Introduction
In December 2019, the COVID-19 epidemic emerged in Wuhan, China, causing global alterations not only in the field of healthcare, but also in all walks of life. The viral agent responsible for this clinical illness is described as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It was documented that SARS-CoV-2 is associated with neurologic manifestations, including headache, dizziness, hypogeusia, and hyposmia. Reference Mao, Wang and Chen1 Beside hypogeusia and hyposmia, there has been increased reporting of distinct peripheral nervous system (PNS) diseases in COVID-19 patients.
Guillain Barre syndrome (GBS) is an inflammatory disease of the PNS, characterized by rapidly progressive, symmetrical, and typically ascending weakness of the limbs with reduced or absent deep tendon reflexes, and upper and lower extremities non-length-dependent paresthesia and sensory symptoms at onset. Cranial nerves involvement can also be present in GBS patients, with facial and bulbar muscles often being affected. Reference Leonhard, Mandarakas and Gondim2 GBS can be classified into different distinct clinical variants including classical sensorimotor, paraparetic, pure motor, pure sensory, Miller Fisher syndrome (MFS), pharyngeal-cervical-brachial variant (PCB), bilateral facial palsy with paranesthesia, and Bickerstaff brainstem encephalitis. Reference Hiew, Ramlan, Viswanathan and Puvanarajah3 Another classification of GBS based on the electromyography (EMG) findings has also been described, with acute inflammatory demyelinating polyneuropathy (AIDP) being the most common variant. Other EMG variants of GBS according to this classification include acute motor axonal neuropathy (AMAN) and acute motor and sensory axonal neuropathy (AMSAN). Reference Dimachkie and Barohn4
GBS has been linked to a variety of causative pathogens; campylobacter jejuni (C. jejuni), cytomegalovirus (CMV), hepatitis E virus, mycoplasma pneumoniae, Epstein–Barr virus (EBV), and Zika virus. Reference Nachamkin, Allos and Ho5–Reference Orlikowski, Porcher and Sivadon-Tardy8 The emergence of Zika virus epidemic in 2016 was noticeably linked to increased incidence of GBS. Reference Counotte, Meili, Taghavi, Calvet, Sejvar and Low9 GBS has also been linked to Middle East respiratory syndrome coronavirus (MERS-CoV) which is genetically similar to SARS-CoV-2 and was responsible for the outbreak of Middle East Respiratory Syndrome in 2013. Reference Kim, Heo and Kim10 In January 2020, the first case of GBS due to SARS-CoV-2 infection was documented in China. Reference Zhao, Shen, Zhou, Liu and Chen11 In this article, we are reviewing all the published cases of GBS that have been linked to SARS-CoV-2, to study their clinical presentations, the average latency period till the onset of GBS symptoms, the global distribution of these cases, and the findings of the ancillary GBS investigations.
Methods
We searched PubMed, EMBASE, and Google scholar and included all papers with full text available in English or Spanish and reporting original data of patients with GBS and recent COVID infection. This systematic literature review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Figure 1). Reference Moher, Liberati, Tetzlaff and Altman12 We used the following keywords on our search: GBS, MFS, COVID-19, SARS-CoV2, and neurological manifestations, and these databases were searched from August 26, 2020 and to February 7, 2021. Titles and abstracts were screened by two researchers (M. Aladawi and M. Elfil). The full texts of the selected papers were read in full by five researchers (M. Aladawi, B. Abu-Esheh, D. Abu Jazar, A. Armouti, and A. Bayoumi), and their extracted data were then revised by M. Aladawi.
