A 60-year-old, right-hand-dominant male presented to hospital with two consecutive episodes of acute-onset transient right hand and lower extremity weakness each lasting approximately 30 minutes over a 2-hour period. His past medical history included coronary artery disease managed with coronary artery bypass grafting, peripheral vascular disease, type 2 diabetes mellitus on insulin, hypertension, dyslipidemia, obstructive sleep apnea, and smoking. By the time of assessment, his blood pressure was 179/91, and his neurological examination was normal, except for length-dependent sensory loss in the lower extremities bilaterally.
Computed tomography (CT) demonstrated a remote infarct in the anterior limb of the right internal capsule and corona radiata, and chronic microangiopathic white matter changes in both cerebral hemispheres, but no acute abnormalities. CT angiography (CTA) demonstrated complete occlusion of the left internal carotid artery (ICA), extending 1 cm from its origin to the cavernous segment, and occlusion of the left vertebral artery, extending from its origin to the level of the C5 vertebral body (Figure 1). There was also 50% stenosis of the right ICA at the origin. The circle of Willis was patent. Brain magnetic resonance imaging (MRI) the following day demonstrated acute ischemic infarcts involving the left parietal cortex and corona radiata in watershed territory (Figure 2A). Given the patient's occluded left ICA, the infarcts were thought to be secondary to large-artery atherosclerosis and thromboembolism. He was discharged with dual antiplatelet therapy using aspirin and clopidogrel and maintained on therapies for diabetes mellitus, hypertension, and dyslipidemia.
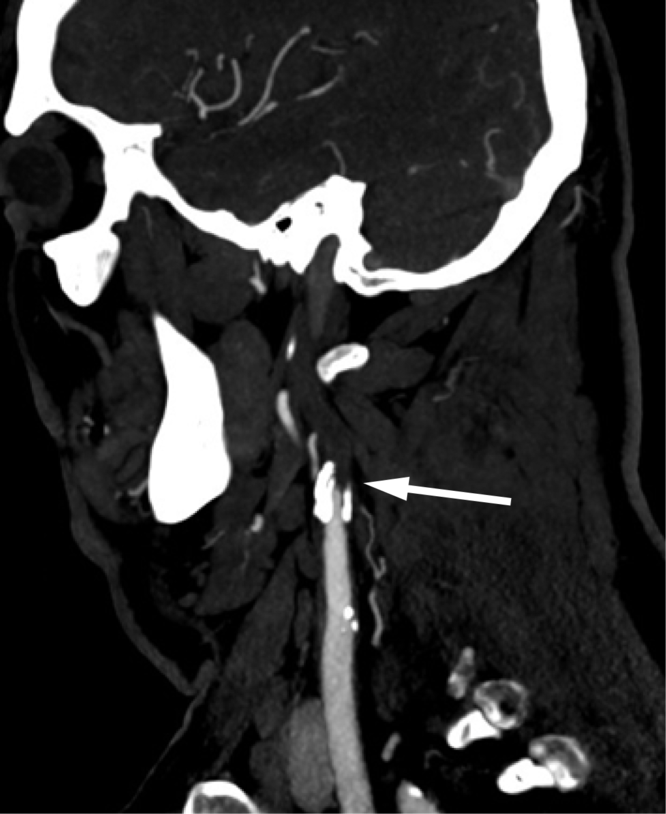
Figure 1: CT angiography (CTA) demonstrated a tapered, heavily calcified narrowing of the left internal carotid artery (ICA) at its origin and occlusion 1 cm distal to its origin (arrow). The occlusion extended to the cavernous segment of the ICA.
