Maximizing extent of resection and minimizing residual tumour volume has been shown to improve overall survival of patients with glioblastoma multiforme,Reference Sanai, Polley, McDermott, Parsa and Berger 1 , Reference Grabowski, Recinos and Nowacki 2 leading to cytoreductive surgery followed by adjuvant radiation and chemotherapy becoming standard of care when feasible. Surgical treatment for low-grade gliomas (LGGs) remains controversial, but a growing number of proponents are advocating for an “aggressive attitude” to LGG treatment with early surgery as initial therapy.Reference Duffau and Taillandier 3 , Reference Hervey-Jumper and Berger 4
Advanced imaging techniques such as functional magnetic resonance imaging (fMRI) provide noninvasive means by which to assess the relative eloquence of brain tumours, and are being increasingly used for surgical planning. When performed preoperatively, these modalities have the potential to help augment the surgical approach to allow for safer surgery and determine whether surgery is even feasible. When an appropriate task can be chosen, fMRI provides important surrogate information about the proximity of a lesion to areas of eloquence, including language and motor regions.
Clinical implementations of fMRI protocols continue to be quite variable because of a lack of standardization. A few systematic reviews have summarized the utility of fMRI techniques,Reference Dimou, Battisti, Hermens and Lagopoulos 5 , Reference Stippich 6 but there remains a lack of high-quality evidence to recommend routine use.
We sought to review our institutional experience with acquiring clinical fMRI sequences for patients with brain tumours at London Health Sciences Centre (LHSC). The objective of this paper is to characterize the cohort of brain tumour patients that have undergone task-based functional MRI in the preoperative surgical planning stages.
MATERIALS AND METHODS
We conducted a retrospective cohort review of all patients evaluated with pre-operative clinical fMRI by the senior author (JFM) from 2002 to 2013 at LHSC. To identify the patients, we used a prospectively maintained database of all patients having undergone fMRI under a clinical protocol. Chart review was subsequently performed on site at University Hospital, London, Ontario.
Baseline demographics were recorded including age at diagnosis, gender, handedness, presenting symptoms and signs, location of tumour, histopathological diagnosis, indication for advanced neuroimaging, functional imaging paradigms acquired, and type of surgical intervention. Handedness was determined using the Edinburgh Handedness Inventory. This study was LHSC Health Sciences Research Ethics Board approved at Western University.
Structural MRI
T1-weighted magnetic resonance imaging (MRI) scans were acquired on a clinical 1.5 Tesla General Electric scanner (GE Healthcare) using a three-dimensional spoiled gradient recalled echo sequence. The following parameters were used: echo time=1.5 ms, repetition time=6.3 ms, matrix size=256×256, field of view=24 cm×24 cm, resolution=1 mm×1 mm×1.5 mm, slice number was variable. The average scan time for the anatomical T1 was 5 minutes varying slightly depending on participant head size.
Functional MRI
At our centre, clinical fMRI acquisitions are completed at University Hospital, LHSC (London, Ontario, Canada). There is a dedicated block of time each week for completion of fMRI on a clinical 1.5T GE magnet (Thursday mornings) with 1 hour of total MRI time allocated per patient, including anticipated downtime between fMRI tasks for setup. Our experienced fMRI coordinator is present for all clinical scans and performs quality control of data at the time of acquisition and is able to repeat studies at that time, if necessary. Before scanning, each patient is briefed on the fMRI protocol, completes a typical screening MRI checklist, and signs consent for the diagnostic scan. This interaction allows for the screening of any gross language or cognitive deficits. If relevant, a basic motor exam of relevant motor functions (i.e. finger, toe tapping) is performed. Language and memory fMRI tasks are simulated non-covertly. The Edinburgh Handedness Inventory is completed. For memory paradigms, picture recall is performed postscan to screen for compliance.
Functional MRI studies acquired depended on patient-specific considerations, but included a full range of conventional block-designed paradigms (motor, speech, memory), and are summarized as the following:
-
∙ Speech: covert verb generation, sentence completion, naming, listening
-
∙ Motor: finger tapping, foot tapping
-
∙ Memory: scene paradigm
-
∙ Sensory
Non-block paradigm datasets that were acquired included resting state and an event-related autobiographical memory task. Examples of the main fMRI tasks used in this study are shown in Figure 1.

