brush border, through which ATTDua is improved. This hypothesis fits the data on the aforementioned trial, and is in accordance with literature data on pigs, which describe a more pronounced effect of emulsifiers on apparent faecal digestibility of non-fat fractions compared with the fat fraction(Reference Dierick and Decuypere8). Likewise, addition of 1 g Na-DMG/kg to a sow diet showed, compared with the control, a relative increase by only 8·3 % in the apparent faecal digestibility of crude fat, but a more profound relative increase by 20·9 and 16·0 % in the apparent faecal digestibility of crude protein and N-free extract, respectively(Reference Cools, Maes and Buyse9).
The improvement in ATTDua of crude protein in broilers through dietary supplementation with 167 mg Na-DMG/kg feed did not concomitantly result in an increase in uric acid excretion. Instead, the total N-to-inert marker ratio in the excreta of DMG-supplemented birds demonstrated a 14 % decline relative to the control. As a consequence, the N load of broiler manure was substantially diminished through dietary DMG(Reference Kalmar, Cools and Buyse6). The latter effect is of particular interest, as it diminishes N pollution of surface water through livestock manure and hence provides for an environmental benefit in addition to the economical advantage of a reduction in feed cost(Reference Kalmar, Cools and Buyse6, Reference Nahm10).
A following trial on broilers showed a positive, linear dose–response relationship between dietary Na-DMG and the meat:fat ratio within the tested range of 0–1 g Na-DMG/kg feed, when added to a diet with vegetable oil as a main fat source(Reference Kalmar, Cools and Verstegen7). Improved feed:gain ratio has been demonstrated in three feeding trials in which broilers of three different strains were fed either a control diet or this diet supplemented with 1 g Na-DMG/kg(Reference Kalmar11). However, none of these trials tested for toxicity or bioaccumulation of DMG.
The aims of the present trial were to evaluate tolerance and safety of dietary DMG in broilers when added at the highest dose tested to date and at a dose 10-fold higher.
Materials and methods
The present safety study was conducted at the trial facility of the Institution for Animal Nutrition at the Free University of Berlin in Germany. The implementation of the trial and experimental design complied with Good Clinical Practice criteria based on the consensus guidelines of the International Cooperation on Harmonization of Technical Requirements for Registration of Veterinary Medicinal Products(12) and lasted 4 d longer than required, as stipulated in the Commission Regulation (EC) no. 429/2008(13).
Experimental design
A total of 480 1-d-old Cobb 500 broiler chicks were fed a diet added with 0, 1 or 10 g Na-DMG/kg (DMG was added in the form of the additive Taminizer® D (Taminco NV, Ghent, Belgium), which is a preparation of dimethylglycine sodium salt (Na-DMG, ≥ 97 %) produced by chemical synthesis) during a production period of 39 d. Technical performance, haematology and clinical biochemistry, as well as histopathology of liver, kidney and muscle were evaluated and compared with the control. Carcass traits were only assessed in the 0 and 1 g Na-DMG dose groups. Finally, the accumulation of DMG and glycine – the metabolite of DMG after complete demethylation – was determined in breast meat and organs from all test groups. To facilitate interpretation of the broiler tissue results, DMG content was also determined in several unrelated food items for human consumption.
The chicks were randomly allocated to twenty-four pens (twenty male or twenty female chicks/pen). The pens were then assigned to one of three dietary treatment groups (eight replicate pens consisting of four male and four female pens/diet group) for the entire production period of 39 d. Common starter, grower and finisher diets were formulated and each of these was divided into three batches to which either 0, 1 or 10 g Na-DMG was added. Feed was offered ad libitum as a mash in flat, plastic feeders during the first 8 d, and afterwards in automatic feeders that were refilled with pre-weighed amounts when required. Fresh water was provided ad libitum from drinking nipples. The floor pens with dimensions 2·2 m × 1·8 m (length × width) and surface area ± 4 m2/pen were strewed with softwood shaving litter. The lighting schedule was 24 h of light during the first 3 d, followed by 23 h of light until day 7 and 18 h of light until the end of the trial. Ambient temperature was maintained between 32 and 32·5°C during the 1st week of the trial, was gradually reduced to 31°C during the 2nd week, and from day 15 onwards, it was reduced by about 0·4°C/d until 22°C was reached at day 39. Surface temperature of the bedding was maintained at 34°C by IR heaters until day 21. Relative humidity started at 50 % on day 1 and reached 60 % at the end of the trial. All birds were vaccinated against coccidiosis with Paracox (Essex Pharma GmbH, Munich, Germany) by individual oral application at the dose level of 0·1 ml/bird at 9 d of age.
