Introduction
Moderate or severe atrioventricular valve regurgitation in patients with univentricular heart is not uncommon and represents a well-known risk factor for increased long-term morbidity and mortality. Reference Tseng, Siddiqui and Di Maria1–Reference Buratto, Ye, Brizard, Brink, d’Udekem and Konstantinov4 The risk of failure of Fontan circulation is significantly increased in the majority of these patients leading to death or heart transplantation. Reference King, Ayer and Celermajer5 Yet, most of the published research about atrioventricular valve regurgitation in surgical palliation of univentricular heart is centred either on patients with a common atrioventricular valve or on patients with hypoplastic left heart syndrome with a single tricuspid valve. Reference King, Winlaw and Alphonso6 However, patients with two separate atrioventricular valves and univentricular heart undergoing surgically staged palliation are prone to valve failure with a cumulative incidence of atrioventricular valve failure of 26% at 25 years of age. Reference Tseng, Siddiqui and Di Maria1 Atrioventricular valve repair in this specific subset of univentricular heart patients is challenging, and freedom from reoperation has been reported as 57% at 5 years after initial atrioventricular valve repair. Reference Nakata, Fujimoto and Hirose7 Another option for valve repair in patients with univentricular heart and two separate atrioventricular valves is closure of the diminutive atrioventricular valve. Closure of the diminutive valve should be performed in case of significant regurgitation or stenosis with concomitant valve dysplasia. Another prerequisite for this surgical technique is that the other atrioventricular valve is of appropriate size. King and colleagues could demonstrate in a cohort of 36 patients with univentricular heart and atrioventricular valve closure that valve closure is associated with no operative mortality and freedom from reintervention at 18 years post-valve closure of 83%. Reference King, Winlaw and Alphonso6 Our group could show that early valve closure is an effective surgical alternative with no incidence of atrioventricular valve reoperations in 17 patients with two separate atrioventricular valves and single-ventricle physiology. Reference Ono, Cleuziou and Pabst von Ohain8 However, caution should be taken in atrioventricular valve closure as it is linked with a 17% risk of patch dehiscence and a 14% risk of heart block. Reference King, Winlaw and Alphonso6 Despite these risks and in contrast to the limited durability of atrioventricular valve repair in patients with univentricular heart and two separate atrioventricular valves undergoing surgical palliation, valve closure may represent an effective surgical tool for the management of atrioventricular valve regurgitation. Yet, data on the outcome of atrioventricular valve surgery in patients with univentricular heart and two separate atrioventricular valves are still limited. The aim of our study was to examine our experience with atrioventricular valve repair and closure in children with univentricular heart and two separate atrioventricular valves.
Materials and method
Ethical statement
This study was approved by the Institutional Review Board of the Technical University of Munich (approved number of 2022-303-SKH on 27 June, 2022). Because of the retrospective nature of the study, the need for individual patient consent was waived.
Patients
We reviewed the medical records of patients with univentricular heart and two separate atrioventricular valves who underwent staged surgical palliation at the German Heart Center Munich between May 1994 and November 2021. Univentricular heart and two separate atrioventricular valves were present in patients with double inlet left ventricle, double inlet right ventricle, double outlet right ventricle, and congenitally corrected transposition of the great arteries. Patients with a common atrioventricular valve were excluded from this study. Medical records including in-hospital and outpatient notes and echocardiography data were retrospectively collected and reviewed. Operative mortality was defined according to the Society of Thoracic Surgeons. Reference Overman, Jacobs and Prager9
Atrioventricular valve anatomy and function
Atrioventricular valve and ventricular morphology were determined from echocardiographic reports and were confirmed by intraoperative inspection. Based on morphologic criteria, the atrioventricular valve was defined either as a left or right atrioventricular valve. After atrioventricular valve surgery, mechanisms of regurgitation and stenosis were based on the surgical reports. The extent of regurgitation was classified as 0 (none), 1 (trivial), 2 (mild), 3 (moderate), or 4 (severe). Significant regurgitation was considered to be present when regurgitation was moderate or severe.
Operative techniques
Atrioventricular valve surgery was performed under standard cardiopulmonary bypass with cardioplegic cardiac arrest. Surgical techniques were individualised according to the valve pathology. Intraoperatively, saline was injected into the ventricle to confirm the mechanism of regurgitation. Attention was paid to the annular dimension, commissural leak, prolapse or restriction of the leaflets, and leaflet and subvalvular abnormalities. Partial and semi-circular annuloplasties were performed. For partial annuloplasty, a 5-0 or 6-0 Prolene horizontal mattress suture was placed along the commissure where the predominant regurgitant jet was detected. Another technique used for partial annuloplasty was the Kay-Wooler plasty. Semi-circular annuloplasty was performed using the De Vega technique. Semi-rigid partial annuloplasty ring (Edwards Lifesciences, Inc., Irvine, CA, USA) was also implanted. Commissuroplasty was achieved with edge-to-edge approximation of the leaflet tissue using 6-0 or 7-0 Prolene sutures. In patients with poor coaptation of two opposing leaflets, edge-to-edge repair was performed. Interrupted stitches or continuous locked sutures were used for direct closure of clefts and fenestrations. In case of significant regurgitation or stenosis of the diminutive atrioventricular valve, closure of the valve was achieved either with a patch or by direct suture. Patch materials used included Gore-Tex (W. L. Gore & Associates, Inc., Flagstaff, AZ, USA) and Dacron (DuPont, Midland, MI, USA). In case of severe residual regurgitation or irreparable leaflet abnormalities, valve replacement was performed. Implanted valve prostheses were the mechanical ATS valve (ATS Medical, Inc., Minneapolis, Minn, USA) and the porcine Medtronic Intact valve (Medtronic, Inc., Minneapolis, Minn, USA). Intraoperative transesophageal echocardiography was performed routinely.