Figure 1 Examples of each of the main fMRI block paradigms included in this study: (A) motor, (B) sensory, (C) language (verb generation, sentence completion, naming, listening), (D) memory, and (E) visual/retinotopy. Complementary details are provided in Table 4.
Paradigms were presented visually using SuperLab Pro (version 2.0) with fiber optic goggles. fMRI images were acquired at the same session as the T1 dataset using the spiral-in method with the following parameters: echo time=40 ms, repetition time=2500 ms, matrix size=64×64, field of view=24 cm×24 cm, resolution=3.75 mm×3.75 mm×5.0 mm, flip angle=90°, 30 slices/volume.
During the course of the fMRI, attentiveness and gross noncompliance (movement, sleeping, seizures) were monitored using video surveillance visible at the MRI console (ViewPoint Eyetracker, Arrington Research, Inc.). Non-compliance to the fMRI memory paradigm was evaluated using postassessment recall.
fMRI Processing
A single volume was constructed from the MRI volumes using MRIcro (http://www.psychology.nottingham.ac.uk/staff/cr1/mricro). The resulting image was flipped to display right hemisphere on the right side of the image. The data were normalized and co-registered with the normalized mean fMRI image in SPM to display the activation map, depending on the paradigm. The anatomical scan was used for registration for subsequent fMRI processing.
Functional MRI data were preprocessed using in-house scripts. The technical details of fMRI processing evolved over the course of the 12-year study period. SPM2 was used for fMRI analysis (http://www.fil.ion.ucl.ac.uk/spm) (UCL, London, UK).
fMRI datasets were subjected to realignment to the mean fMRI volume. The mean fMRI image was further registered to the patient’s structural T1 scan so that regions of functional activation were mapped in the space of the structural MRI scan. fMRI images were corrected for head motion, and smoothed with a 7.5 mm and 10 mm Gaussian kernel. Contrast images were derived by comparing blood oxygenation level dependency (BOLD) signal during the task versus at rest using a Student t test with family-wise group error, thresholds being set at p<0.05 and voxel cluster size>25. The data were analyzed without normalization, resampled into the resolution of the patient’s structural anatomical scan using SPM2, and displayed in the Atamai viewer (Atamai, http://www.atamai.net).
Our fMRI coordinator performed all the processing. The fMRI analysis results were interpreted and reported by the Clinical Functional MRI Team consisting of three members: our fMRI coordinator, a neurologist, and neuroradiologist. The reports and selected images were available through the electronic medical record and picture archive and communication system (PACS).
RESULTS
A total of 60 patients were identified who were evaluated with preoperative fMRI with baseline characteristics summarized in Table 1. Four patients were missing clinical fMRI report data, two of which were scheduled for fMRI but never had the test performed, leaving 56 patients. In that same time period, the senior author had performed 1004 tumour surgeries, combining biopsies and resections. Thus, fMRI evaluation was performed in 5.6% of tumour cases. Representative cases are shown as Figures 2 through 4.

Figure 2 A 51-year-old female presenting with multiple simple partial seizures with right hand numbness and clumsiness found to have a left frontoparietal meningioma. Representative preoperative T1-weighted and T2-weighted MRI scans are shown (A). Selective axial T1-weighted MRI scans with fMRI overlay (t value thresholded at 4.76) for a sentence completion language task. Bilateral activation was noted for this task (B). Left hemispheric surface view of fMRI activation areas for verb generation (red), sentence completion (green), naming (blue), right finger tap (yellow), and left finger tap (cyan). As expected, there was no activation of the tumour mass itself (C). Representative postoperative MRI scans showing gross total resection (D).

Figure 3 An 18-year-old male presenting with headache found to have a right frontal mixed astrocytoma/oligodendroglioma. Representative preoperative T1-weighted and T2-weighted MRI scans are shown (A). Selective axial T1-weighted images are shown for a left finger tap motor task (t value thresholded at 3.87). The lesion appears anterior and lateral to the area of fMRI activation (B). This patient underwent gross-total resection of the lesion with a craniotomy under general anesthesia with post-operative images shown (C).