A three-phase feeding schedule was applied, with a starter diet being fed from day 1 to day 14, followed by a grower feed from day 15 to day 28 and a finisher feed from day 29 to day 39. Treatments for each growth phase comprised the unsupplemented control diet, or this diet supplemented with 1 or 10 g Na-DMG/kg feed. The natural supply of DMG through the dietary ingredients was only 40 mg DMG/kg feed. The control diets were formulated to meet energy and nutrient requirements according to the Gesellschaft für Ernährungsphysiologie(14).
Ingredient composition of diets is presented in Table 1. Proximate analysis was performed on starter, grower and finisher diets according to the Verband Deutscher Landwirtschaftlicher Untersuchungs und Forschungstantalten(15) (Table 1). Starter, grower and finisher diets were divided into three batches to which 0, 1 or 10 g Na-DMG/kg was added. All test diets were analysed for Na, K, Cl, choline, DMG, methionine and glycine content. Choline, Cl, Na and K were analysed by ion chromatography using ICS-3000 (Dionex, Sunnyvale, CA, USA). Dietary electrolyte balance ((mEq/kg) = mEq Na++mEq K+ − mEq Cl−) was calculated(Reference Mongin16). DMG content was analysed by GC using flame ionisation detection (Agilent 6890; Agilent, Glendale, CA, USA). Methionine and glycine contents were determined by ion-exchange chromatography using a Biochrom 30 amino acid analyser (Biochrom Limited, Cambridge, UK; Table 2).
Table 1 Ingredient composition and analysed nutrient composition of the diets as fed*

ME, metabolisable energy; CP, crude protein; CF, crude fat; St, starch; Su, sugars.
* All diets were divided into three batches to which either 0, 1 or 10 g N, N-dimethylglycine sodium salt was added.
† Per kg diet: 167·5 μg vitamin A; 1200 μg cholecalciferol; 50·4 mg vitamin E; 2·4 mg menadione; 2·4 mg vitamin B1; 3·0 mg vitamin B2; 42 mg niacin; 4·8 mg vitamin B6; 0·04 mg vitamin B12; 240 mg biotin; 18 mg calcium pantothenic acid; 1·2 mg folic acid; 60 mg Zn, 90 mg Fe; 60 mg Mn; 14·4 mg Cu; 0·60 mg I; 0·48 mg Co; 0·42 mg Se; 1·6 g Na; 2·0 g Mg; choline: starter 1300 mg, grower: 1000 mg, finisher: 700 mg.
‡ ME (MJ/kg) = 0·1551 CP (%)+0·3431 CF (%)+0·1301 Su (%)+0·1669 St (%)(Reference Larbier and Leclercq43).
Table 2 Analysed content of selected intermediate metabolites in the choline-to-glycine pathway* as well as sodium and dietary electrolyte balance (DEB) of the test diets expressed on an as-fed basis

DMG, N, N-dimethylglycine.
* Betaine content was not analysed, but is presumed to be negligible based on available literature data reporting its absence in the maize and soyabean meal (88·3–90·3 % of current diets)(Reference Chendrimada, Neto and Pesti17).
† Choline requirements: 0·830–1·330 g/kg (1 to 21–21 to 42 d)(14).
‡ Methionine requirements: 3·8–4·4 g/kg (1 to 21–21 to 42 d)(14).
Health status and performance in target species
Mortality and culled birds were recorded daily and macroscopic necropsies were performed on dead or culled birds. Technical performance traits were assessed at pen level with body weight (BW), offered feed and feed remainders measured weekly from day 1 to day 35, and on day 39. On day 39, all birds were weighed individually (BW). Average daily gain and average daily feed intake were determined from measured values. The feed:gain ratio was calculated as:
At the end of the trial, carcass traits were evaluated in eight replicate birds (one random bird/pen) from the control and 1 g Na-DMG/kg feed groups. BW with empty crop was determined after an 8 h fasting period. Then, birds were humanely euthanised by stunning followed by exsanguination. The carcasses were then immersed in hot water, mechanically plucked and manually eviscerated. The weight of abdominal fat was measured and the remaining carcass was chilled for 24 h at 3°C in a cooling chamber. The heads, necks and feet at the hock joint were removed from the chilled carcasses before obtaining carcass for grilling weights. Finally, the breast meat, legs (drumsticks and thighs) and wings were manually dissected and weighed to determine meat parts.
