Emergencies in the paediatric cardiac ICU require highly specialised care, understanding of complex physiology, and multidisciplinary teamwork. Complex scenarios involving numerous specialists including cardiac intensivists, cardiac surgeons, cardiologists, nurses, and respiratory therapists require not just cognitive and technical skill but also efficient communication, clear leadership, and coordination of care. Reference Prince, Hines, Chyou and Heegeman7 A break down in teamwork and communication and unclear responsibilities may contribute to medical error. Reference Bognar, Barach and Johnson1 In situ simulation and Crisis Resource Management training in the paediatric cardiac ICU have been associated with improved comfort and team function during resuscitations. Reference Allan, Thiagarajan and Beke2
One such highly complex resuscitation scenario in the cardiac ICU involves emergency cardiac resternotomy. Emergent cardiac resternotomy is performed in 0.8–2.7% of all adult patients who have undergone cardiac surgery. 3 The number of emergent cardiac resternotomies performed in the paediatric cardiac surgical population is not well known. Both the European Resuscitation Council and the Society of Thoracic Surgeons Expert Task Force on Resuscitation after Cardiac Surgery suggest emergency resternotomy should be performed for non-ventricular fibrillation/ventricular tachycardia arrests that do not resolve after exclusion of airway or respiratory problems within 5 minutes. 3,Reference Dunning, Fabbri and Kolh4,Reference Dunning, Levine and Ley10 The American Heart Association Scientific Statement for the Cardiopulmonary Resuscitation in infants and children with cardiac disease suggests early urgent opening of the sternum in the immediate postoperative period within the first minutes of resuscitation if indicated. Reference Marino, Tabutt and MacLaren5
In this study, we report a novel educational intervention performed in the cardiac ICU to train a multidisciplinary team to more efficiently perform cardiac resternotomy. We aimed to evaluate the efficiency of the intervention to decrease time to perform critical tasks and improve caregiver comfort.
Materials and methods
Educational intervention design
First, the literature was reviewed to identify standardised resternotomy processes and reported challenges encountered during bedside cardiac resternotomy. Identification of key stakeholders and barriers to process improvement were discussed. Then a multidisciplinary meeting with cardiac ICU leadership including cardiac intensivists, cardiothoracic surgeons, cardiac ICU nurses, cardiovascular nurse practitioners, perfusionists, and nursing leadership was held to discuss and understand the current process and potential challenges. The scope, goals, requirements, equipment, and personnel required were delineated. The educational tool was then developed by the primary study personnel and reviewed and revised by the multidisciplinary group.
The final educational tool consisted of (1) Cards with seven clear roles and responsibilities for cardiac ICU nursing (Supplemental Table 1) (2) a labelled diagram of the sternotomy cart (Fig. 1) (3) a unit-specific video of a simulated resternotomy scenario with a demonstration of each role and responsibility. The roles and responsibilities and sternotomy cart were reviewed weekly via a standardised script over a three-month period during morning and evening nursing huddles. The video was circulated via the hospital educational module system and all nurses were required to view it.

Figure 1. Resternotomy cart overview.
In addition to the educational tools, direct educational sessions were performed weekly during morning and evening nursing huddles over a three-month period and encompassed all cardiac ICU nurses. Cardiac ICU charge nurses received personalised education and were required to demonstrate competence in performance of all seven key roles and performance of the critical tasks.
To collect pre-intervention data about the current practice, in situ simulations were conducted in the cardiac ICU. Simulations involved a low-fidelity simulator with a realistic sternal dressing, central line access, and endotracheal tube. The scripted scenario included a bedside nurse who was engaged in manually ventilating the patient and a cardiac ICU fellow who relayed the medical information to the responders and ran the code. One research assistant was responsible for timing all interventions with a standardised scoring sheet. Additional assistants answered the phone calls posing as the cardiovascular surgeon, operating room charge nurse, etc. The in situ approach used an actual cardiac ICU bed space and the same equipment (defibrillators, surgical instrument trays, supplies, code cart medications, medical dispenser) used for real patients. The cardiac ICU was not aware that a simulated scenario would occur but responded to the code button similar to a real code scenario. Participants were expected to perform the actual roles they would perform during a real scenario and were instructed to draw up and administer medications, perform cardiopulmonary resuscitation, and use sterile barrier precautions to enhance realism. Post-intervention simulations employed similar methods but included a structured debrief. In addition, additional simulations were performed 6 months after the initial intervention.
