Marfan syndrome (MFS) is a rare, autosomal dominant connective tissue disorder caused by a fibrillin gene mutation. Reference Groth, Hove and Kyhl1 Complications can include cardiac disease, ocular disease, and skeletal disease. Reference Stark, Hensen and Kutsche2 Dural ectasia (DE) is well reported in MFS, varying between 50 and 90% and often seen in the lumbosacral region. Reference Böker, Vanem and Pripp3 While spinal deformities and DE are common, here we present a rare case of a teenager with MFS who was found to have multiple multiloculated, intradural thoracic spinal cysts causing cord compression and myelopathy. The postoperative course was complicated by spinal epidural hematoma from anticoagulation initiated 6 hours postoperatively. This presentation underscores the incredibly rare neurologic sequelae of MFS and the clinical challenges of balancing anticoagulation with a high-risk neurosurgical procedure.
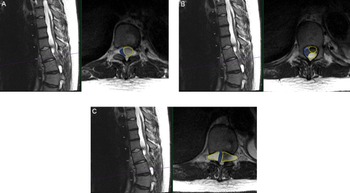
A 15-year-old male with MFS, scoliosis, and an extensive surgical history involving his thoracoabdominal aorta presented with a 9-month history of imbalance. He had myelopathy with right leg pain/weakness eventually requiring assistance for walking. His examination was remarkable for hyperreflexia and inability to tandem walk along with loss of proprioception. Magnetic resonance imaging (MRI) spine revealed thoracic DE and tethering of the spinal cord at T9-L1 with multilevel loculated cysts causing compression from the thoracic cord to the conus causing a 2-mm width cord at T10/T11 (Figure 1). He was admitted to the Pediatric Intensive Care Unit (PICU) for preoperative anticoagulation bridging due to his mechanical valves.

Figure 1: Preoperative findings of multiloculated intradural spinal cysts on T2-weighted axial and sagittal thoracic MRI (A) at T10 level with compression of the cord (blue outline) to the right due to large cyst on the left (yellow outline); (B) at superior T11 level with two large left-sided cysts (yellow outlines) compressing the cord to the right (blue outline); and (C) at T11/12 level with the cord compressed by bilateral loculated cysts (yellow outlines) to a diameter of 2 mm in the cord (blue outline).
The patient’s cardiac history is quite extensive including aortic dissection, aortic root dilatation, mitral and aortic valve replacements with mechanical valves, aortic arch replacement, and open repairs of thoracoabdominal aorta. Due to this extensive cardiac history, the patient needed to resume anticoagulation in a timely manner after his spinal surgery. The cardiologists, cardiac surgeons, neurosurgeons, hematologists, and intensivists comprised the multidisciplinary collaborative team that carefully managed re-initiating his anticoagulation.
The patient underwent T9-L1 laminectomy for resection of intradural cysts and adhesions, fenestration of the multiloculated cysts, and detethering of the thoracic spinal cord at T9-T11. Pathology demonstrated benign intradural cysts. He was taken to the PICU and then to MRI for evaluation of any post-op fluid collection or blood at the surgical site (Figure 2A). The postoperative MRI showed no hematoma and excellent decompression of the spinal cord. Heparin infusion started 6 hours after surgery; aspirin and warfarin started on postoperative day (POD) one.

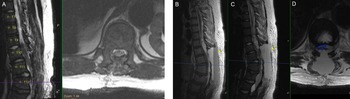
Figure 2: Postoperative findings of cord decompression and subsequent expansive epidural hematoma on T1-weighted sagittal and T2-weighted sagittal/axial thoracic MRI. (A) Postoperative day (POD) 0 imaging of T2-weighted sagittal/axial series showing cord decompression and detethering of the cord. (B–D) POD2 imaging of T1-weighted sagittal, T2-weighted sagittal, and T2-weighted axial series, respectively, showing distinct fluid level with massive layered epidural hematoma (yellow arrows) causing cord compression (blue outline).
The patient was ambulating on POD1. On POD2, he noted numbness and parasthesias in bilateral lower extremities and difficulty voiding but no weakness. STAT MRI was obtained (Figure 2B–D), and the patient was taken emergently to the operating room and lost bilateral extremity motor function en route. In surgery, he had a hematoma that spanned from T8 to L2 with significant compression of the cord and conus. The patient emerged from anesthesia with full strength in bilateral lower extremities and resolution of the numbness. Anticoagulation was resumed slowly 48 hours after this second intervention, first with a heparin infusion followed by aspirin (81 mg) and then warfarin. After stabilization, the patient was transferred from the ICU for ongoing rehabilitation, pain management, and anticoagulation titration.
The fibrillin gene is responsible for encoding proteins crucial for intercellular matrix integrity. Reference Hayward and Brock4 These proteins are widespread and form filaments and fibers important to the structure of most tissues including skin, muscles, and ligaments. However, many variants are known to exist which may contribute to the variable phenotypes seen in MFS. DE, one of the more common manifestations, is the result of a weakened dura mater in the face of cerebrospinal fluid pulsations with subsequent progressive widening of the sac. Reference Fattori, Nienaber and Descovich5 Cysts may begin to form when the dilated dura bulges through the intervertebral foramen, as in this patient. Though there are reports in the literature describing this phenomenon, Reference Arnold and Teuber6–Reference Di Lazzaro, Pilato and Dileone8 to our knowledge, this is the first case report in the literature describing multilevel cyst formation in the intradural thoracic space leading to cord compression in a pediatric MFS patient.
Acknowledgments
No acknowledgments or financial support to disclose.
Disclosures
The author(s) have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Statement of Authorship
JG planned and conceived the manuscript. JG and TGP performed the literature review, wrote the paper, and developed the figures.