Haematology, clinical biochemistry and histopathology in target species
At the end of the trial, blood samples (2 ml) were collected after an overnight fast to determine haematology and clinical biochemistry. Blood from twelve replicate broilers (six males and six females) per dietary treatment (one or two per pen) was sampled from the ulnar vein and immediately submerged in iced water (4°C). Samples were collected in 5 ml plastic tubes (Sarstedt, Nümbrecht, Germany) either without anticoagulant for serum separation or containing EDTA for determination of haematological parameters. To obtain plasma, the tubes were centrifuged at 3000 rpm for 10 min. Hb, erythrocyte count, leucocyte count and white blood cell formula were analysed using systemic K 4500 (Sysmex, Norderstedt, Germany). Next, plasma was analysed for electrolytes (Na, K, Cl, Ca and P) and the activities of alanine aminotransferase, asparatate aminotransferase, γ-glutamyl transferase and alkaline phosphatase were analysed by flame photometry and spectrophotometric methods using AAS vario 6 (Analytik Jena AG, Jena, Germany) and AU 800 Olympus (Olympus Diagnostica GmbH, Hamburg, Germany), respectively. Finally, the plasma metabolites cholesterol, TAG, uric acid, glucose, total protein and albumin were analysed spectrophotometrically using AU 800 Olympus (Olympus Diagnostica GmbH).
After blood collection, these thirty-six birds (twelve per treatment) were humanely euthanised, as described above, and representative samples of liver, kidney and heart were taken. Immediately after excision, tissue samples were fixed in 4 % buffered formalin, embedded in paraffin and cut into 5–7 μm slices. After conventional haematoxylin–eosin staining with an automatic Leica ST 4040 (Leica Microsystems Nussloch GmbH, Nussloch, Germany), tissue sections were evaluated using light microscopy. Samples were first screened for pathological changes. Next, histological appearance was described in detail. Degree of hepatocellular vacuolisation and extramedullar haematopoiesis in liver sections was scored per sampled bird (none, 0; discrete, 1; mild, 2; moderate, 3; marked, 4) and per treatment group (sum of grading scores multiplied by the number of birds graded with the respective scores).
Assessment of potential bioaccumulation of N, N-dimethylglycine in target species
At the end of the trial, eight broilers (one per pen) from each of the three dietary treatment groups were humanely euthanised, as described above, after an 8 h fasting period. Samples of blood, kidney, liver, abdominal adipose tissue and breast muscle from each bird, as well as fresh excreta from each pen at study termination and subsamples of all test diets were analysed for DMG content and for the content of its metabolite glycine. DMG and glycine were isolated from the freeze-dried samples by aqueous extraction. After drying, the DMG in the extracted residues was derivatised with N, O-bis(trimethylsilyl)trifluoro-acetamide to (CH3)2NCH2COO-Si(CH3)3 and analysed via GC using flame ionisation detection (Agilent 6890; Agilent, Glendale, CA, USA). Glycine content was determined by derivation with 9-fluorenylmethoxycarbonyl chloride and analysis via liquid chromatography using UV detection (Agilent HPLC 1200; Agilent, Waldbronn, Germany). To put DMG content in broiler tissue in perspective with common food items for human consumption, DMG content was also determined in the following: beef (sirloin steak), salmon (steak), egg, whole milk, maize, wheat, wheat bran and spinach. Because these food items were raw when analysed, homogenates of three prepared meals for human consumption were also analysed for DMG content. These meals included: (1) hamburger, beans and potatoes, (2) roast beef, peas, carrots and potatoes, and (3) chicken, rice and curry.
Statistics
Data on technical performance were statistically analysed with data per pen as the experimental unit, whereas sampled birds were used as the experimental unit for carcass traits and analysis on blood and tissue samples. Finishing BW was measured per bird, thus this parameter was also analysed with bird as the experimental unit. Normality and homoscedasticity were tested with the Kolmogorov–Smirnov and modified Levine test, respectively. All parameters (excluding mortality) were analysed using one-way ANOVA, and if significant followed by Tukey's post hoc tests to determine the significance of differences between groups. Statistical analysis of technical performance and carcass traits were analysed with sex and Na-DMG level as independent variables. There were no significant interactions between sex and Na-DMG level; hence, the models were generated without the interaction term. Mortality was not normally distributed, hence these data were analysed with the non-parametric Friedman rank sum test with DMG dose as the grouping variable and sex as the blocking variable. Average values are expressed as means with their standard errors of the mean. All statistics were performed using S-PLUS 8.0 (TIBCO Software Inc., Palo Alto, CA, USA) and SPSS 16.0 (SPSS Inc., Chicago, IL, USA). Significance was set at P < 0·05.