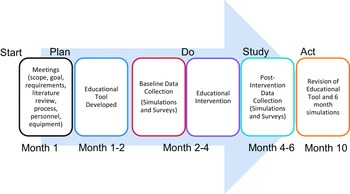
A pre-intervention survey was sent to all the nurses with questions involving their knowledge of resternotomy and comfort level with performing responsibilities and critical tasks and used a 5-point Likert scale. The scale was 1 = very uncomfortable, 2 = uncomfortable, 3 = comfortable, 4 = very comfortable, and 5 = extremely comfortable. Post-intervention surveys were again sent to all the nurses regarding their comfort level with the critical tasks. The study timeline is shown in Figure 2.
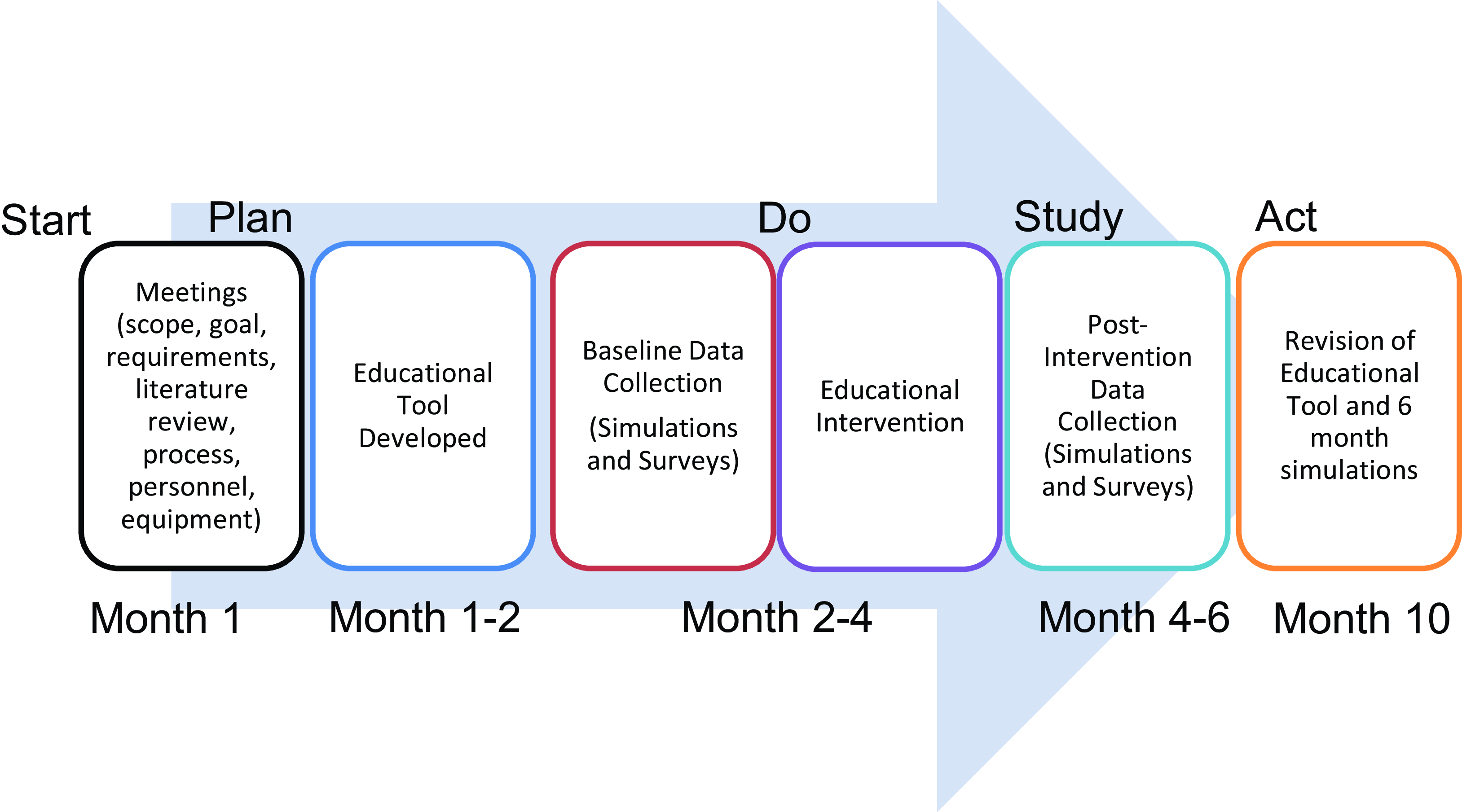
Figure 2. Flow chart of the educational tool development and implementation.
Statistical analyses were performed using analysis of variance to compare the different study timepoints and time to critical tasks. The Wilcoxon signed ranks test was used to compare pre and post-intervention survey results. For analysis, Likert scales 3,4, and 5 were grouped into a composite response of comfortable with task.
Results
A total of 186 providers participated in the intervention (168 nurses, 10 physicians, four nurse practitioners, and four respiratory therapists). A total of 10 pre-intervention simulations