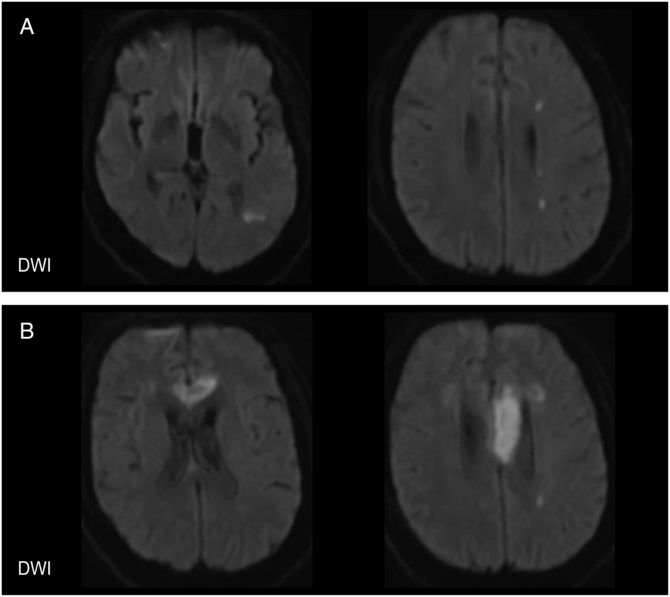
Figure 2: Brain MRI with diffusion-weighted imaging (DWI). (A) 1 day after the patient's initial presentation, MRI demonstrated acute infarction of the left parietal cortex (left) and corona radiata in a watershed distribution (right). (B) During the patient's second presentation, MRI demonstrated acute infarction of the anterior corpus callosum involving the left genu (left) and the body (right).
Two months later, the patient re-presented to hospital with acute-onset altered level of consciousness, mutism, a blood pressure of 140/80, and a random blood glucose of 3.4 mmol/L. His level of consciousness improved with the administration of dextrose, but his speech and language did not return to baseline. Language examination demonstrated occasional word finding difficulties, and slowed speech, hesitations, and articulatory errors consistent with an apraxia of speech. The remainder of the neurological examination was not significantly changed from his previous admission. CT and MRI demonstrated an acute ischemic infarct involving the left lateral aspect of the genu and body of the corpus callosum (Figure 2B). CTA did not show any interval change. Further workup with transthoracic echocardiogram and 48-hour Holter monitoring was unremarkable.
Notably, between the patient's initial discharge and second presentation, he began experiencing episodes of shaking in his right, left, or both lower extremities that were provoked by standing and immediately relieved by sitting. These episodes were accompanied by light-headedness, and he also had recurrent falls. Outpatient assessment determined that the patient was normotensive at baseline, and orthostatic vital signs did not reveal orthostatic hypotension.
Following the patient's second discharge, his ambulation declined and he became dependent on a walker due to increased frequency of lower limb shaking with standing and falls. He also had increasing difficulty with attention and short-term memory. Three months later, he experienced acute-onset painless vision loss in his left eye. Examination 3 weeks after revealed a nonreactive left pupil and retinal ischemia with narrowed vessels and pallor, without a macular cherry-red spot. These features suggested occlusion of the ophthalmic artery, rather than just the central retinal artery. CT and CTA did not show any interval change.
In summary, the patient experienced recurrent ischemic events in the territory of an occluded left ICA and associated sequalae, including limb-shaking transient ischemic attacks (TIAs) and cognitive dysfunction. The chronic ICA occlusion ultimately prompted assessment of hemodynamic status using CT perfusion (CTP) with an acetazolamide challenge. CTP demonstrated increased mean transit time and cerebral blood volume in the left cerebral hemisphere, with decreased cerebral blood flow in left frontoparietal regions corresponding to cortical watershed zones. Administration of acetazolamide decreased cerebral blood flow, particularly in the left hemisphere, and was consistent with cerebrovascular autoregulation failure. The patient did not undergo transcranial Doppler ultrasound monitoring for microemboli.