Results
The content of DMG in the test diets was very close to the intended level (Table 2). Analysed choline and methionine contents were above requirements(14). Betaine content was not analysed, but is presumed to be negligible based on available literature data reporting its absence in maize and soyabean meal (88·3–90·3 % of current diets)(Reference Chendrimada, Neto and Pesti17). Supplementation of Na-DMG at the highest tested dose resulted in a substantial increase in dietary electrolyte balance (Table 2).
Health status and performance in target species
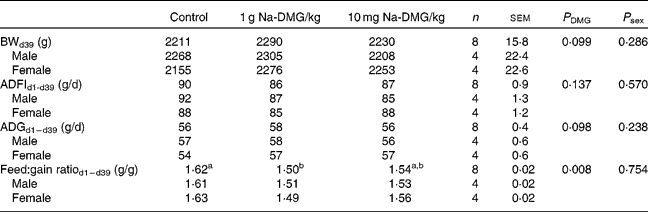
Mean starting weight was 39·4 (sem 0·4) g and did not differ significantly between the dietary treatment groups (P = 0·995), but was significantly higher in female birds compared with males (P < 0·001). Overall mortality was only 0·83 (sem 0·39) % and was not significantly different between the treatments (P = 0·368).
Supplementation with 1 g Na-DMG/kg feed resulted in a significant improvement in feed:gain ratio compared with the control or 10 g Na-DMG/kg feed groups (P = 0·008). Herein, the feed:gain ratio was diminished by 8 % when fed the diet supplemented with 1 g Na-DMG/kg feed compared with the control (Table 3). Carcass traits did not reveal significant differences between the control and 1 g Na-DMG/kg groups (P>0·05). Neither technical performance traits nor carcass traits showed significant interactions between DMG-dose and sex (P>0·05). Breast meat yield was significantly higher (P = 0·26) and abdominal fat accretion significantly lower (P = 0·009) in female compared with male birds (Table 4).
Table 3 Effects of dietary supplementation with N, N-dimethylglycine (DMG) on technical performance in broilers
(Mean values with their standard errors)
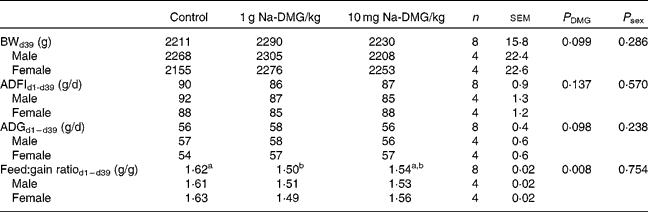
BW, body weight; ADFI, average daily feed intake; ADG, average daily gain.
a,b Values with unlike superscript letters within a row were significantly different (P < 0·050).
Table 4 Effects of dietary supplementation with N, N-dimethylglycine (DMG) on carcass traits in broilers
(Mean values with their standard errors)

BW, body weight; CfG, carcass for grilling.
Haematology, clinical biochemistry and histopathology in target species
Erythrocyte and leucocyte counts, differential leucocyte counts and Hb were similar between the dietary treatments (P>0·05). Likewise, electrolytes, enzyme activities and metabolites in plasma of fasted birds were not significantly altered by dietary supplementation with DMG at either of the tested doses (P>0·05; Table 5).
Table 5 Effects of dietary supplementation with N, N-dimethylglycine (DMG) on blood cell numbers and plasma chemistry in broilers
(Mean values with their standard errors)

ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyl transferase; ALP, alkaline phosphatase.