Figure 4 A 36-year-old male presenting with seizure found to have a left frontoparietal tumour. Representative preoperative T1-weighted and T2-weighted MRI scans are shown (A). Selective axial T1-weighted MRI scans with fMRI overlay (t value thresholded at 3.87) for a right finger tap motor task (B). Left hemispheric surface view of fMRI activation areas for verb generation (red), sentence completion (green), and right finger tap (blue) (C). After discussion, the patient opted for stereotactic biopsy using a Leksell frame. Pathology returned as oligodendroglioma with 1p19q loss of heterozygosity. He has remained symptom-free at the 6-year follow-up with seizures under control.
Table 1 Patient demographics
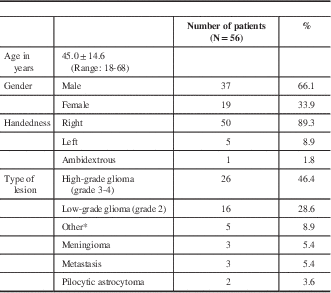
* Other diagnoses included neurocysticercosis, clinically isolated syndrome (presumed demyelinating disorder), ganglioglioma, cavernous malformation, PXA, and primary central nervous system fibrosarcoma.
There were 37 males (66.1%) and 19 females (41.1%) with an average age of 45.0±14.6 years (range, 18-68). In terms of handedness, 50 (94.6%) were right-handed, five (8.9%) were left-handed, and one (1.7%) was ambidextrous.
We defined low-grade glioma as World Health Organization grade II and high-grade gliomas as World Health Organization grade III or IV. Pilocytic astrocytoma, ganglioglioma and pleomorphic xanthoastrocytoma were treated as distinct from our definition of low-grade glioma. There were 26 patients (46.4%) with high-grade glioma, 16 (28.6%) with low-grade glioma, three (5.4%) with meningioma, two (3.6%) with metastasis, and two (3.6%) with pilocytic astrocytoma. Other diagnoses included neurocysticercosis, clinically isolated syndrome (presumed demyelinating disorder), ganglioglioma, cavernous malformation, pleomorphic xanthoastrocytoma, and primary central nervous system fibrosarcoma.
Preoperative clinical status is summarized in Table 2. Most commonly presenting symptoms encountered were seizures in 37 patients (66.1%), language deficits in 12 (21.4%), change on surveillance imaging in seven (12.5%), headache in 11 (19.6%), and weakness in six (10.7%). In five patients (8.9%), the intracranial lesion was found incidentally. Other symptoms included nausea and vomiting, personality changes, visual disturbances, and fatigue.
Table 2 Patient symptoms at presentation
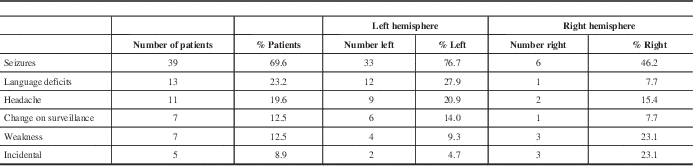
Other symptoms encountered: nausea/vomiting, personality changes, visual deficits, fatigue.
The lesions investigated with preoperative fMRI were predominantly left hemispheric in 43 patients (76.8%) compared with 13 right hemispheric patients (23.2%) (Table 3). Lobes most commonly involved were frontal in 36 (64.3%), temporal in 19 (33.9%), and parietal in 12 (21.4%). Because a tumour can involve more than one lobe, the total number of locations is greater than the number of subjects. Examining the left hemispheric lesions in isolation, the distribution of locations followed the same trend as in the combined analysis, occurring most often in the frontal lobe in 25 (58.1%), temporal lobe in 18 (41.9%), then parietal lobe in eight (18.6%).
Table 3 Lesion location
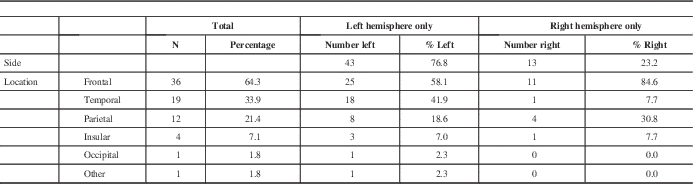
Right hemispheric lesions were distributed differently, occurring in the frontal lobe most commonly in 11 (84.6%), followed by parietal lobe in four (30.8%), and then temporal lobe in one (7.7%). Patients with right-sided lesions tended to be younger (36.7±13.7 years) compared with the whole fMRI group (45.0 ± 14.6 years). All of the patients with right-sided lesions had motor paradigms assessed using fMRI, and eight (61.5%) had language paradigms assessed. Four (30.8%) of the patients with right-sided lesions had recurrent lesions, compared with nine (20.9%) recurrent lesions in patients with left-sided lesions group. Three of the patients (23.1%) with right-sided lesions presented with weakness, compared with four (9.30%) patients with left-sided lesions. Similar to the group of patients with left-sided lesions, the most common presentation for patients with right-sided lesions was seizure (six patients, 46.2%).