Follow-up data
Patients were regularly followed by paediatric cardiologists in an outpatient setting. Mortality, cause of death, and morbidity were tracked using our institutional single-ventricle patient database system. The severity of atrioventricular valve regurgitation was regularly evaluated with transthoracic echocardiography. Listwise deletion was used for missing data.
Statistical analysis
Continuous variables are expressed as mean with standard deviation or median with interquartile range. Distribution of continuous variables was tested with the Kolmogorov–Smirnov test, histograms as well as P–P plots for graphical testing. For time-to-event analysis, the mean with standard deviation was reported for follow-up time. Categorical variables are presented as absolute numbers and percentages. Kaplan-Meier analysis was applied to calculate estimated survival. Log-rank test was used to compare survival between groups. Competing risk analysis was used to calculate the cumulative incidence of reoperation. Cumulative incidence of reoperation with standard error of the mean was described at the mean follow-up time after atrioventricular valve surgery. Statistical significance was set at p < 0.05. Data were analysed using Statistical Package for the Social Sciences, version 28.0 for Windows (IBM, Ehningen, Germany) and R-statistical software (version 3.5.2; R Foundation for Statistical Computing, Vienna, Austria).
Results
Patient characteristics and timing of initial atrioventricular valve surgery
In 202 children with univentricular heart and two separate atrioventricular valve morphology, single-ventricle palliation was performed during the study period, with 15.8% (32/202) requiring atrioventricular valve surgery. Main indications for surgery of the left atrioventricular valve were moderate or greater regurgitation in nine patients (28.1%), and severe stenosis in one patient (3.1%). Indications for surgery of the right atrioventricular valve were severe and moderate regurgitation in six (18.8%) and 17 patients (53.1%), respectively. Median age and weight at initial atrioventricular valve surgery were 1.9 years (interquartile range, 0.9–5.7) and 10.6 kg (interquartile range, 7.9–18.9), respectively. Nine patients (28.1%) were below 1 year of age. The primary diagnoses were double inlet left ventricle (n = 14, 43.8%), double outlet right ventricle (n = 7, 21.9%), congenitally corrected transposition of the great arteries (n = 7, 21.9%), and Shone Complex (n = 2, 6.3%). Frequently associated cardiac malformations were coarctation of the aorta in nine patients (28.1%) and subvalvular pulmonary stenosis in eight patients (25%). Atrioventricular valve surgery was performed at Fontan completion in 17 patients (53.1%), concomitant with bidirectional cavopulmonary shunt in 11 (34.4%), at Norwood procedure in one (3.1%), and during interstage in three (9.4%). Further baseline demographics are depicted in Table 1.
Table 1. Baseline characteristics of patients at initial atrioventricular valve surgery
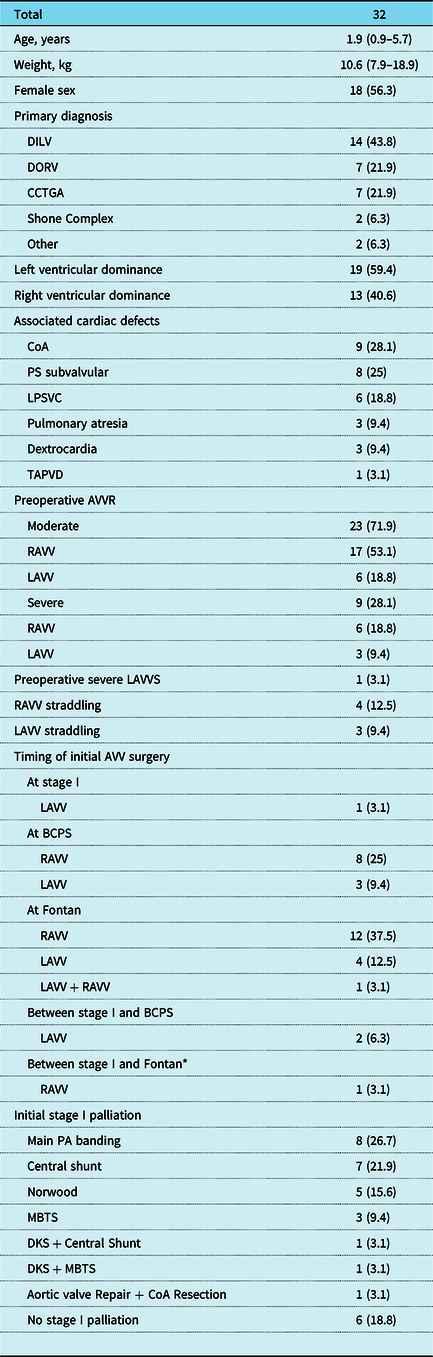
Values are expressed as n (%) or median (interquartile range). AVV = atrioventricular valve; AVVR = atrioventricular valve regurgitation; BCPS = bidirectional cavopulmonary shunt; CCTGA = congenitally corrected transposition of the great arteries; CoA = coarctation of the aorta; DILV = double inlet left ventricle; DKS = Damus-Kaye-Stansel anastomosis; DORV = double outlet right ventricle; kg = kilogram; LAVV = left atrioventricular valve; LAVVS = left atrioventricular valve stenosis; LPSVC = persistent left superior vena cava; MBTS = modified Blalock-Taussig shunt; PA = pulmonary artery; PS = pulmonary stenosis; RAVV = right atrioventricular valve; TAPVD = total anomalous pulmonary venous drainage; VSD = ventricular septal defect.