Histological examination of kidney and heart sections showed normal tissue structures and evidence of mild extramedullary granulopoiesis in all samples but no pathological changes were noted. Independent of the dietary treatment groups, most liver samples showed a discrete to marked hepatocellular vacuolisation and about half the samples showed evidence of discrete to moderate extramedullary granulopoiesis. In the control group, liver sections showed occasional heterophilic granulocyte (heterophil) infiltration in the sinusoidal spaces and mild perivascular lymphocytic cell infiltration. Multifocal lymphocyte aggregations were seen in four control birds. None of the control samples showed an increase in fibrous tissue, but one sample showed a focal area with extensive haemorrhage and parenchymal necrosis. In chickens fed the diet supplemented with 1 g Na-DMG/kg, histopathological examination revealed occasional sinusoidal heterophils and multifocal lymphocyte aggregations. No additional changes were noted in the samples of birds fed the 1 g Na-DMG/kg feed diet. Liver sections of birds fed the diet supplemented with the 10-fold DMG dose showed occasional sinusoidal heterophils and multifocal portal lymphocyte infiltrations. In this group, three samples showed mild bile duct proliferation. There was no evidence of hepatocellular damage or increase in fibrous tissue in the liver sections of birds fed a diet supplemented with DMG at 10 g Na-DMG/kg (Table 6).
Table 6 Histopathological degree of hepatocellular vacuolisation, extramedullary haematopoiesis and inflammatory cell infiltration in the liver samples of 39-d-old broilers fed a control diet or the same diet supplemented with N, N-dimethylglycine (DMG) at a dose of 1 or 10 g Na-DMG/kg feed (n 12)

* Diffuse, hepatocellular micro- and macrovesicular infiltration.
† Sum of grading scores multiplied by the number of birds graded with respective scores (grading scores: none, 0; discrete, 1; mild, 2; moderate, 3; marked, 4).
Assessment of potential bioaccumulation of N, N-dimethylglycine in target species
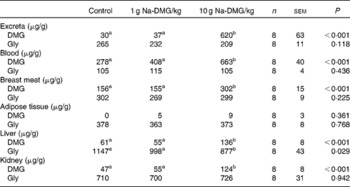
DMG content was highest in fasted blood, followed by muscle tissue, whereas free glycine was predominantly present in liver and kidney tissue. A dose of 1 g Na-DMG/kg feed did not significantly affect DMG or glycine content in excreta, fasted blood, breast meat, liver or kidney, compared with the control (P>0·05 for all). However, at the 10-fold of this dose, DMG content was significantly increased in all these samples (P < 0·001 for all), whereas glycine content was significantly lowered in liver tissue (P = 0·029). Dietary DMG did not affect DMG or glycine content in adipose tissue (P = 0·361 and P = 0·768, respectively; Table 7).
Table 7 Assessment of bioaccumulation of N, N-dimethylglycine (DMG) or its metabolite glycine in the excreta of broilers and in the tissue of fasted broilers when fed a control diet or the same diet supplemented with 1 or 10 g Na-DMG/kg feed*
(Mean values with their standard errors)

a,b Values with unlike superscript letters within a row were significantly different (P < 0·050).
* Values are expressed on a DM basis.
The DMG content in the analysed prepared meals for human consumption (30–40 μg/g) was comparable with the DMG content in the control broiler diet (40 μg/g) of the present trial (Tables 2 and 8). DMG content in the breast meat of broilers fed the 10 g Na-DMG/kg diet (302 μg DMG/g) was comparable with the DMG content in wheat bran (300 μg DMG/g). In comparison, salmon and beef contained much lower DMG (20 and 50 μg DMG/g, respectively) compared with broiler meat from the control group (156 μg/g) (Tables 7 and 8).
Table 8 N, N-dimethylglycine (DMG) content in raw food items and in prepared meals for human consumption*

* Values are expressed on an as-fed basis and are analytical data of one sample per item.
Discussion
Dietary supplementation with DMG did not result in apparent impaired health at the tested doses, observations that are in agreement with those of other trials where DMG was added at 1 g Na-DMG/kg feed or less to broiler diets(Reference Kalmar, Cools and Buyse6, Reference Kalmar, Cools and Verstegen7, Reference Kalmar11). This is consistent with a clinical trial with human subjects in which DMG was orally administered at 300 mg/d for 14 d followed by 600 mg/d for another 14 d and a study using New Zealand white rabbits that were force-fed DMG at 20 mg DMG/kg BW per d for 44 d(Reference Gascon, Patterson and Yearwood18, Reference Reap and Lawson19). In the highest DMG dose group of the present trial, total daily intake of DMG was much higher compared with these previous studies, namely 329, 892 and 1745 mg DMG/d in the starter, grower and finisher phases, respectively.