and 10 post-intervention simulations were conducted during both day and night shifts in situ in the cardiac ICU. Additional post-intervention simulations were performed 6 months after the initial intervention. The critical tasks were identified a priori during the multidisciplinary planning stage.
Median time in seconds to performance of critical tasks pre and post-educational intervention is detailed in Table 1. There was a decrease in time to have defibrillator in the room, setup the resternotomy equipment and internal defibrillator paddles, and decreased time to deliver sedation and fluid after the intervention that was sustained in the 6-month simulations (all p < 0.05). Time to escort family from the room and having blood at the bedside were significantly decreased after intervention and at 6 months (p < 0.05). There was no difference in time to first dose of epinephrine, defibrillator pads on the patient, or time to call the cardiovascular surgeon or blood bank.
Table 1. Pre, post, and 6 month intervention time to complete critical tasks during simulations
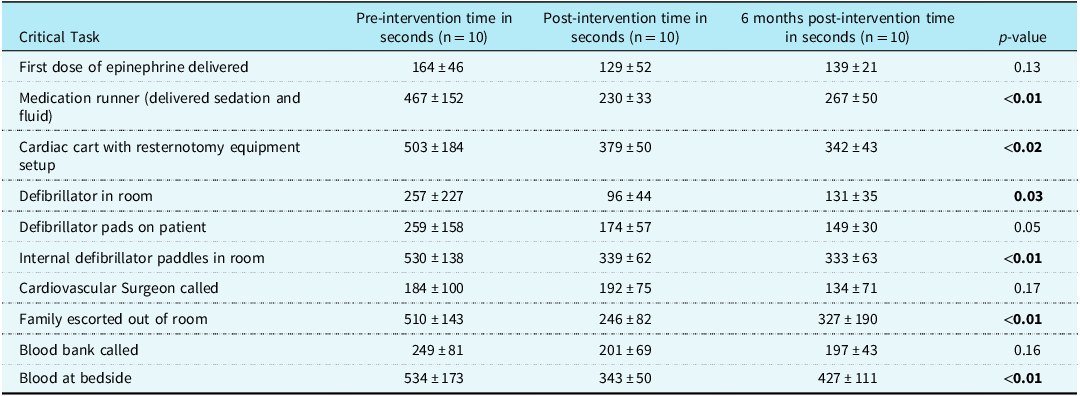
Continuous variables presented as mean ± standard deviation.