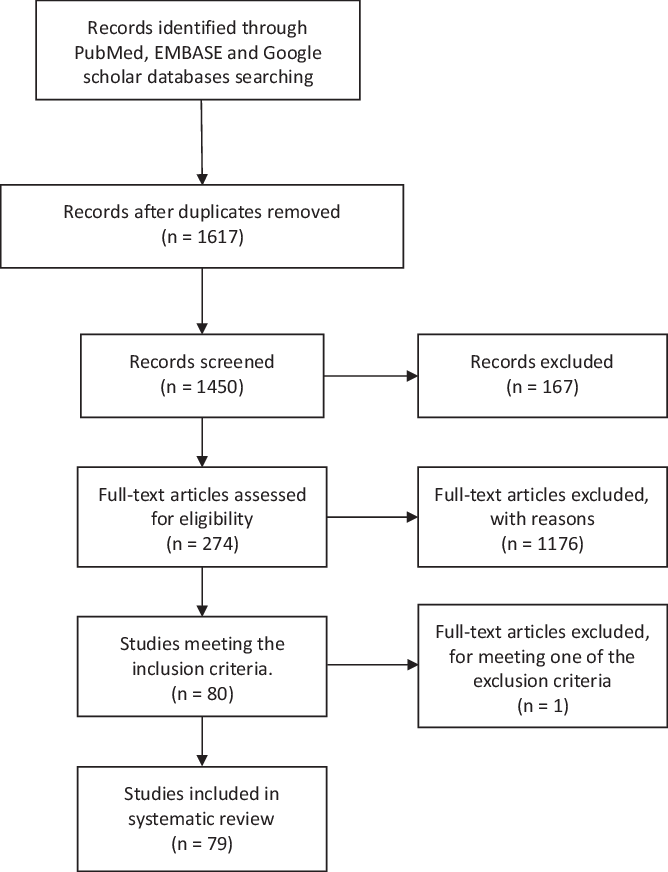
Figure 1: PRISMA figure showing the steps of literature search and paper selection for the systematic review.
We included all papers, reports, or bulletins with the full text available in English or Spanish, reporting data of patients with GBS and a probable or confirmed recent COVID-19 diagnosis. Preidentified exclusion criteria were: (1) GBS with proven triggering infection other than SARS-CoV2 (e.g., C. jejuni), (2) presence of alternative diagnosis for weakness (e.g., critical illness neuropathy), and (3) latency period between COVID-19 infection and the onset of GBS symptoms of more than 6 weeks. Variables of interest were demographics, COVID-19 diagnostic investigations, latency between constitutional viral symptoms and neurological symptoms, presence of a negative SARS-Cov2 polymerase chain reaction (PCR) at the time of neurological manifestations (Table 1). Studied variables of cases with confirmed COVID-19 infection were pooled into another table to identify clinical characteristics (viral symptoms and neurological symptoms), GBS ancillary diagnostic investigations (cerebrospinal fluid [CSF] findings and testing for antiganglioside antibodies), the predominant clinical and electrophysiological variants of COVID-19-related GBS, received immunomodulatory therapy, disease progression, and clinical outcome (Table 2).
Table 1: Demographics, diagnostic confirmation of COVID-19, latency duration of neurologic symptoms, and PCR testing at the time of neurological manifestations of both suspected and confirmed cases of COVID-19

Table 2: Demographics, clinical features, and GBS classification in patients with confirmed cases of COVID-19

AIDP= acute inflammatory demyelinating polyneuropathy; AMAN=acute motor axonal neuropathy; AMSAN=acute motor and sensory axonal neuropathy; CSF=cerebrospinal fluid; GBS=Guillain Barre syndrome; ICU=intensive care unit; IVIG=intravenous immunoglobulin; PLEX=plasmapheresis.
Cases were classified according to the reported diagnostic certainty levels for GBS and COVID-19 infection. To classify the diagnosis of GBS, we employed the Brighton Collaboration Criteria. Reference Fokke, van den Berg, Drenthen, Walgaard, van Doorn and Jacobs13 The diagnostic certainty of COVID-19 infection was classified as confirmed and suspected. As confirmed cases were identified by the presence of positive PCR at the time of arboviral symptoms or the presence of positive SARS-CoV2 antibodies whether during arboviral or neurological presentation as in some cases GBS was the presenting manifestation.
Results
We identified 1450 articles in the databases researched, of which 79 papers were included in our systematic review (66 case reports and 13 cases series). The selected studies reported on a total of 109 GBS cases with a confirmed or a suspected COVID-19 infection. One case was excluded as it met one of the exclusion criteria; the latency between the onset of COVID-19 infection and the GBS onset of symptoms was 53 d (>6 weeks). Reference Raahimi, Kane, Moore and Alareed93
The applied investigations in confirming COVID-19 infection at the time of arboviral symptoms were COVID-19 PCR testing, detection of SARS-CoV2 antibodies, and suggestive features on chest radiography. Cases with either positive PCR or SARS-CoV2 antibodies were categorized as confirmed cases, whereas patients diagnosed based on abnormal chest radiographs or clinical suspicion only were categorized as suspected cases. We have identified 99 cases of COVID-19 complicated by GBS that has been confirmed with either PCR testing or serology (Table 1). Table 1 also includes the latency period between arboviral symptoms and neurologic manifestations, the country of reported cases, and repeat COVID-19 PCR at the time of neurological symptoms either from nasopharyngeal, swabs, or in the CSF.
