Paediatric heart failure is defined by the International Society for Heart & Lung Transplantation (ISHLT) as “a clinical and pathophysiologic syndrome that results from ventricular dysfunction, volume, or pressure overload, alone or in combination”. Reference Kirk, Dipchand and Rosenthal1 Kantor et al describe it as “as the failure of the heart to supply blood to either systemic or pulmonary circulation at an appropriate rate of flow or to receive venous return at an appropriate filling pressure, resulting in adverse effects on the heart, the circulation, and the patient”. Reference Kantor, Lougheed and Dancea2
In high-income countries, paediatric heart failure is primarily caused by congenital heart disease (CHD) or cardiomyopathy. In low- and middle-income countries, rheumatic heart disease and infective endocarditis remain the most common causes of paediatric heart failure. It is important to understand the background pathophysiology to paediatric heart failure before considering the role of various plasma biomarkers in this disease process. In clinical medicine, we routinely measure various plasma components which have an active role in the body to diagnose and monitor a patient’s health. In adult patients, cardiac biomarkers are recommended in the diagnosis of heart failure and to aid clinical management. However, in paediatric patients their use has remained mostly scientific as a result of differences in the underlying mechanism of cardiac dysfunction and influence of age affecting the assay levels.
This review provides a comprehensive overview of paediatric heart failure caused by CHD and cardiomyopathy and considers the available evidence for cardiac biomarkers in this complex, multifactorial condition.