The pre-intervention and post-intervention survey data were also collected and analysed.
Before the intervention was implemented, the lowest comfort level was reported in identifying equipment needed for resternotomy, setting up the defibrillator with internal paddles, selecting appropriate size paddles, and opening the resternotomy equipment. Table 2 shows the pre and post-intervention survey results. Nurse participants reported increased comfort in identifying equipment needed for resternotomy (p = 0.007) and setting up the internal defibrillator paddles (0.003) after the intervention. All respondents reported increased comfort after intervention.
Table 2. Pre- and post-intervention survey results
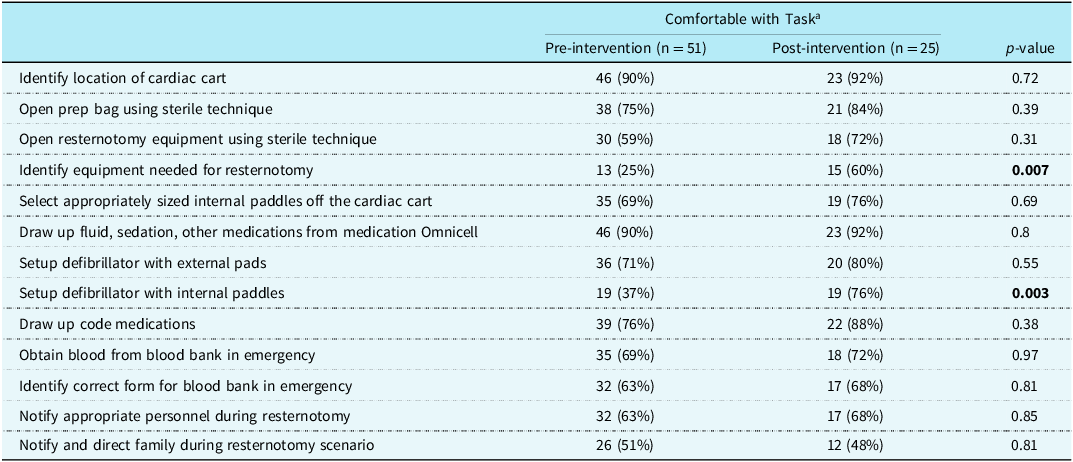
a Likert scale 3, 4, and 5.
Discussion
This report describes a novel educational intervention using in situ simulation to practice emergent cardiac resternotomy in the paediatric cardiac ICU. We have identified that it is feasible, decreases time to performance of critical tasks, and improves participant comfort.
Resuscitation of postoperative cardiac patients requires understanding of complex physiology, specialised skills, and multidisciplinary teamwork. Bedside resternotomy occurs rarely but is a specialised procedure required for resuscitation and little if any education is routinely performed to assist in this complex intervention. Provider inexperience, discomfort, and distress when the event occurs may lead to a chaotic situation.
The European Resuscitation Council and the Society of Thoracic Surgeons Expert Task Force on Resuscitation after Cardiac Surgery suggest emergency resternotomy should be performed for non-ventricular fibrillation/ventricular tachycardia arrests that do not resolve after exclusion of airway or respiratory problems within 5 minutes. 3,Reference Dunning, Fabbri and Kolh4 Multiple organisations have developed a postoperative cardiac surgical algorithm for resuscitation that includes emergent resternotomy. 3,Reference Dunning, Fabbri and Kolh4,Reference Dunning, Fabbri and Kolh11,15
Utilising an adult Cardiac Surgical Advanced Life Support protocol Maccaroni et al. reported significantly improved survival in their postoperative cardiac surgical patients. Reference Maccaroni, Watson and Mukherjee12 Cardiac resternotomy, which is a low frequency, high-acuity scenario, can be practised via simulation. Studies have shown that simulation is an effective teaching tool. Reference Mickelsen, McNeil, Parikh and Persoff8,Reference Herbers and Heaser9,Reference Figueroa, Sepanski, Goldberg and Shah14 Simulation improves comfort, team skills, confidence, and communication. Reference Bognar, Barach and Johnson1,Reference Allan, Thiagarajan and Beke2,Reference Kane, Pye and Jones6,Reference Figueroa, Sepanski, Goldberg and Shah14 Other paediatric studies report improved clinical performance and patient outcomes with high-fidelity simulation. Reference Gilfoyle, Koot and Annear16–Reference Knight, Gabhart, Earnest, Leong, Anglemyer and Franzon18 This is especially important in low-frequency and high-acuity scenarios such as cardiac resternotomy.