Functional MRI Paradigms Acquired
The most commonly performed tasks were in the language and motor domains. At least one language paradigm was acquired in 47 patients (83.9%) and at least one motor paradigm in 42 patients (75.0%). Details are provided in Figure 1 and Table 4. Other paradigms included sensory in nine patients (16.1%), memory in six patients (10.7%), visual in one patient (1.8%), and resting state in one patient (1.8%).
Table 4 Clinical fMRI Paradigms

NA, not applicable.
The average number of fMRI tasks performed was 4.5±1.2 (range: 2-8) tasks across all patients in the series. We stratified by tumour localization and did not find any relationship with number of fMRI tasks except for temporal lobe lesions (33.9%; N=19) where the average number of tasks was slightly decreased at 3.9±1.3 (range: 2-6). The mean scan time for functional MRI paradigms was 30:10 minutes (with a standard deviation of 7:20 minutes; range: 11:08 to 49:12 minutes). A total in scanner time of 1 hour was allocated per patient.
Thirty-two subjects (53.3%) had language lateralization index values calculated. With a lateralization index set at ±0.2, language lateralization index was concordant with hand dominance in 59.4% of patients. 18.8% of patients with right hand dominance had language lateralization index showing bilateral hemispheric dominance.
Management: Surveillance versus Biopsy versus Resection
Forty-two patients (75.0%) ultimately underwent a craniotomy, whereas a smaller subset underwent stereotactic biopsy (five patients; 8.9%) and non-surgical management (nine patients; 16.1%), outlined in Table 5. Time to completion of fMRI and fMRI to intervention are summarized in Table 6. Time from request for fMRI to actual fMRI acquisition was 3.1±2.3 weeks. Time from fMRI to intervention across the entire intervention group was 18.5±49.1 weeks. Excluding those that first underwent a period of watchful waiting, time from fMRI acquisition to intervention was 4.9±5.5 weeks. All cases that had initial watchful waiting were either previously diagnosed LGG or presumed LGG. In all cases, patients and their families were provided options for management including surveillance, tissue diagnosis with biopsy, and surgical resection.
Table 5 Surgical interventions performed
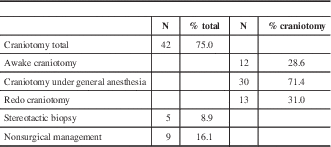
Table 6 Time to completion of fMRI and intervention from initial presentation

Patients with Missing or Poor Quality fMRI Data
Details regarding the four patients with missing fMRI data are outlined as follows. In one case, the workstation with the fMRI protocol software (SuperLab Pro) was not functioning, so the test was aborted. Two had missing fMRI details and clinical reports. The last patient had poor-quality fMRI results with plans to repeat the study, but was not completed before their craniotomy procedure (pathology returned as fibrosarcoma).
Four patients in our study group had fMRI protocols repeated at the time of scanning because of acquisition problems detected while the scan was in progress. For one additional patient, acquisition problems were detected at the time of fMRI data postprocessing, for a total of five patients with poor quality fMRI data (8.9%), and thus a usability rate of 91.1%.
DISCUSSION
The clinical role of functional magnetic resonance imaging for preoperative surgical planning in tumour patients has not been clearly identified. Evaluations for efficacy continue to be limited to single-centre experiences because of differing practice patterns, resulting in a continued lack of standardization. The results reported here reflect practice patterns at our Canadian centre integrating functional magnetic resonance imaging into the surgical planning process.
Overall, the decision to perform fMRI and complete the clinical fMRI scan were within reasonable limits considering that these patients are usually minimally symptomatic and can be booked on an elective basis. Our fMRI usability rate of 91.1% is higher than that reported in most existing studies. In our typical clinical fMRI protocol, an experienced fMRI coordinator (FB) is present for all clinical scans, and is able to detect any issues with data acquisition at the time of the scan, limiting the need for repeat scans at a later date.