A literature review was completed using MEDLINE ALL, EMBASE, and PubMed on 10th November 2022. Search terms included biomarkers, heart failure, heart defects, CHD, Fontan circulation, single ventricle circulation, cardiomyopathy, and child.
Paediatric heart failure pathophysiology
Pulmonary over-perfusion
Children with significant left to right shunting of blood secondary to congenital cardiac conditions such as large septal defects and persistent ductus arteriosus may experience clinical heart failure secondary to pulmonary over-circulation (See Figure 1). Significant atrioventricular valvar regurgitation may also cause increased volume overload in the atria and exacerbate pulmonary over-circulation. For affected infants, the reduction in pulmonary vascular resistance a few weeks after birth results in symptoms of respiratory distress, failure to thrive, and recurrent lower respiratory tract infections. Importantly, whilst these children may experience significant comorbidities secondary to congestive heart failure, their ventricular function is usually well preserved, and they will make a good recovery post-surgical repair. Reference Spaziani, Bennati and Marrone3
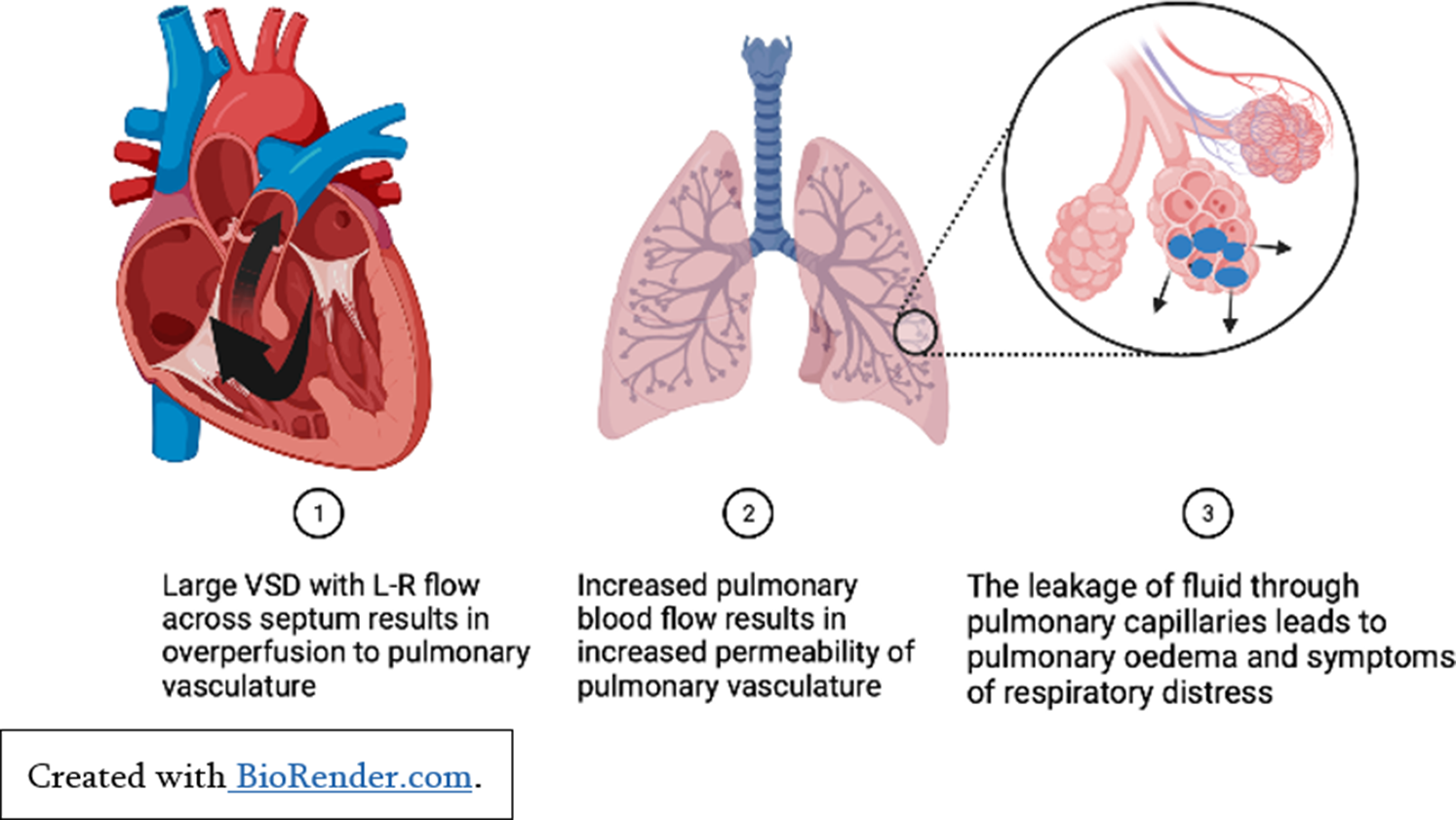
Figure 1. Pulmonary over-perfusion secondary to left to right shunt.
Pressure overload