The global distribution of cases was as follows: 32 cases in Italy, 16 cases in the United States, 12 cases in Spain, 9 cases in Iran, 6 cases in France, 6 cases in the United Kingdom, 5 cases in India, 4 cases in Germany, 4 cases in Switzerland, 2 cases in China, 1 case in Guinea, 1 case in Austria, 1 case in Brazil, 1 case in Canada, 1 case in Columbia, 1 case in Japan, 1 case in Morocco, 1 case in Netherlands, 1 case in Sudan, 1 case in Tanzania, 1 case in Turkey, and 1 case in Saudi Arabia.
At the time of the patient’s demonstrated neurologic signs and symptoms, repeat SARS-CoV2 PCR swab was negative in 23 cases. Reverse transcription PCR (RT-PCR) for SARS-CoV-2 in the CSF was performed in 50 cases in which it was negative. The average latency period between the arboviral symptoms and neurologic manifestations for confirmed COVID-19 cases was 12.2 d (Table 2). There were two cases where neurological manifestations have preceded arboviral symptoms, and nine cases where patients only presented with neurologic deficits with no symptoms of COVID-19, but they had positive COVID-19 testing.
Table 2 shows the pooled data of GBS cases that have been preceded by a confirmed COVID-19 infection. There was a total of 99 cases (71 males and 28 females), the average age was 56.07 years. The most common arboviral symptoms prior to GBS were fever, dry cough, dyspnea, and gastrointestinal symptoms. There were four cases which did not report patient’s arboviral symptoms prior to GBS manifestations. The most commonly reported neurological signs and symptoms were ascending motor weakness (tetraparesis and paraparesis), diminished deep tendon reflexes, sensory disturbances (paresthesia), sensory loss, and facial palsy. GBS was complicated by respiratory failure in 30 cases and dysautonomia in 20 cases.
Clinical GBS variants have been identified in these cases. The most commonly reported GBS variants were classical sensorimotor GBS (64 cases), followed by paraparetic GBS (16 cases), MFS (9 cases), facial diplegia with paresthesia (3 cases), pharyngeal-cervical-brachial GBS (2 cases), and pure sensory GBS (1 case). There were four cases that could not be classified into any of the GBS clinical variants. CSF analysis was performed in 86 cases. Seventy-four cases have shown albuminocytologic dissociation (normal CSF protein <45 mg/dl Reference Bourque, Breiner and Moher94 ), 2 cases have shown oligoclonal band, and 10 cases had no abnormalities in the CSF analysis. Antiganglioside antibodies were investigated in 50 cases. The majority of cases had negative antiganglioside antibodies (43 cases). Each of anti-GM1, anti-GD1a, and anti-GD1b were positive in three cases; anti-GM2 was positive in two cases; and each of anti-GD3, anti-GQ1b, anti-GT1b, and anti-Gal-C were positive in one case.
Electromyography (EMG) was performed in 77 cases. The predominant EMG variant of GBS was AIDP (59 cases), followed by AMSAN (10 cases), and AMAN (8 cases). Eighty-nine reports confirmed the use of immunomodulatory treatment for GBS. Seventy-two cases received intravenous immunoglobulin (IVIG) therapy, 10 cases were treated with plasmapheresis (PLEX), and 7 cases were treated with both IVIG and PLEX. In terms of disease progression and the clinical outcomes, 40 cases required admission to the intensive care unit (ICU), 33 cases required mechanical ventilation, and 6 cases were complicated by death.
Brighton criteria were applied to improve the diagnostic certainty for the cases; valid symptomatology included bilateral and flaccid weakness of limbs at the time of presentation, decreased deep tendon reflexes in affected limbs, the presence of a monophasic course of neurologic symptoms, CSF cell count <50/μl, elevated CSF protein, EMG findings consistent with one of the subtypes of GBS, and the absence of alternative diagnosis. Accordingly, cases were classified from level 1–4 of diagnostic certainty. Reference Fokke, van den Berg, Drenthen, Walgaard, van Doorn and Jacobs13 Cases with MFS where the complete triad of ophthalmoplegia, ataxia, and areflexia was not present were classified as level 4. Reference Tan, Razali, Goh and Shahrizaila95 Cases with other variants such as facial diplegia with paresthesia, PCB variant, and pure sensory GBS has been excluded. Accordingly, 51 cases have fulfilled level 1 of diagnostic certainty, 26 cases have fulfilled level 2, 7 cases have fulfilled level 3, and 9 cases fulfilled level 4. We have concluded that the reported cases have a high-diagnostic certainty of GBS as most of the cases have been classified into level 1–3 of Brighton criteria.