Although males usually show enhanced growth traits compared with females, technical performances were similar between males and females in the present trial(Reference López, Schilling and Corzo20). Finishing BW and feed:gain ratio in females met the breeder's performance objectives, whereas finishing BW of males was below expectations. Possibly, suboptimal quality of males with a low average starting weight (39 g instead of 41 g) contributed hereto. Also, the full growth potential of the normally faster growing males might not have been reached as a result of the use of mash diets instead of pellet diets(Reference Mirghelenj and Golian21). Still, a dose of 1 g Na-DMG/kg resulted in a significant improvement in feed:gain ratio, which is in agreement with previous trials(Reference Kalmar11), and did not show a sex × DMG interaction. This improvement of technical performance concurred with preservation of carcass yield and dressing percentage. This denotes a true beneficial effect on production efficiency instead of an apparent beneficial effect on account of an increase in offal tissue or accumulation of transudate fluid within the abdominal cavity.
Technical performance was not significantly influenced by the addition of DMG at the 10-fold dose compared with the control. This indicates, on the one hand, a high tolerance range for DMG in broilers and, on the other hand, that a dose of 10 g Na-DMG/kg is above the optimal dose to improve broiler performance, for which an explanation remains to be elucidated. The increase in dietary electrolyte balance as a result of a high dose of dietary Na-DMG is possibly a contributing factor. Reported data on ideal dietary electrolyte balance for broiler diets are, however, highly divergent, ranging from 120 to 330 mEq/kg(Reference Borgatti, Albuquerque and Meister22, Reference Jankowski, Zdunczyk and Juskiewicz23).
Neither of the tested doses of DMG resulted in a significant effect on carcass traits. Previous trials showed either similar or improved carcass traits when DMG is added to broiler diets. Similarly to technical performance traits, the results on carcass traits did not show a sex × DMG interaction. With respect to the observed sex effects, higher breast meat yield in females compared with males is consistent with literature data. In contrast, most authors have described either no effect of sex on abdominal fat percentage or a higher percentage in females, rather than a lower percentage in females compared with males(Reference Abdullah, Al-Beitawi and Rjoup24, Reference Kubena, Chen and Deaton25).
Leucocyte counts were not significantly changed by dietary DMG and remained in all treatment groups well within the normal reference range of 19·8–32·6 × 103/ml(Reference Apo and Samour26). Differential leucocyte counts showed high similarity between treatments. The heterophil:lymphocyte ratio, a reliable indicator of chronic stress in birds, was also not affected by dietary DMG(Reference Gross and Siegel27, Reference Maxwell28).
Likewise, erythrocyte and Hb concentrations were similar between the test groups and remained within the normal reference ranges of 2·2–3·3 × 106/ml and 5·52–8·39 mmol/l, respectively(Reference Apo and Samour26). Toxicologically, altered enzyme activity in plasma is of high importance because it reflects organ function and indicates leakage from cells resulting from damaged cellular membrane integrity(Reference Danishefsky29, Reference Hoffmann, Kramer, Main, Loeb and Quimby30). Plasma enzymology is of special interest because increased activity in plasma may occur even without morphological evidence of necrosis or cellular damage(Reference Hoffmann, Kramer, Main, Loeb and Quimby30). In avian species, plasma asparatate aminotransferase activity is considered a very sensitive, but not specific, indicator of hepatocellular damage(Reference Jaensch, Cullen and Raidal31). In the present trial, asparatate aminotransferase activity was similar between the treatment groups. γ-Glutamyl transferase activity was also not affected by dietary DMG; increased γ-glutamyl transferase activity in birds indicates cholestasis or biliary epithelial disorders(Reference Harr32). Non-specific cellular damage as indicated by increased plasma alanine aminotransferase activity was also absent in the present trial(Reference Samour and Samour33). Plasma electrolytes and metabolites were analysed to investigate eventual detrimental effects on functional organ capacity(Reference Samour and Samour33), but the results did not reveal an effect of dietary DMG.
Concordant with plasma results, histological examination of liver, kidney and heart tissue did not reveal pathological changes. The degree of hepatocellular degeneration or necrosis and oval cell proliferation at the limiting plate are important parameters in toxicity studies. In the present trial, a discrete to marked degree of hepatocellular vacuolisation, without evidence of apoptotic or necrotic hepatocytes, was observed in the livers of eleven out of twelve chickens of each group. The presence of these intra-hepatocytic micro- and macrovesicles is considered normal in broiler chickens(Reference Hodges and Hodges34). Although foci of heterophils and lymphocytes in the liver can be associated with disease conditions such as ascites, they are a common and non-specific histological finding in the liver of normal broiler chicken(Reference Maxwell, Robertson and Spence35, Reference Crespo, Shivaprasad and Saif36). In one control animal, a focal area with haemorrhage and parenchymal necrosis was detected in the liver, which is an incidental finding. Mild bile duct proliferation as seen in three liver samples at the 10-fold dose, without concurrent evidence of hepatocellular necrosis or deviant plasma biochemistry, can also be considered an incidental finding(Reference Doupnick and Peckham37).