Children with significant left-sided cardiac obstructions may show signs of heart failure secondary to reduced cardiac output. Critical aortic stenosis or neonatal coarctation of the aorta both put abnormal pressure on the left ventricle, cause left ventricular remodelling, and reduced ventricular function if untreated (See Figure 2). Reference Law and Tivakaran4 Patients with hypertrophic cardiomyopathy can develop left ventricular hypertrophy secondary to marked fibrosis and disarray of cardiac myocytes. For some patients, this causes left ventricular outflow tract obstruction, subsequent pressure overload, and heart failure symptoms. Reference Yuan5
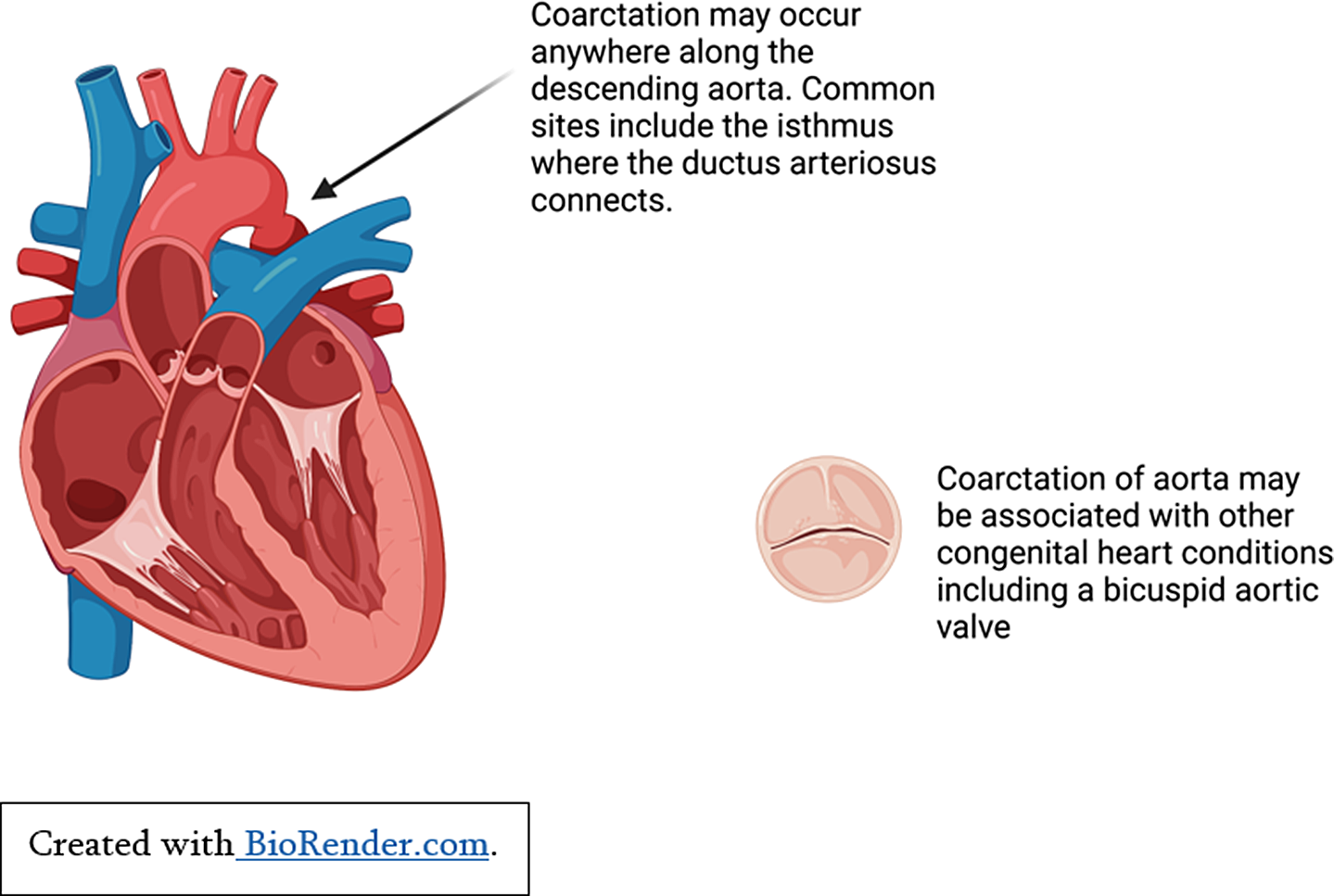
Figure 2. Pressure overload secondary to coarctation of aorta.
Pump failure
Ventricular dysfunction can result from ischaemic injury, arrhythmias, and myocardial failure which leads to systemic and/or pulmonary hypoperfusion. Ischaemic myocardial injury in children is caused by conditions such as myocarditis, Kawasaki disease, and abnormalities of the coronary arteries. Ischaemia causes ventricular remodelling and reduced ventricular pump function.
Children with single ventricle physiology may also experience pump failure during their staged palliation or following the completion of Fontan circulation. This is a result of reliance on the morphological right ventricle to maintain the systemic circulation and subsequent ventricular dysfunction over time.
Cardiomyopathies in children cause atrial and ventricular remodelling which in turn affects pump function. Reference Lipshultz, Law and Asante-Korang6 Dilated cardiomyopathy may be inherited, acquired, or associated with autoimmune and metabolic conditions. Reference Molina, Shrader and Colan7 Dilated cardiomyopathy is characterised by ventricular remodelling and haemodynamic overload as a result of reduced sarcomere