Discussion
Our systematic review shows that the published literature on COVID-19-related GBS commonly report a classic sensorimotor variant of GBS with often facial palsy and a demyelinating electrophysiological subtype. The disease course is frequently severe with high rates of respiratory dysfunction and ICU admission. Reference Shang, Zhu, Baker, Feng, Zhou and Zhang96 The time elapsed between infection and neurologic manifestations, and a negative PCR in spinal fluid might suggest that there is a postinfectious mechanism implicated in the etiology of COVID-19-related GBS. However, these results should be interpreted with caution as the cases included in this systematic review varied widely in diagnostic ascertainment and reporting of different variables. Moreover, the reported cases were limited to certain geographical areas, which might provide a source of bias.
The constellation of sensorimotor signs with facial palsy, respiratory insufficiency, and a demyelinating electrophysiological subtype has been described in GBS patients with other viral infections such as CMV and Zika virus, which might indicate that this clinical and electrophysiological variant of GBS is related to viral infections in general. Reference Orlikowski, Porcher and Sivadon-Tardy8,Reference Leonhard, Bresani-Salvi and Lyra Batista97 On the other hand, C. jejuni is typically associated with pure motor and axonal type of GBS. Reference Rees and Hughes98 Although GBS is generally more common in men as compared with women, Reference Katirji, Ruff and Kaminski99 in our systematic review, we have found that the male to female ratio was 2.5:1 which is significantly higher than what is usually reported. Reference Piccione, Salame, Katirji, Katirji, Kaminski and Ruff100 This suggests that men might be more prone to COVID-19-related GBS.
In our review, the most common arboviral symptoms were fever and dry cough, which is typical in COVID-19 infection. Reference Eastin and Eastin101 We could not identify a specific arboviral symptom that could be typically preceding the development of GBS. However, we have identified two cases in which GBS manifestations preceded COVID-19 arboviral symptoms, and nine cases that did not present with arboviral symptoms initially. This chronology of GBS preceding the arboviral symptoms has not been previously reported with GBS related to other viral agents. In addition, the asymptomatic infection of COVID-19 might limit the ability to accurately determine the latency period between viral symptoms and the GBS presentation.
The mean duration between the onset of COVID-19 infectious symptoms and GBS presentation was 2 weeks, which is similar to other infections preceding GBS. Reference Rees, Soudain, Gregson and Hughes102 The latency between COVID-19 infection and GBS was more than a week for most cases, but it should be taken into consideration that COVID-19 can initially be asymptomatic which makes the latency duration arguably longer than reported. This suggests a postinfectious immunopathogenesis rather than direct neuronal damage or a parainfectious mechanism. The fact that COVID-19 PCR of the CSF was not positive in a single report, the negativity of repeat nasopharyngeal PCR at the time of symptoms in almost one-third of the cases, and the absence of elevated white blood cell count in the CSF in majority of cases, further argues against the assumption of COVID-19 infection being directly responsible for the GBS development in this proportion of patients.
Despite the fact that previous epidemiological studies have suggested that COVID-19 might not be associated with GBS, Reference Keddie, Pakpoor and Mousele103 the chronology of publication of the COVID-19-related GBS cases followed the same pattern of the global spread of COVID-19, as the first cases report was from China followed by Italy, Iran, and USA indicates a positive association. Reference Zhao, Shen, Zhou, Liu and Chen11,Reference Sedaghat and Karimi24,Reference Toscano, Palmerini and Ravaglia48,Reference Virani, Rabold and Hanson65 GBS has been historically related to various pathogens including C. jejuni, M. pneumoniae, EBV, CMV, Hepatitis E virus, and Zika virus. Reference Nachamkin, Allos and Ho5–Reference Counotte, Meili, Taghavi, Calvet, Sejvar and Low9 However, in certain pathogens such as Hepatitis E virus, this association has not been established globally, as it was only reported in Netherlands and Bangladesh. Reference Liu and Ma104 Therefore, immunogenicity of COVID-19 in the development of GBS should consider the variations between different populations, Reference Hardy, Blum, McCombe and Reddel105–Reference Nyati, Prasad and Verma108 as epidemiologic studies involving certain populations might introduce bias in reporting results.