* In this patient, Stage II bidirectional cavopulmonary shunt was not performed.
Mechanisms of atrioventricular valve regurgitation and stenosis
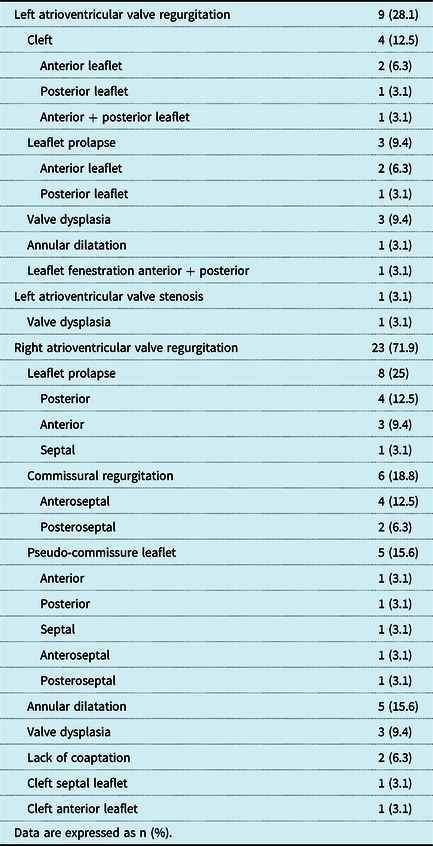
Mechanisms of atrioventricular valve regurgitation and stenosis according to surgical findings at the initial surgical procedure are shown in Table 2. Leaflet cleft was identified in four patients (12.5) and posed the most common pathology of left atrioventricular valve regurgitation. The second most common cause was leaflet prolapse, which was observed in three patients (9.4%). Stenosis of the left atrioventricular valve was due to extensive valve dysplasia in one patient (3.1%). Main mechanisms of right atrioventricular valve regurgitation were leaflet prolapse and commissural regurgitation in eight (25%) and six patients (18.8%), respectively.
Table 2. Atrioventricular valve pathology at initial surgery
Surgical procedures
Details of the initial atrioventricular valve surgery and detailed description of the surgical techniques for each palliative stage are specified in Table 3. Surgery of the left atrioventricular valve was performed in nine patients (28.1%) with valve repair in five patients (15.6%) and valve closure in four patients (12.5%). Main techniques for repair of the left atrioventricular valve were either closure of clefts or leaflet adaptation in two patients (6.3%) each. Closure of the left atrioventricular valve was performed with the use of a Gore-Tex patch in two patients (6.3%) and with direct suture in two patients (6.3%). Surgery of the right atrioventricular valve was conducted in 22 patients (68.8%) with valve repair in 20 patients (62.5%) and valve closure in two patients (6.3%). The most common technique for repair of the right atrioventricular valve was commissural approximation in six patients (18.8%), followed by annuloplasty in five patients (15.6%). Closure of the right atrioventricular valve was achieved by suture in two patients (6.3%). Concomitant right atrioventricular valve Dacron patch closure and repair of the left atrioventricular valve was performed in one patient (3.1%) during Fontan completion. The median cardiopulmonary bypass time was 100.5 minutes (interquartile range, 78.3–136) and the median aortic cross-clamp time was 40.5 minutes (interquartile range, 27–60.8).