contractility and increased ventricular volumes to maintain cardiac output (See Figure 3). This progressive ventricular dilatation leads to tricuspid and mitral valve insufficiency which further reduces systolic function, increases ventricular wall stress and myocardial injury, and contributes to ventricular dysfunction and pump failure. Reference Mahmaljy8
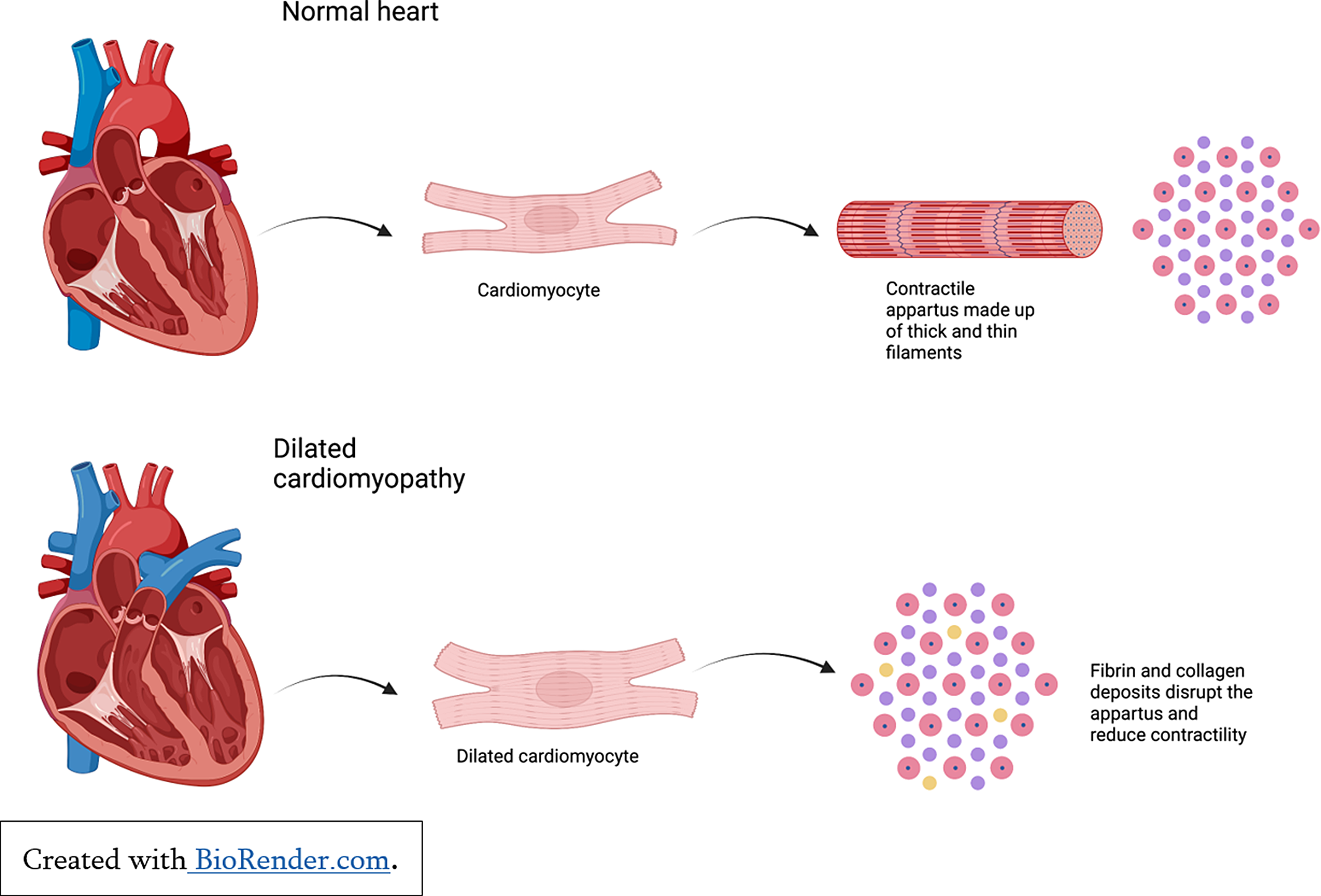
Figure 3. Cardiac remodelling in the setting of dilated cardiomyopathy.
Role of biomarkers
Current adult NICE guidelines advise measuring NT-proBNP as the first-line investigation in suspected heart failure because of its high diagnostic accuracy. Similarly, cardiac troponin I and T are recommended by NICE for diagnosis of acute coronary syndrome and screening of chest pain. However, the current ISHLT paediatric guidelines advise brain naturetic peptide/NT-proBNP can be used as an adjunctive marker in the integrated evaluation and monitoring of children with known heart failure but should not be used as a stand-alone test for diagnosis. Reference Kirk, Dipchand and Rosenthal1 Therefore, cardiac biomarkers are routinely used in adults but not in paediatric patients. This is due to the complex, multifactorial mechanism of heart failure in children and the limited number of large paediatric research studies. Given that heart failure may be the first presentation of structural heart disease in children, Reference Bakker, Bergman and Krikov9 and is often a complicated clinical state to monitor, a widely available blood biomarker to aid diagnosis and prognosis would improve outcomes in paediatric patients as it has in adults.