Although chronic ICA occlusions are uncommon, the risk of ischemic stroke is up to 5% per year with two-thirds ipsilateral to the occlusion.Reference Furtan, Whisnant and Baker1,Reference Cote, Barnet and Taylor2 Recurrent ischemic strokes in the territory of an occluded ICA are thought to be due to cerebral hypoperfusion, thromboembolism, or a combination of the two. In ICA occlusion, collateral circulation is provided across the circle of Willis via the anterior communicating artery (ACA) and posterior communicating artery (PCA), from reversed flow in the ipsilateral ophthalmic artery, and from increased flow through leptomeningeal collaterals.Reference Liebeskind3 Insufficient collateral compensation leads to cerebral hypoperfusion and watershed infarcts. Thromboembolic mechanisms include embolization from the proximal occlusion through external carotid artery to ICA anastomoses or reversed flow in the ophthalmic artery (carotid stump syndrome),Reference Barnett, Peerless and Kaufmann4 distal propagation or embolization of the thrombus, or embolization from the contralateral circulation through the circle of Willis.Reference Nicholls, Kohler, Bergelin, Primozich, Lawrence and Strandness5 In a minority of cases, the ICA occlusion may recanalize and allow for reperfusion, but with risk of ischemic stroke due to thromboembolism from an atherosclerotic and stenotic ICA.Reference Morris-Stiff, Teli and Khan6 Patients presenting with ischemic stroke and ICA occlusions have been found to have radiographically determined watershed infarcts, non-watershed infarcts, or both. Notably, microembolic signals were found in equal rates in watershed and non-watershed infarcts using transcranial Doppler, suggesting a role for embolization in ischemic infarcts where cerebral hypoperfusion is suspected as a primary mechanism.Reference Liberman, Zandieh and Loomis7
Regarding the mechanism of strokes in this patient, multiple CTAs demonstrated a persistently occluded left ICA with a proximal “stump.” Although the patient initially had multifocal infarcts suggestive of a thromboembolic process, cerebral hypoperfusion was a likely etiology for many of his infarcts and TIAs and was supported by impaired hemodynamics observed using CTP. The left brain relied entirely on collateral circulation given occlusion of the left ICA and vertebral artery, and the pattern of cerebral infarcts was consistent with cortical and deep border zone watershed infarcts. Furthermore, limb-shaking TIAs, or short-duration limb-shaking involving the upper or lower extremities, often associated with limb paresis and provoked by activities that reduce cerebral perfusion such as postural changes, are indicative of impaired hemodynamics in the brainReference Persoon, Kappelle and Klijn8 and are a risk factor for recurrent stroke in patients with ICA occlusion.Reference Persoon, Luitse and de Borst9 Concurrently, this patient had several infarcts that favored an embolic mechanism, including his ophthalmic artery occlusion and corpus callosum infarct. Although corpus callosum infarctions are rare and hypoperfusion cannot be excluded, the corpus callosum is supplied by the ACA, PCA, and abundant anastomoses, and infarction has been associated with large-artery atherosclerosis and thought to be embolic.Reference Li, Sun and Bai10 Taken together, this patient likely had multiple mechanisms for recurrent ischemia, which contributed to challenging management for secondary stroke prevention.
When patients present with acute stroke secondary to ICA occlusion, timely CTA and endovascular thrombectomy in eligible patients provide a method for recanalization and preventing a chronic occlusion. Patients who develop a chronic ICA occlusion should be assessed in follow-up using carotid ultrasound or CTA for spontaneous recanalization that may be subsequently managed with carotid endarterectomy or stenting. Patients with persistent ICA occlusions, recurrent strokes, and accumulating disability ultimately pose a challenge. Surgical interventions such as extracranial–intracranial bypass surgery have not been shown to reduce the risk of recurrent ipsilateral stroke compared to medical therapy alone.Reference Powers, Clarke and Grubb11 Antiplatelet therapy and statin therapy are routinely used to decrease the risk of embolization and ischemic stroke. Patients with cerebral hypoperfusion may benefit from more liberal, individualized blood pressure targets based on reducing hypoperfusion-related clinical symptoms. Our case demonstrates that multiple mechanisms may be operative, and patients should be considered individually to optimize secondary stroke prevention. Contributing factors require further investigation, and characterizing carotid occlusions in terms of location, length, and duration, the presence of a “stump,” collateral circulation, and hemodynamic measures may aid in predicting the mechanism and risk of stroke recurrence and consequently guide management in chronic ICA occlusions.
Disclosures
No conflicts of interest, financial or otherwise, are declared by the authors.
Statement of Authorship
JLC and GBY were involved in reviewing the literature and drafting the manuscript. JLC, KMJ, and GBY were involved in data collection. All authors revised and approved the manuscript for submission.