Table 3. Details of initial surgical techniques on the atrioventricular valves
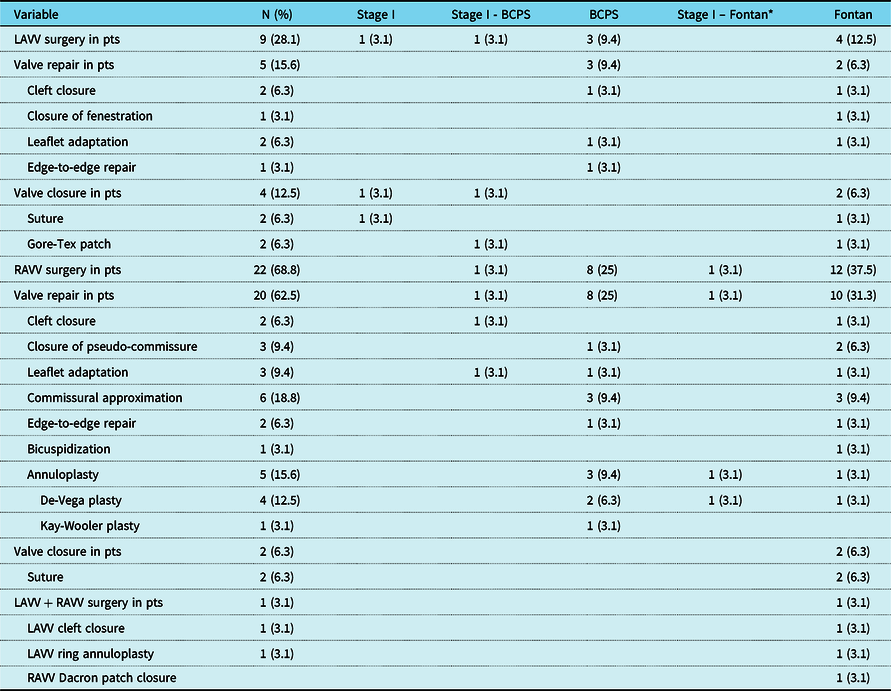
Data are expressed as n (%). BCPS = bidirectional cavopulmonary shunt; LAVV = left atrioventricular valve; pts = patients; RAVV = right atrioventricular valve.
* In this patient, Stage II bidirectional cavopulmonary shunt was not performed and atrioventricular valve surgery was performed between stage I and Fontan.
In-hospital outcomes
Operative mortality was 3.1% (1/32). Cause of death was basal ganglia infarction in a 3.4-year-old girl who died on the day of concomitant right atrioventricular valve repair and Fontan completion. Echocardiographic data at discharge were available in 31 patients (96.9%). After right atrioventricular valve repair, mild or less valve regurgitation was recorded in 16 patients (50%) and moderate valve regurgitation in four (12.5%) (Fig. 1A). After closure of the right atrioventricular valve with suture, moderate valve regurgitation was seen in one patient (3.2%). After repair of the left atrioventricular valve, moderate valve regurgitation was seen in two patients (6.5%), and mild regurgitation in one (3.2%) (Fig. 1B). After closure of the left atrioventricular valve with suture, mild valve regurgitation was diagnosed in one patient (3.2%). After concomitant closure of the right atrioventricular valve and repair of the left valve, no atrioventricular valve regurgitation was detected in the echocardiogram at discharge. Two patients required redo repair of the right atrioventricular valve during Fontan completion because of recurrent moderate regurgitation at 13.6 and 12.5 months after initial repair. In one patient (3.1%), epicardial ventricular pacemaker implantation was necessary due to second-degree atrioventricular block 9 days after DeVega annuloplasty.

Figure 1. Echocardiographic evaluation and course of right (A) and left (B) atrioventricular valve pathology. FU = follow-up; LAVVR = left atrioventricular valve regurgitation; LAVVS = left atrioventricular valve stenosis; RAVVR = right atrioventricular valve regurgitation.
Follow-up outcomes
The mean follow-up time after atrioventricular valve surgery was 10.3 years (standard deviation: 8.3) (median 9.7; interquartile range, 1.5–18.6). Late mortality was 9.7% (3/31). Death was cardiac-related in two patients (6.5%) and non-cardiac-related in one patient (3.2%). The first patient was a 2-year-old boy who underwent cleft closure of the anterior left atrioventricular valve leaflet and bidirectional cavopulmonary shunt at 9 months of age and died subsequently due to heart failure. The second patient was a 4-year-old girl who died from heart failure 3.4 months after closure of the left atrioventricular valve with a Gore-Tex patch in combination with Fontan completion. In the third patient, cause of death was protein-losing enteropathy 10.5 years after closure of the left atrioventricular valve with a Gore-Tex patch. Ten-year survival after atrioventricular valve surgery was 89.4% (standard deviation: 5.8) (Supplemental Fig. 1), and in all patients, completion of Fontan circulation was achieved. However, patients with surgery of the right atrioventricular valve showed a significant better 10-year survival than patients with surgery of the left atrioventricular valve (95.5% [standard deviation: 4.4] vs. 71.4% [standard deviation: 17.1], respectively; p = 0.02) (Supplemental Fig. 2).