Interestingly, almost half of the cases were tested for the presence of antiganglioside antibodies in serum. There were only seven cases have tested positive for different antiganglioside antibodies. Historically, different antigangliosides have been linked to different variants of GBS, such as anti-GQ1b in MFS and anti-GD1a in PCB variant. Reference Willison and O’Hanlon109,Reference Nagashima, Koga, Odaka, Hirata and Yuki110 Antiganglioside antibodies are considered to be biomarkers of axonal injury rather demyelination, as they directly target the neuronal membrane gangliosides. Reference Willison and Yuki111 Because most of the COVID-19-related GBS cases reported a demyelinating variant of GBS, it can be anticipated that the presence of antiganglioside antibodies would be low. Thus, the spectrum of immune cascade in COVID-19-related GBS should be expanded by studying other different antibodies affecting the myelin sheath, Schwann cell components, and the neuronal axolemma. Reference Soliven112,Reference Stathopoulos, Alexopoulos and Dalakas113 One case was reported with positive NF-155 and NF-186 antibodies, which are structural proteins in the node of Ranvier. Reference Tard, Maurage and de Paula22
The possible role of host immunogenetic background in the development of GBS and its variants has been related to human leukocyte antigen (HLA) polymorphism in different populations, this observation might explain the increased reporting of COVID-19 related GBS in the Italy, as one-third of the cases identified in our review were Italian. Reference Rodríguez, Rojas and Pacheco114,Reference Safa, Azimi, Sayad, Taheri and Ghafouri-Fard115 The role of HLA polymorphism in COVID-19 related GBS has been emphasized in one of the cases reported by Gigli et al., Reference Gigli, Vogrig and Nilo36 in which SARS-CoV2 antibodies were detected in the CSF. Interestingly, HLA analysis of the reported case showed several HLA alleles that are known to be associated with GBS, such as: HLA-A33, Reference Guo, Wang, Li, Liu and Wang116 DRB1 * 03:01, Reference Hasan, Zalzala and Mohammedsalih117 and DQB1 * 05:01. Reference Schirmer, Worthington and Solloch118
With the emergence of COVID-19 pandemic, there have been increasing reports of various neurological complications in infected patients, which was well documented and studied in other coronaviruses. Reference Mao, Wang and Chen1 Genomic analysis shows that SARS-CoV-2 is in the same beta-coronavirus (βCoV) clade as MERS-CoV and SARS-CoV, and shares a highly homological sequence with SARS-CoV. Reference Hu, Liu, Zhao, Zhuang, Xu and He119 There has been clinical evidence of neuromuscular sequela in SARS CoV and MERS infection and the most documented neuromuscular syndromes related to these viruses are critical illness polyneuropathy and myopathy, which are hypothesized to occur in the context of severe inflammatory response syndrome (SIRS). Reference Tsai, Hsieh and Chang120 Cases of MERS-related GBS have been reported, yet GBS in these cases has been linked to the treatment received for MERS infection, such as interferon alpha2 and Lopinavir/ritonavir. Reference Kim, Heo and Kim10 In contrast to MERS, SARS-CoV2 is likely associated with GBS.
Conclusion
Based on this systematic review, most cases of COVID-19-related GBS are of the sensorimotor demyelinating subtype with frequent facial palsy. The latency between infection and onset of neurologic symptoms as well as the absence of viral genome detected by PCR suggest a postinfectious, rather than a direct infectious or para-infectious mechanism. Global reporting of COVID-19-related GBS cases, in addition to testing for different antibodies to different structural proteins and glycolipids in the peripheral nerves, would improve the understanding of the immunological cascade of COVID-19-related GBS. Finally, early diagnosis and identification of GBS in COVID-19 patients is important as COVID-19-related GBS might be associated with a severe disease course that frequently requires ICU admission and mechanical ventilation.
Disclosures
The authors declare no conflicts of interest.
Statement of Authorship
MA: contributed with the conception and design of the study, acquisition, analysis, and interpretation of data, drafting, revising, and final approval of the article.
ME: contributed with the conception and design of the study, acquisition, analysis and interpretation of data, drafting, revising, and final approval of the article.
BA: contributed with acquisition and extraction of data and drafting the article.
DA: contributed with extraction of data and final approval of the article.
AA: contributed with extraction of data and final approval of the article.
AB: contributed with extraction of data and final approval of the article.
EP: contributed with conception and design of the study, drafting, revising and final approval of the article.