As a result of growing governmental compulsion to decrease N pollution of surface water caused by animal production and in view of the increased competition for cereal grains between livestock and humans, the livestock industry is now more than ever driven to search for new feed additives(Reference Nahm10, Reference Wheeler and Campion38). Yet, increased public concerns towards possible deleterious effects of residues of feed additives in foods of animal origin require investigation of eventual bioaccumulation, as this may result in involuntary intake of those additives through consumption of animal products(Reference Bird39). Although DMG is a natural metabolite in human cells and oral treatment in human subjects does not show toxic effects, bioaccumulation in broilers as a result of dietary supplementation with DMG was investigated in the present trial(Reference Gascon, Patterson and Yearwood18). The DMG content in excreta was found to be similar between broilers fed a control diet and 1 g Na-DMG/kg feed, indicating a high efficiency of absorption, as described in the literature(Reference Cupp, Tracy, Cupp and Tracy5, Reference Budavari, O'Neil, Smith, Heckelman and Kinneary40). This finding also supports the hypothesised predominant route of DMG clearance by mitochondrial oxidative demethylation, rather than urinary clearance(Reference Lever, Atkinson and Slow41). An increase in the DMG content of fasted blood through dietary supplementation with DMG is in agreement with data on human subjects(Reference Slow, McGregor and Lever42). However, the present data demonstrated that supplementation of broiler diets with DMG at a dose of 1 g Na-DMG/kg feed does not result in an elevated DMG content of breast meat or liver of the target species. The observed increase in the DMG content of breast meat, liver and kidney of birds fed the diet supplemented with 10 g Na-DMG/kg feed indicates incomplete metabolisation of DMG at this dose. The higher DMG content in the excreta of this test group is thus more probably the result of increased urinary excretion, rather than incomplete intestinal absorption. Although DMG content in the breast meat of broilers supplemented with 10 g Na-DMG/kg feed was almost 2-fold compared with the control or 1 g Na-DMG/kg feed group, this DMG content is not excessively high when compared with some common ingredients in human nutrition. The significant reduction in the glycine content of liver samples when fed the 10 g Na-DMG/kg feed diet compared with the control or 1 g Na-DMG/kg feed diet is unclear.
In conclusion, in agreement with previous studies, supplementation of a broiler ration with DMG at a dose of 1 g Na-DMG/kg feed resulted in improved technical performance without compromising carcass traits. However, technical performance and carcass traits were similar between the control and 10-fold DMG dose groups. The results of both haematology and plasma chemistry, as well as histological examination of liver, kidney and heart tissue did not reveal pathological changes or indications of a toxic effect of dietary supplementation with DMG at 1 g Na-DMG/kg feed or at the 10-fold of this dose. Moreover, DMG supplementation at 1 g Na-DMG/kg feed did not accumulate in consumer parts of the target species, thus consumption of chicken meat or liver from these broilers will not increase DMG intake by the consumer. In addition, chicken meat or liver from broilers supplemented at the 10-fold dose did not contain a higher amount of DMG than other common food items, e.g. spinach. The present trial confirms previously reported favourable effects on the rearing efficacy of broilers, reveals a high level of tolerance and safety for DMG in the target species and demonstrates no consumer risk of unintentionally increased DMG intake through consumption of chicken meat or liver from poultry supplemented with DMG at a dose of 1 g Na-DMG/kg feed.
Acknowledgements
The present study was funded by Taminco NV. The authors' responsibilities were as follows: I. D. K. and K. M. designed the study; K. M. conducted the trial, collected all data and planned and supervised all analysis; I. D. K. monitored the trial site, performed the statistical analysis and wrote the manuscript; G. M. interpreted the histopathological results; J. Z. was the study director; M. W. A. V., K. M., J. Z., G. M. and G. P. J. J. revised the manuscript; G. P. J. J. supervised I. D. K. The authors declare that there is no conflict of interest.