As for reoperations during follow-up, there were eight patients who required redo atrioventricular valve surgery at a median interval of 1.2 years (interquartile range, 0.6–4.7) after initial atrioventricular valve surgery. Redo surgery of the left atrioventricular valve included repair (n = 1) due to recurrent moderate valve regurgitation and valve suture closure (n = 1) due to severe left atrioventricular valve regurgitation. Indications for redo surgery of the right atrioventricular valve were moderate valve regurgitation (n = 2) and consisted of valve closure (Suture = 1, Gore-Tex patch = 1). One patient developed severe regurgitation of the right atrioventricular valve due to a significant restriction of the septal leaflet 1.2 years after bicuspidization of the anterior and posterior right atrioventricular valve leaflet and the valve had to be replaced with a 31 mm large porcine Medtronic Intact valve prosthesis. In another patient, replacement of the right atrioventricular valve with a 27 mm large mechanical ATS valve prosthesis was necessary due to suture line dehiscence with severe regurgitation 5 months after DeVega annuloplasty. Details of the surgical redo procedures of the atrioventricular valves are depicted in Table 4. An overview of the clinical course after atrioventricular valve surgery in patients with two separate atrioventricular valves and univentricular heart is shown in Figure 2. The reoperation-free survival 10 years after atrioventricular valve surgery was 62.3% (standard error of the mean: 6.9), and the cumulative incidence of reoperation at 10 years was 30.9% (standard error of the mean: 9.6), respectively (Fig. 3). Details on the atrioventricular valve pathologies at redo surgery are listed in Supplementary Material, Table S1.
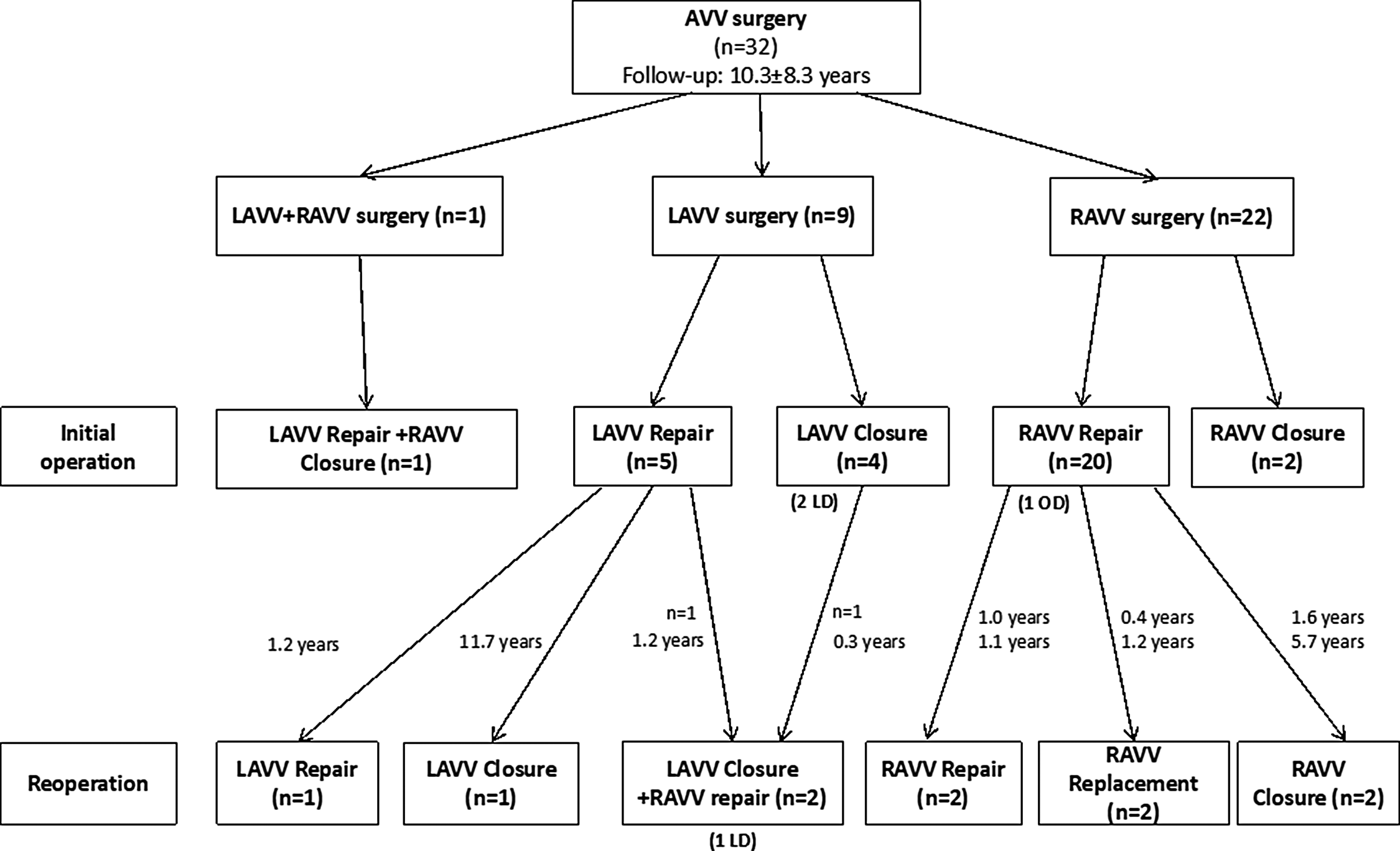
Figure 2. Clinical course and outcomes for patients with surgery of the atrioventricular valves. AVV = atrioventricular valve; LAVV = left atrioventricular valve; LD = late death; OD = operative death; RAVV = right atrioventricular valve.
