Introduction
Meningiomas are tumors of arachnoidal cap cell origin. They account for approximately 20% of primary brain tumors with an overall incidence of 1.8 and 3.4 per 100,000 for males and females, respectively.Reference Surawicz, McCarthy, Kupelian, Jukich, Bruner and Davis 1 They are classified into three grades based on pathological features predicting recurrence. Grade I tumors are considered to be benign and grade III tumors are malignant, but grade II, atypical meningioma (AM) behave variably. After the WHO meningioma classification was updated in 2007, the incidence of AM increased to about 20% of all meningiomas.Reference Zaher, Abdelbari Mattar, Zayed, Ellatif and Ashamallah 2 , Reference Louis, Ohgaki and Wiestler 3 Atypical meningioma is defined as mitotically active cells (≥4 mitoses per HPF) with three or more of the following: loss of lobular architecture (sheeting), prominent nucleoli, increased cellularity, small cells with high nuclear to cytoplasmic ratio, foci of spontaneous necrosis, or brain invasion. Chordoid and clear cell histologies are considered AMs.Reference Mawrin and Perry 4 This definition has not been revised in the recent 2016 WHO classification of tumors of central nervous system.Reference Louis, Perry and Reifenberger 5
The postoperative management of benign and malignant meningiomas are well defined; observation for the former and post-operative radiation therapy (PORT) for the latter. However, the post-resection management of patients with AM is heterogeneous. Several studies have demonstrated inferior outcomes with subtotal resection (STR) alone compared to when STR was followed by PORT.Reference Jo, Park and Nam 6 - Reference Sun, Kim and Cai 10 Some series have shown that adjuvant PORT improved outcomes after gross total resection (GTR),Reference Jo, Park and Nam 6 , Reference Park, Kang and Kim 9 , Reference Aghi, Carter and Cosgrove 11 - Reference Aizer, Arvold and Catalano 13 while others showed no benefit from PORT after STRReference Hardesty, Wolf and Brachman 14 , Reference Hammouche, Clark, Wong, Eldridge and Farah 15 or GTR.Reference Lee, DePowell, Air, Dwivedi, Kendler and McPherson 7 - Reference Sun, Kim and Cai 10 , Reference Hammouche, Clark, Wong, Eldridge and Farah 15 , Reference Jenkinson, Waqar and Farah 16 Some advocate for delayed radiotherapy or to only use PORT after a second resection for recurrence in patients who initially had GTR.Reference Sun, Hawasli, Huang, Chicoine and Kim 17 This approach aims to minimize the use of radiotherapy to reduce the risk of toxicity among patients who may not develop recurrence.
In an attempt to standardise practice, the Alberta Provincial Central Nervous System Tumour Team, which consists of neurosurgeons, radiation and medical oncologists, neuropathologists, neurologists, nurses, and pharmacists, developed an evidence-based clinical practice guideline (CPG) for the management of meningomas in November 2009 which was then updated in 2012. 18 That guideline, which was disseminated to all team members including neurosurgeons via emails and at annual provincial meetings, was based on a systematic literature review and in both 2009 and 2012, recommended PORT for WHO grade II, consistent with other guidelines. 19 , 20 This means that all patients with AM should have been referred to a radiation oncologist (RO) for a PORT discussion. The objective of the current study was to document population-based care and outcomes for patients with AM and to determine whether CPG influenced RO referral or the use of PORT in southern Alberta.
Methods
This quality improvement study was conducted at the Tom Baker Cancer Centre which has a catchment area including the entire southern part of Alberta, Canada. All patients diagnosed with intracranial meningioma and treated with maximum safe resection at our centre between January 2003 and December 2013 were identified using a local brain tumor database (n=526). All patients with a new diagnosis of intracranial AM and >18 years old were included (n=83). Using the provincial electronic medical record, demographic and tumor characteristics, extent of surgical resection (EOR), PORT, date of first recurrence, and treatment of recurrence were manually abstracted. The date of recurrence or progression was the date of the first MRI showing new enhancement, or increase in tumour size. In order to assess the influence of the CPG, the patients were divided into two groups based on date of initial surgery; pre- and post- January 2010 to correspond with availability of the local CPG. Patients’ characteristics and RO referral rates were analysed by these two groups. Progression free survival (PFS) and overall survival (OS) according to EOR were assessed by the Log-Rank test of Kaplan-Meier survival. The relationships between the survival outcomes and variables of interest were evaluated using a multivariate Cox regression model. This study was approved prior to conduct, by the Alberta Privacy Office after using the ARECCI Ethics Screening tool.Reference Hagen, O’Beirne, Desai, Stingl, Pachnowski and Hayward 21
All financial and material support for the conduct of this study were provided through operational funding by CancerControl Alberta, Alberta Health Services.