The utility of preoperative fMRI in planning tumour surgery has been evaluated in a number of independent studies. In 1999, one study demonstrated the role of preoperative fMRI in decision-making in three key categories: assessment of feasibility of resection, surgical planning, and selection of patients for invasive functional mapping procedures.Reference Lee, Ward and Sharbrough 7 fMRI was helpful in at least one of the categories in 89% of patients with sensorimotor eloquent lesions. Separately, Petrella and colleagues prospectively evaluated treatment decision-making in brain tumour patients as influenced by preoperative fMRI. They demonstrated that treatment plans were altered by the use of preoperative fMRI resulting in reduced surgical time, increased extent of resection, and decreased craniotomy size.Reference Petrella, Shah and Harris 8
Substantial evidence has been gained through the Norway experience, where a group of neurosurgeons has evaluated the use of fMRI, among other advanced imaging paradigms, in a number of studies over the past decade. After quality control of data, fMRI results were reported to be helpful for surgical planning in 75% of cases.Reference Håberg, Kvistad and Unsgård 9 They found that postoperative deficit was more likely when the distance between the tumour and regions of activation was less than 10 mm. In a separate prospective study, this group evaluated patients with high-grade glioma in eloquent locations discovering that when fMRI was combined with diffusion tensor imaging (DTI) and intraoperative ultrasound, significant tumour debulking to 16% of the original volume could be achieved while minimizing deficits.Reference Gulati, Berntsen and Solheim 10
Preoperative Assessment of Motor Function by fMRI
Motor task activation has generally been found to be the most reliably measured fMRI paradigm for a number of reasons. Task simplicity increases patient compliance during activity blocks. Compliance can also be assessed from the viewing area by the fMRI coordinator and MRI technician. Presurgical planning has been shown to be influenced in one or more clinical decision-making categories in 89% of patients with sensorimotor eloquent tumours.Reference Lee, Ward and Sharbrough 7 Wengenroth et al. found that an optimized fMRI motor paradigm showed superiority to identification by structural MRI alone in a cohort of patients with lesions proximal to the precentral gyrus.Reference Wengenroth, Blatow, Guenther, Akbar, Tronnier and Stippich 11
Several studies have validated results with intraoperative direct cortical stimulation. Regions of significant functional activation have been confirmed to identify concordant motor findings in more than 90% of cases.Reference Pujol, Conesa, Deus, López-Obarrio, Isamat and Capdevila 12 , Reference Lehéricy, Duffau and Cornu 13 Using intraoperative electrocortical mapping, Bizzi et al. reported that fMRI hand motor activation sensitivity and specificity were 88% and 87%, respectively.Reference Bizzi, Blasi and Falini 14
Preoperative Assessment of Language Function by fMRI
Current literature indicates that language function assessment using fMRI is a valuable tool for patients with tumours affecting their language cortical network.Reference Dimou, Battisti, Hermens and Lagopoulos 5 , Reference Petrella, Shah and Harris 8 The goal of preoperative fMRI language mapping is twofold: identify cerebral hemispheric language lateralization and determine the proximity of the lesion to regions critical to language functions.Reference Zacá and Pillai 15 Although fMRI assessment of motor function has been shown to be reliable in numerous validation studies, fMRI language mapping remains more controversial.Reference Stippich 6 , Reference Zacá and Pillai 15 , Reference Wehner 16
For the purpose of language lateralization, fMRI is generally regarded as reliable. A recent meta-analysis showed a good sensitivity and specificity (83.5% and 88.1%, respectively) compared with Wada testing, the clinical gold standard.Reference Dym, Burns, Freeman and Lipton 17 A strong predictor of lateralization discordance between fMRI and Wada testing is atypical lateralization on fMRI assessment. If fMRI suggests typical left language dominance, Wada testing is very likely to be concordant.Reference Janecek, Swanson and Sabsevitz 18 This observation has important clinical implications. It suggests that Wada testing and fMRI have complementary value, with fMRI being particularly valuable for reliably identifying preoperative patients with typical language lateralization and eliminating the need for Wada testing. However, it is important to note that individual language lateralization validation studies have varied widely in their results. This variety can be explained by small sample sizes, as well as variability in subject populations, paradigms used, test protocols, and postprocessing techniques.Reference Stippich 6 , Reference Dym, Burns, Freeman and Lipton 17 , Reference Giussani, Roux, Ojemann, Sganzerla, Pirillo and Papagno 19 Wada testing remains a valuable option for determining the lateralization of memory, as no fMRI paradigm has been shown to reliably assess this noninvasively.