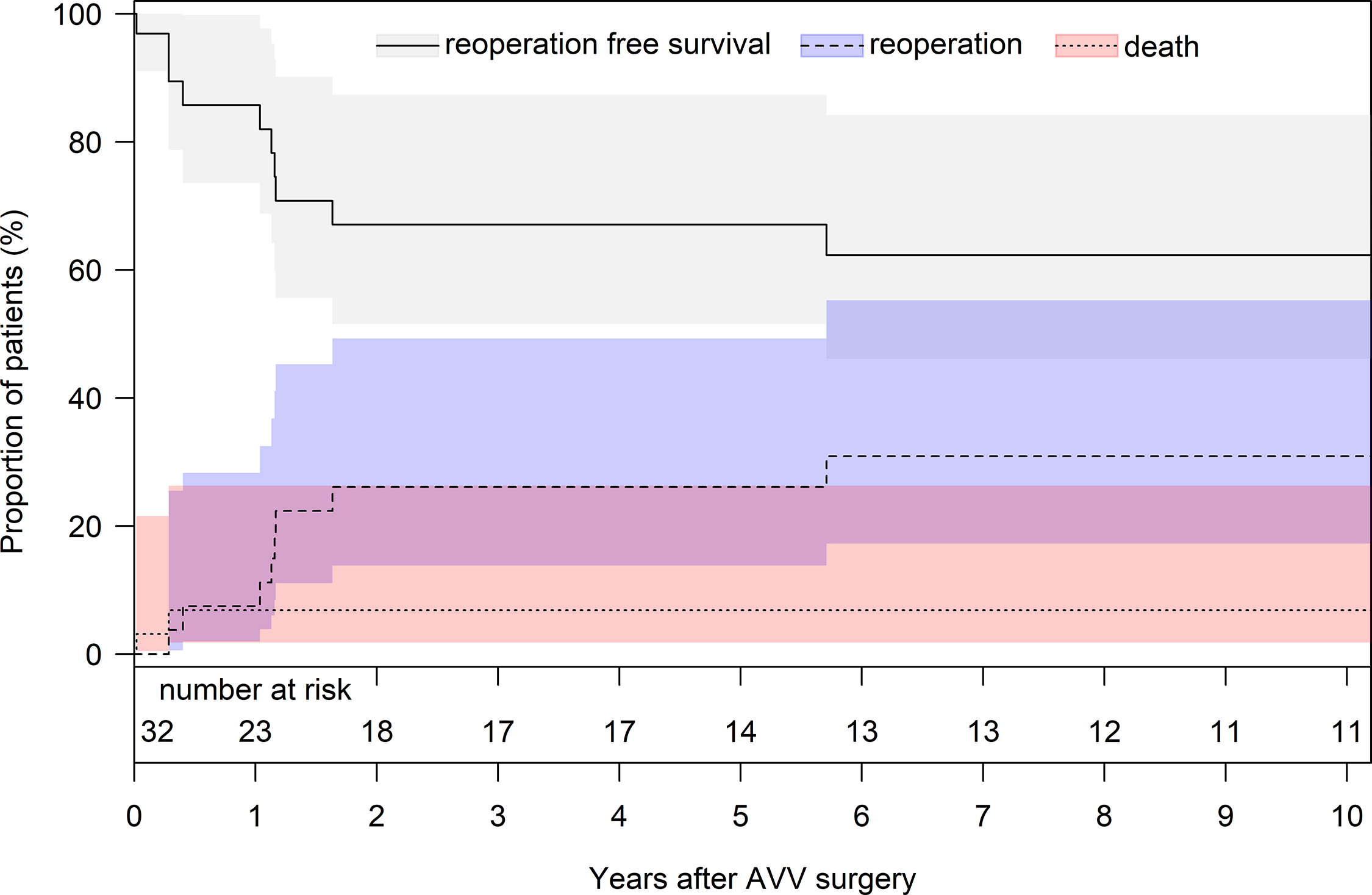
Figure 3. Competing risk analysis for death and reoperation at the atrioventricular valves. Cumulative incidence of reoperation (blue) and death with no reoperation (red). The grey curve shows the patients being alive without reoperation. AVV = atrioventricular valve.