Results
Patient and tumor characteristics
Among 526 patients diagnosed with intracranial meningiomas and surgically treated between January 2003 and December 2013, there were 83 patients (16%) with intracranial AM and those were included in the study. The median age at diagnosis of AM was 57 years. Females represented 59% of the patients. Convexity tumors were found in 70% of patients. Table 1 summarizes the patient and tumor characteristics.
Table 1 Patients ‘and tumors’ characteristics and treatment
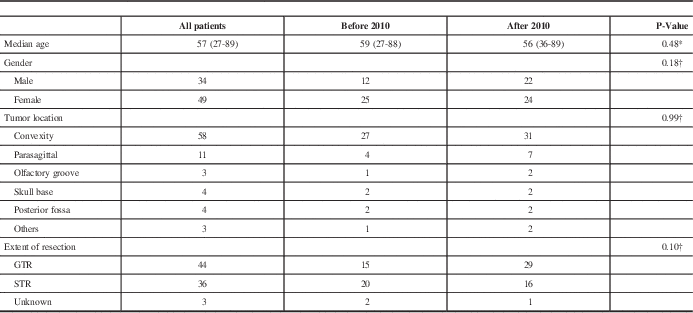
GTR: gross total resection, STR: subtotal resection
† P-values obtained from Fisher exact test
* P-values obtained from Wilcoxon test
Referral rate to radiation oncology
Only twenty-two patients (26.5%) were referred to RO after initial surgery before any signs of radiological recurrence or progression; 5 patients after GTR and 17 after STR. The referral rate to an RO at first recurrence or progression (based on MRI) was 64% (18/28). Table 2 summarises RO referral rates.
Table 2 Referral rates to Radiation Oncologists
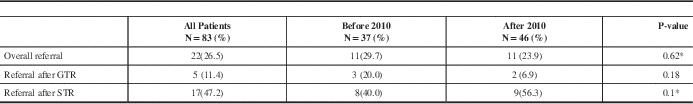
GTR: gross total resection, STR: subtotal resection.
* P-values obtained from Fisher exact test
Referral rate according to pre- and post- guideline development
Patients’ characteristics were similar between both groups. Overall, there was no statistically significant difference in RO referral rates between both groups (p= 0.62). Prior to the CPG development, 11/37 (29.7%) patients received RO referral; 20% (3/15) among patients with GTR and 40% (8/20) among patients with STR patients and two patients were referred with unknown EOR. After the development of the CPG, 11/46 (23.9%) patients were referred to RO; 7% (2/29) among patients who had GTR and 56% (9/16) among patients with STR and one patient was referred with unknown EOR, summarised in Table 2. The difference in numbers of referred patients to an RO after initial GTR or STR was statistically not significant between pre- and post- guideline developments groups (p=0.1).
Treatments
All patients were initially treated with maximum safe surgical resection. No patients had a biopsy alone. Forty-four patients had GTR, 36 patients had STR and 3 patients had an unknown EOR. Only 7 patients (8.4%) received PORT after the initial diagnosis of AM and prior to recurrence or progression. Of these patients, one had GTR and 6 had STR. The radiation therapy (RT) doses were 54 Gy, 55.8 Gy, and 60 Gy in 4, 1 and 2 patients, respectively. All RT was delivered as 2 Gy per day, Monday to Friday.
Recurrences
The median follow up time was 29 months (range 4.3-121 months). Overall, recurrence or progression was documented in 28 patients (33.7%); 4/44 (9%) after GTR, 21/36 (58%) after STR and among 3 patients with unknown EOR. Among patients who received PORT after initial surgery, 2/7 (28.5%) patients had recurrence or progression, both after STR plus PORT.
Survival
The 2-year PFS rates were 95% after GTR and 55% after STR and the corresponding rates at 5 years were 52% vs. 33%, respectively, (p=002). Median progression free survival was 100 months after GTR and 33 months after STR. The 2-year overall survival rates were 100% after GTR and 93% after STR and the corresponding rates at 5 years were 92% vs. 83%, respectively, (p=0.45).