The American Heart Association Scientific Statement for the Cardiopulmonary Resuscitation in infants and children with cardiac disease suggests early urgent opening of the sternum in the immediate postoperative period within the first minutes of resuscitation if indicated. Reference Dunning, Fabbri and Kolh4 For the first time, this guideline gives recommendations specifically for paediatric cardiac patients. Unlike the adult guidelines, a specific timeline is not proposed and very few studies detail a systematic approach to resternotomy education. Lo et al. report a low-fidelity simulation training scheme to evaluate standard paediatric resuscitation including cardiac pacing and chest re-opening. Reference Lo, Morrison and Atkins13 In this study, new medical leaders took significantly longer to order chest re-opening than experienced team leaders. As noted previously, bedside paediatric cardiac resternotomy is a rare intervention and the opportunities for learning are few in most cardiac ICUs. In our cardiac ICU, 75% of nurses surveyed had never witnessed a cardiac resternotomy.
In our study, we found a significant reduction in time to performance of tasks needed for cardiac resternotomy including faster sterile setup of the resternotomy cart equipment for the cardiac surgeon and less time to sterile preparation of the correct sized internal defibrillator paddles. We found that most nurses knew where the actual cardiac resternotomy cart was located and performed well on standard resuscitation measures including time to initiate chest compressions, time to bring the code cart to the bedside, time for epinephrine delivery, and time to bring the defibrillator in the patient room. However, we did find significant improvement in tasks that were not only important for cardiac resternotomy but also tasks that were important for other emergent resuscitations. For example, after completion of the intervention, it took less time to deliver fluid, sedation, blood to the bedside, and family support. All of these tasks may be important during a paediatric medical resuscitation but receive less emphasis and education during code training than standard roles (code cart, recorder, etc.)
The results of our survey showed that many nurses had never participated in a real or simulated cardiac resternotomy scenario. After the educational intervention, nurses reported significantly increased comfort in identifying equipment needed for resternotomy and setting up the defibrillator with internal paddles. All participants reported feeling that the intervention was useful and improved overall confidence.
Strengths of this study include the large number of participants and multidisciplinary input. Limitations include challenges of simulation education. This was a simulation scenario, not a real patient scenario. Although we demonstrated decreased time to perform critical tasks during simulation, we do not know if this improved performance translates to improved clinical performance or improved patient outcome. In addition, the educational intervention and feedback were primarily aimed at nursing staff, which may be a limitation of the study. Future studies focused on whether this educational intervention leads to improved clinical outcomes are needed.
Conclusion
This novel standardised educational intervention decreased time to perform critical tasks during emergent paediatric cardiac resternotomy and improved nursing comfort.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S1047951124000891.
Acknowledgements
We would like to thank Dr Henry Walters III, Michelle Dokas, Krissy Richards, Kacee Harris, Keith Larson, Krista Fell, Andrea Kline, and all of the nurses, perfusionists, respiratory therapists, and other ICU staff who participated in this project.
Financial support
None.
Competing interests
None.
Ethical standard
The authors assert that all procedures contributing to this work comply will all ethical standards of the relevant national guidelines on human experimentation (United States National Institutes of Health Belmont report) and with the Helsinki Declaration of 1975, as revised in 2009, and have been approved by the Wayne State Institutional Review Board.