Table 4. Details of redo operations on the atrioventricular valves
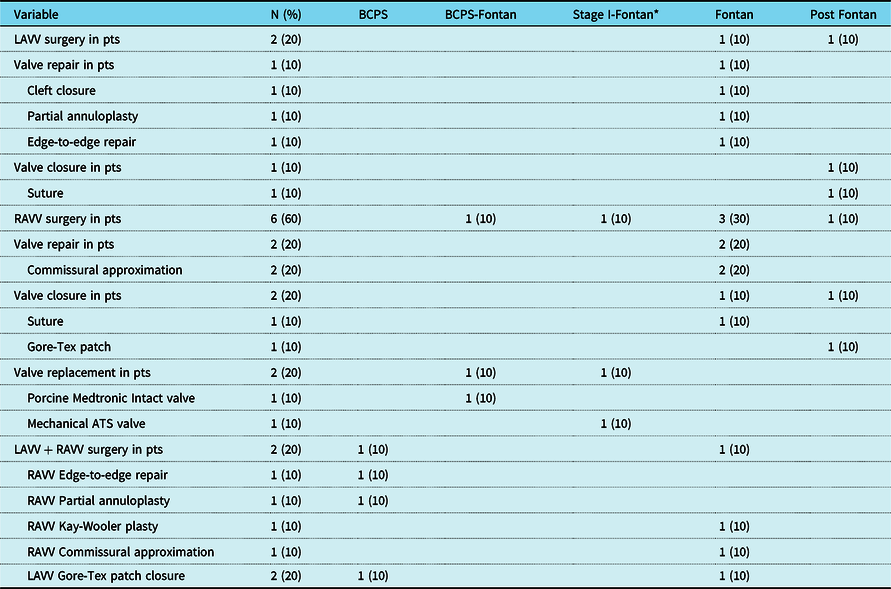
Data are expressed as n (%). BCPS = bidirectional cavopulmonary shunt; LAVV = left atrioventricular valve; pts: patients; RAVV = right atrioventricular valve.
* In this patient, Stage II bidirectional cavopulmonary shunt was not performed and atrioventricular valve surgery was performed between stage I and Fontan.
In one patient (3.1%), epicardial dual chamber pacemaker implantation was necessary during concomitant redo repair of the right atrioventricular valve and Gore-Tex patch closure of the left atrioventricular valve. In another patient, upgrade to a dual chamber pacemaker during suture closure of the right atrioventricular valve was performed. Indication for pacemaker implantation was complete atrioventricular block in both patients.
At the last follow-up, echocardiographic results were available for 22 patients (68.8%). No severe regurgitation of the left or right atrioventricular valve was seen. Moderate regurgitation of the left atrioventricular valve was visible in one patient (4.5%) and moderate regurgitation of the right atrioventricular valve was diagnosed in one patient (4.5%). Mild and less regurgitation of the left atrioventricular valve was seen in six patients (27.3%) and mild or less regurgitation of the right atrioventricular valve was found in 14 patients (63.6%) (Fig. 1A, 1B).
Discussion
In the present investigation, 16% of children with two separate atrioventricular valves and univentricular heart required surgery of the atrioventricular valves during staged palliation and surgical intervention was most commonly performed as a concomitant procedure during Fontan completion. Atrioventricular valve closure was performed in 22% of children. Significant regurgitation of the left atrioventricular valve was mainly due to clefts of the anterior leaflet and posterior and anterior leaflet prolapse of the right atrioventricular valve were the main pathologies for regurgitation of the right atrioventricular valve. Ten years after atrioventricular valve surgery survival was 62%, however, associated with a 31% incidence of reoperation at 10 years after the initial valve surgery.
Despite improvements in treatment strategies, surgery, and management of patients with two separate atrioventricular valves and univentricular heart, significant atrioventricular valve regurgitation and stenosis remain a surgical challenge in this highly specific patient population. Reference Tseng, Siddiqui and Di Maria1,Reference Nakata, Fujimoto and Hirose7 Atrioventricular valve morphology is complex and often associated with valve dysplasia in these patients. Most published studies about atrioventricular valve repair in single-ventricle patients focus on patients with a common atrioventricular valve or on patients with hypoplastic left heart syndrome and a single tricuspid valve. Reference Honjo, Atlin and Mertens10–Reference Mayr, Burri and Strbad12 Patients with two separate atrioventricular valve morphology such as double inlet left ventricle or double outlet right ventricle represent only a small subgroup of univentricular heart patients with atrioventricular valve surgery and significant atrioventricular valve regurgitation is more frequently associated in patients with a systemic tricuspid or a common atrioventricular valve. Reference Ono, Cleuziou and Pabst von Ohain8 In the retrospective study by Nakata and colleagues, atrioventricular valve repair in 29 consecutive patients with double outlet right ventricle and right atrial isomerism was associated with 5-year freedom from reoperation rate of 57%. Reference Nakata, Fujimoto and Hirose7 Survival at 5 years after atrioventricular valve repair was 70% and body weight less than 4 kg was identified as an independent risk factor for mortality. Reference Nakata, Fujimoto and Hirose7 In patients with double outlet right ventricle and hypoplastic left ventricle, coincidence of total anomalous pulmonary venous drainage has been shown to be an independent risk factor for mortality. Reference Takeuchi, McGowen and Bacha13 According to Lim and colleagues, significant valve regurgitation in patients with two atrioventricular valve morphology and functional single ventricle is associated with an early mortality of 2.6% and late mortality of 18.4% after atrioventricular valve surgery. Reference Lim, Kwak, Min, Cho and Kim14 Difference in late mortality to the present investigation is due to the higher rate of atrioventricular valve replacement in the study by Lim and colleagues.