Multivariate analysis
Based on Cox regression results, older age and STR were associated with inferior PFS rate, while receiving RO referral was statistically significant for better PFS. Table 3 summarises the results of a multivariate analysis on PFS.
Table 3 Multivariate Analysis (PFS)
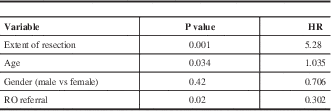
RO: radiation Oncologist
Discussion
To the best of our knowledge, this is the first study to report RO referral rates for patients with AM. We observed that overall RO referral rate was low and did not increase following development of a provincial tumor team consensus and a CPG recommending that all patients with WHO grade II should have a discussion with an RO regarding PORT 18 . Even after recurrence, only 62% of the patients were referred to an RO. The low RO referral rates are not consistent with provincial, national and international guidelines 19 , 20 . We attribute this low referral rate to the inherent biases against PORT by the local neurosurgical team as there has never been conclusive evidence for the benefit of PORT. Even among patients who saw an RO in our current study, only 32% of patients received PORT which may be one explanation for the low rate by the neurosurgical team in Alberta. Although Alberta provincial CPGs have been around since 2012, they were never formally disseminated and knowledge of their existence has been limited. Because of the lack of good evidence for PORT, even the multidisciplinary tumour board did not always follow these guidelines. Moreover, in the last couple of years, a stronger emphasis has been placed on creating and updating provincial cancer guidelines through the Guidelines Resource Unit (GURU) in Alberta. Guidelines are far more accessible on the Alberta Health Services website now and the presence of the GURU team at every annual provincial tumour meeting has helped create awareness of provincial CPGs. Multipdisciplinary meetings are held inter-provincially throughout the year to update these guidelines. It is anticipated that adherence to these guidelines will improve and referral rates for AM patients will increase. In a survey conducted among neurosurgeons in the UK and Ireland, 80% indicated that they would not advocate PORT for patients with AM after GTR and only 59% would recommend it after STR.Reference Marcus, Price, Wilby, Santarius and Kirollos 22 Most of the patients who received PORT had an initial STR (6/7). In an analysis of SEER data for patients with AM or malignant meningiomas from 1988-2007, PORT was received by 37% of patients (244/657), consistent with the use of PORT in the current series.Reference Stessin, Schwartz and Judanin 23 In comparison, the rate of PORT for AM was 18% after GTR and 74% after STR in a large, German, multicenter, retrospective series.Reference Simon, Bostrom, Koch and Schramm 24
The PFS and OS outcomes in the current study were consistent with other institutional experiences.Reference Aghi, Carter and Cosgrove 11 , Reference Adeberg, Hartmann and Welzel 25 , Reference Yoon, Mehta and Perumal 26 However, we could not determine the effect of PORT on outcomes of AM patients due to the small number of patients who received that therapy. Two recent meta-analyses have shown a benefit from PORT;Reference Kaur, Sayegh and Larson 27 , Reference Hasan, Young and Albert 28 in terms of reducing tumor recurrence and improving local control, but not survival, even after GTR;Reference Hasan, Young and Albert 28 however, the concern about the low statistical power of the included studies remains a significant issue. Many retrospective series showed that PORT was beneficial after STR Reference Jo, Park and Nam 6 - Reference Sun, Kim and Cai 10 , and some showed a benefit after GTR,Reference Jo, Park and Nam 6 , Reference Park, Kang and Kim 9 , Reference Aghi, Carter and Cosgrove 11 - Reference Aizer, Arvold and Catalano 13 while some others did not show any benefit of PORT after either STRReference Hardesty, Wolf and Brachman 14 , Reference Hammouche, Clark, Wong, Eldridge and Farah 15 or GTR.Reference Lee, DePowell, Air, Dwivedi, Kendler and McPherson 7 - Reference Sun, Kim and Cai 10 , Reference Hammouche, Clark, Wong, Eldridge and Farah 15 , Reference Jenkinson, Waqar and Farah 16 In one large series, PORT was associated with worse PFS and OS.Reference Yoon, Mehta and Perumal 26 The authors attributed these findings to the selection bias of referring patients with more aggressive tumors (e.g. elevated Ki67 and brain invasion) for PORT. Overall, the interpretation of the current literature is challenging, given the small numbers of patients included in most studies, their retrospective nature, and inclusion of malignant meningioma. Consistent with published data, older age and STR were associated with inferior outcomes in the current study.Reference Lee, DePowell, Air, Dwivedi, Kendler and McPherson 7 , Reference Pasquier, Bijmolt and Veninga 29 - Reference Goldsmith, Wara, Wilson and Larson 31 The association between receiving RO referral and better PFS might reflect a selection bias of referring patients with better prognosis like younger patients or those who had better performance status rather than a treatment effect.