Analysis of our fMRI data shows a lower concordance of language lateralization index with the Edinburgh Handedness Inventory compared with other studies that evaluate reliability of fMRI in normal subjects.Reference Springer 20 These results are consistent with other previous studies in patients with brain tumours where bilateral language dominance and a functional shift toward the contralesional, nondominant hemisphere is more likely to be found.Reference Ulmer, Hacein-Bey and Mathews 21 - Reference Partovi, Jacobi and Rapps 23 BOLD activation is likely influenced by physiological factors affecting brain tumour patients, including neuroplasticity and neurovascular uncoupling.
The reliability of fMRI studies in determining the spatial proximity of critical language regions to brain lesions in brain tumour patients is quite controversial.Reference Wehner 16 Several studies comparing preoperative fMRI and electrocortical stimulation (ECS), the gold standard, in patients with brain lesions have been performed. The results of these studies have been mixed. In a 2010 review of fMRI language mapping validation studies, Giussani et al. found that sensitivities of 59% to 100% and specificities of 0% to 97% compared with ECS had been measured. Much like the aforementioned fMRI validation studies of language lateralization, investigations into the reliability of fMRI to map specific critical language regions have been highly heterogeneous.Reference Giussani, Roux, Ojemann, Sganzerla, Pirillo and Papagno 19 Studies suggest that fMRI lacks reliability in identification of essential versus participatory language regions and has a high rate of false positives.Reference Zacá and Pillai 15 , Reference Giussani, Roux, Ojemann, Sganzerla, Pirillo and Papagno 19 , Reference Roux, Boulanouar and Lotterie 24 , Reference Spena, Nava and Cassini 25
Although the reasons for the variability in concordance between fMRI and gold standard language assessment are not fully understood, several factors that contribute to this observation have been identified. Different fMRI language task paradigms have been shown to have different degrees of concordance with Wada testing and ECS.Reference Bizzi, Blasi and Falini 14 , Reference Dym, Burns, Freeman and Lipton 17 , Reference Baciu, Watson, Maccotta, McDermott, Buckner and Gilliam 26 , Reference Benke, Köylü and Visani 27 A wide range of different language tasks have been proposed in the literature, resulting in significant heterogeneity between sites.Reference Zacá and Pillai 15 , Reference Stippich, Blatow and Krakow 28 At our centre, the fMRI language tasks are performed covertly—that is, the subject is asked to silently generate words during the task block. This method of assessing language has been used widely to avoid excessive movement artifact during image acquisition related to a verbal response from the participant. As a result, unlike the motor task scenario, task compliance is not easy to assess. Some centres, including our own, have adopted either prescan assessments or postscan interviews to ensure proper task performance.Reference Waites, Briellmann, Saling, Abbott and Jackson 29 Real-time fMRI processing holds tremendous promise for quality control and ensuring compliance related to task completion (see Weiskopf et al.Reference Weiskopf, Sitaram and Josephs 30 for a review). The type of lesion present has also been shown to have an effect on the degree of concordance between fMRI language assessment and Wada testing or ECS. Factors including the degree of infiltration of the lesion and the lesion’s effect on vasculature influence concordance.Reference Wehner 16 , Reference Ulmer, Hacein-Bey and Mathews 21
The optimal role for fMRI in assessing language function seems to be complementary to Wada and ECS. A recent study of 677 epilepsy patients who underwent Wada testing found that 10.9% experienced complications, the most common being encephalopathy (7.2%), seizures (1.2%), and stroke (0.6%).Reference Loddenkemper, Morris and Möddel 31 In preoperative patients with typical language lateralization, fMRI can reliably detect lateralization and prevent the need for Wada testing. fMRI can play a role in guiding the decision to treat based on noninvasive mapping of important eloquent regions. Once a decision to treat has been made, an awake craniotomy with ECS provides an intraoperative mapping of essential language regions that can complement the whole brain map produced with fMRI.Reference Stippich 6
Limitations
Although this article reports initial experiences with preoperative fMRI at our centre, there are several inherent limitations. The data are retrospective and self-reported. Because no guidelines currently exist, the decision to pursue advanced imaging was made completely at the surgeon’s discretion.