Our institutional policy is to preserve the atrioventricular valve as long as possible, but the optimal timing for atrioventricular valve surgery in patients with univentricular heart and two separate atrioventricular valves is currently unclear. In our study, surgical intervention on the left and right atrioventricular valves was most commonly performed during Fontan completion. However, atrioventricular valve surgery can also be performed as a sole operation before the Fontan procedure as the additional procedure of valve surgery may prolong the bypass and aortic cross-clamp time leading to a higher incidence of postoperative complications and increased mortality. Reference Ono, Cleuziou and Pabst von Ohain8 Atrioventricular valve surgery as a separate procedure before Fontan completion also yields the possibility to work on the valve at the time of Fontan procedure to improve suboptimal repairs and to prevent the breakdown of systemic ventricular function at the time of Fontan completion. Reference Mayr, Burri and Strbad12 If one of the valves is not amenable to repair and severe regurgitation or stenosis is present, prompt atrioventricular valve closure should be performed, even between the classic palliative steps.
Despite advances in the treatment of double inlet left ventricle and double outlet right ventricle with univentricular palliation, redo atrioventricular valve procedures are frequently required as these frail valves have a high tendency to fail. In our study, the cumulative incidence of reoperation at 10 years after atrioventricular valve surgery was 31% with valve replacement in only 6.3% of children. In the retrospective study by Ono and colleagues, 32% of single-ventricle patients required atrioventricular valve reoperations with a slightly higher rate of valve replacement (8.7%). Reference Ono, Cleuziou and Pabst von Ohain8 However, atrioventricular valve replacement in single-ventricle patients is associated with a 25–44% rate of complete heart block as well as an early mortality of 29%. Reference Mahle, Gaynor and Spray15,Reference Sughimoto, Hirata and Hirahara16 Another option in patients with two separate atrioventricular valves and univentricular heart is valve closure. Atrioventricular valve closure, either with patch or suture, is associated with low operative mortality and an 18-year freedom from reintervention rate of 83%. Reference King, Winlaw and Alphonso6 In the present investigation, valve closure was performed in 22% of children (7/32), mainly (5/7) during Fontan completion. In one child (1/7), redo closure of the left atrioventricular valve was performed due to suture dehiscence 3.6 months after valve suture closure. In our study, no patch dehiscence (Gore-Tex or Dacron) occurred. In the retrospective study by King and colleagues, atrioventricular valve closure in Fontan circulation was associated with an incidence of patch dehiscence of 17%. Reference King, Winlaw and Alphonso6 In the present investigation, epicardial pacemaker implantation was required in two patients due to postoperative complete heart block after redo atrioventricular valve closure in both patients. After atrioventricular valve closure, King and colleagues reported a 14% risk of heart block. Reference King, Winlaw and Alphonso6 According to Nunez and colleagues, atrioventricular valve closure using suture may be able to decrease the risk of alterations to the heart conduction system with lower need for pacemaker implantation. Reference Nunez, Aguado, Celemin and Larrea17 However, published data on outcomes of atrioventricular valve closure, either with patch or suture, are limited making further comparison with our data difficult.
This study was limited by its retrospective nature and single-centre design. Main limitation of this study is the small sample size and thus differences between subgroups may not be detected. Mechanisms of atrioventricular valve anomalies were inferred from the operation records, and bias among surgeons can be expected. Inconsistencies in preoperative, operative, and postoperative management during the 27-year study period may have affected our outcome parameters.
Surgical atrioventricular valve intervention in patients with two separate atrioventricular valves and univentricular heart is required in 16% of patients during and after the staged Fontan completion and can be performed at various palliation points. Atrioventricular valve closure was performed in 22% and was mainly performed during Fontan completion. No atrioventricular valve replacement was necessary at the initial surgery. Atrioventricular valve surgery in this highly specific patient cohort is associated with low operative and late mortality. However, a high incidence of reoperation was observed due to the complex atrioventricular valve anatomy in these patients. Therefore, thoughtful considerations are needed to evaluate how the atrioventricular valve reintervention affects the long-term results of Fontan circulation in patients with two separate atrioventricular valve morphology and univentricular heart.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S104795112400012X.
Acknowledgements
None.
Financial support
This research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
Competing interests
None.