The lack of phase III randomized controlled trials and the conflicting data from the available retrospective studies, have contributed to the diversity of AM treatments among centres and physicians. The RTOG 0539 is a phase II trial that was closed to accrual in 2009 and is expected to be published soon. All recruited patients in the trial who had atypical meningioma received PORT following either GTR (54Gy in 30 fractions) or STR (60Gy in 30 fractions). Since that trial was not randomized, it cannot provide information on the efficacy of PORT for patients with AM but can provide a benchmark for PFS and OS. The currently open phase III trial, ROAM/EORTC 1308,Reference Jenkinson, Weber, Haylock, Mallucci, Zakaria and Javadpour 32 aims to recruit 172 patients after GTR to be randomized between observation or early PORT. The primary endpoint is disease-free survival and the estimated required follow up is 10 years.
Limitations of the current study include its retrospective nature and the lack of complete data on prognostic factors including histological subtypes, mitotic rates, Ki67, brain invasion and sheeting architecture which were examined in other studies.Reference Jo, Park and Nam 6 , Reference Lee, DePowell, Air, Dwivedi, Kendler and McPherson 7 , Reference Sun, Kim and Cai 10 , Reference Olar, Wani and Sulman 33 , Reference Vranic, Popovic, Cor, Prestor and Pizem 34 These factors have not been used to select patients for PORT in Alberta and should have not affected RO referral rate or utilization of PORT. 18 Another limitation is the lack of systematically recorded performance status. As all of the included patients in this study had maximum safe surgical resection, their initial performance status would presumably have been sufficient to permit PORT; however, surgical complications or clinical deterioration postoperatively, which were not assessed in our study, could have made RO referral and/or PORT not feasible. Furthermore, we used a time cut-off corresponding to the year of CPG publication, perhaps it would take a bit more time for physicians to be familiar with the provincially published guidelines. The referral rate over the more recent years may perhaps be improved.
Compliance with CPGs has been associated with better outcomes in many tumor sites including head and neck,Reference Lewis, Hessel and Roberts 35 melanoma,Reference Erickson Foster, Velasco and Hieken 36 sarcoma,Reference Bagaria, Ashman, Daugherty, Gray and Wasif 37 gastric,Reference Worhunsky, Ma and Zak 38 colon,Reference Boland, Chang and Haynes 39 , Reference Chagpar, Xing and Chiang 40 pancreas,Reference Visser, Ma, Zak, Poultsides, Norton and Rhoads 41 and leukemia.Reference Goldberg, Chen and Guerin 42 Generally, most of these CPGs were based on higher levels of evidence than are available in AM. This could certainly influence the pattern of practice in offering adjuvant PORT for patients with AM but it couldn’t justify omitting an RO referral. While multidisciplinary tumor board discussion can provide general treatment recommendations, ultimately, patients have the right to make an informed decision about PORT after discussing the pros and cons during a visit with an RO.
Conclusion
In Alberta, the RO referral rate (26.5%) and use of PORT (8%) were low for patients with AM diagnosed during 2003 to 2013. Even after the first recurrence, only 62% of patients were referred. The development and web-publication of a CPG did not seem to influence the RO referral rate or use of PORT. Given the lack of conclusive evidence supporting using PORT in such patients, a multidisciplinary approach, including RO consultation and improved adherence to local CPGs, are needed to provide patients with optimal and individualised care. Moreover, when more data becomes available in the future, practice and/or guideline recommendations may change.
Acknowledgements
The Al Baha University (Al Baha, Saudi Arabia) sponsored MA for his residency training at the University of Calgary. The authors thank members of the CNS tumor group in Alberta who treated the patients and provided constructive advice during the design and interpretation of this study. Presented in part at the Canadian Association of Radiation Oncology annual meeting September, 2015 and at the American Society for Radiation Oncology annual meeting, October, 2015.
Disclosures
Majed Alghamdi, Haocheng Li, Ivo Olivotto, Jay Easaw, John Kelly, Robert Nordal, and Gerald Lim do not have anything to disclose.