fMRI is based on the concept of neurovascular coupling. In essence, neuronal activation leads to a downstream hemodynamic response, typically occurring with a 2- to 4-second delay. Local changes in paramagnetic deoxyhemoglobin concentration can be detected as a local magnetic field inhomogeneity, and measured as the blood oxygenation level dependency (BOLD) signal. A single BOLD reading can be quite noisy with magnitude of activation typically 2% to 4% of baseline.Reference Zacá and Pillai 15 Thus, repeated trials typically via a block design can improve the signal-to-noise ratio. The BOLD signal can show whether a region is activated or not, but it cannot distinguish between whether a region is excitatory or inhibitory. Similarly, if a sufficiently small cluster of neurons is activated by a given task at a scale smaller than the resolution of acquisition (typically 2 mm isotropic), the electrophysiological activation may not be detected.
Pujol et al. reported an inability to identify sensorimotor activation in 18% of tumour patients, concerning for the presence of false-negative regions.Reference Pujol, Conesa, Deus, López-Obarrio, Isamat and Capdevila 12 Lack of activation may be attributed to failure of acquisition, when a given patient study has not passed basic quality control metrics. Several studies have reported a failure rate of 10% to 30% in patients with brain tumours.Reference Håberg, Kvistad and Unsgård 9 In our cohort, acquisition problems were detected 8.9% of our total group. Patients with poor data acquisition should be considered for repeat fMRI.
Magnetic resonance properties of the tumour may also adversely influence the BOLD signal leading to inaccuracies.Reference Dimou, Battisti, Hermens and Lagopoulos 5 , Reference Pujol, Conesa, Deus, López-Obarrio, Isamat and Capdevila 12 , Reference Megyesi, Kachur and Lee 32 BOLD signal decreases of 36% have been reported in the lesional hemisphere compared with the unaffected hemisphere.Reference Schreiber, Hubbe, Ziyeh and Hennig 33 Gliomas have been found to have reduced BOLD signal at the periphery of the lesion, correlating with increases in relative cerebral blood volume in the same region.Reference Hou, Bradbury, Peck, Petrovich, Gutin and Holodny 34 This phenomenon has been termed lesion-induced neurovascular uncoupling, and Ulmer et al. have warned that these factors can influence functional maps and lead to erroneous interpretations of fMRI-derived cerebral dominance.Reference Ulmer, Hacein-Bey and Mathews 21 Another study similarly found decreased fMRI activation in glioma patients, particularly high-grade gliomas, with carbogen inhalation, a technique used to assess voxel-wise BOLD response independent of task activation.Reference Hsu, Chang and Jung 35 , Reference Jiang, Krainik and David 36
An ideal surgical workflow for management of eloquent lesions would involve seamless integration of fMRI and DTI. DTI provides important structural information about lesion proximity to critical fiber tracts, such as the corticospinal tract and arcuate fasciculus.Reference Fortin, Aubin-Lemay and Boré 37 , Reference Incekara, Lee, Rigolo and Golby 38 Both MRI sequences can be acquired at a single scan session and provide compatible functional and structural information that can be used to customize a surgical approach.
CONCLUSIONS
We have characterized patient demographics in a retrospective single-surgeon cohort undergoing clinical fMRI at our centre for evaluation of intracranial lesions. Not unexpectedly, these patients tended to have relatively eloquent lesions. Time from scan request to acquisition and scan to treatment appear to be reasonable. Our results represent a unique Canadian perspective on the practical triaging of brain tumour patients for fMRI under a clinical protocol.
ACKNOWLEDGMENTS
The authors thank the members of the Clinical Functional Magnetic Resonance Imaging Team (Dr. Donald H. Lee and Dr. Seyed M. Mirsattari) at London Health Sciences Centre.
Disclosures
JCL is funded through the Western University Clinical Investigator Program accredited by the Royal College of Physicians and Surgeons of Canada. SK is funded through the Western University Schulich Medicine & Dentistry Medical Student Research Training Program (SRTP). JFM is the past chairman of the Brain Tumour Foundation of Canada. The authors report no conflict of interest concerning the materials, methods, or results described in this article.
SUPPLEMENTARY MATERIALS
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/cjn.2016.306