Natriuretic peptides
Atrial naturetic peptide and brain naturetic peptide are primarily produced in cardiac cells, namely atrial and ventricular cardiomyocytes. The cardiac natriuretic hormones are synthesised by cardiomyocytes as prohormones, i.e. proANP and proBNP. They are then split into two fragments at the point of secretion: the inactive precursor, i.e. NT-proANP and NT-proBNP, and the active hormone, i.e. atrial naturetic peptide and brain naturetic peptide. Reference Clerico, Giannoni, Vittorini and Passino10
The main mechanical stimulus for atrial naturetic peptide and brain naturetic peptide secretion is atrial and ventricular distension respectively, although studies suggest multiple other proteins/hormones can promote secretion including endothelin-1, α-adrenergic agonists, and angiotensin II, glucocorticoids, vasopressin, growth factors and cytokines. Reference Fu, Ping, Wang and Luo11
Both atrial naturetic peptide and brain naturetic peptide bind to natriuretic peptide receptor-A (NPR-A or guanylyl cyclase-A). They exert similar effects within target tissues namely they reduce vascular tone, increase electrolyte and water excretion, functionally antagonise the renin angiotensin aldosterone system (RAAS) and have antifibrotic and antihypertrophic effects. Reference Nakagawa, Nishikimi and Kuwahara12
Brain naturetic peptide and NT-proBNP
Early studies of paediatric heart failure in CHD and cardiomyopathy focused on brain naturetic peptide measurements but this has been widely overtaken by focusing on brain naturetic peptide’s precursor NT-proBNP given its longer half-life and stability. Reference Şahin, Portakal and Karagöz13
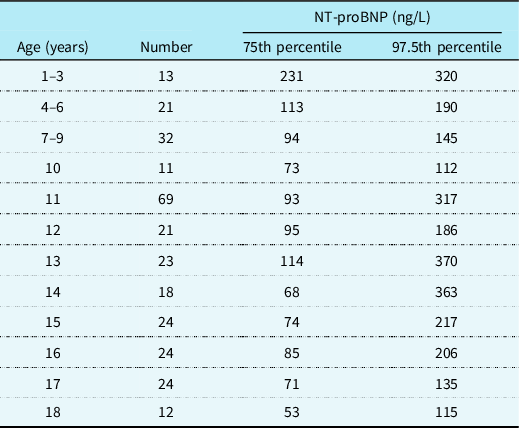
NT-proBNP is the most widely studied cardiac biomarker in paediatric heart failure and although it is influenced by age, sex and underlying cardiac condition, it appears to be the most sensitive and specific biomarker of cardiac dysfunction available. In healthy children, NT-proBNP levels are highest in the first year of life and then decrease steadily throughout childhood. Reference Lam, Higgins and Zhang14,Reference Kiess, Green and Willenberg15 Roche have generated reference values (see Table 1) for NT-proBNP based on the Albers et al study published in 2006 which included 408 participants. These values can be applied to healthy children with structurally normal hearts to aid interpretation of NT-proBNP levels. The LIFE Child study included 2522 children aged 3 months to 18 years and determined reference ranges based on 97.5th percentiles for healthy children. They also compared these to the study by Albers et al. Reference Albers, Mir, Haddad and Läer16 They supported the previous findings that NT-proBNP is elevated in the first year of life and demonstrated a steady decrease in NT-proBNP levels during childhood to adult levels as the child reaches puberty. Given their larger sample population, they had less variability than Albers et al and may therefore be worth considering as the most accurate reference ranges for 97.5th percentiles of NT-proBNP in healthy children based on age.
Table 1. Roche assay NT-proBNP reference ages for healthy children based on age(Reference Albers, Mir, Haddad and Läer16)
Both Albers et al and the LIFE child study highlight that females have higher baseline NT-propBNP than males of the same age.
Whilst it is useful to establish reference ranges for NT-proBNP in healthy children based on age and sex, we must consider the impact of CHD on baseline levels of NT-proBNP. This is because studies suggest that the presence of structural cardiac disease impacts baseline NT-proBNP levels for individual patients and therefore the healthy children reference ranges cannot be relied on for similarly aged children with a structural abnormality.
To improve our understanding of how to interpret NT-proBNP levels in paediatric patients, we must first consider the clinical indication or need for this biomarker. In adult patients, elevated NT-proBNP levels aid diagnosis of heart failure in primary and secondary care. In children, heart failure is multi-factorial and may mimic other pathologies, e.g. respiratory or infectious conditions. Therefore, NT-proBNP needs to be used with a clear clinical question before it can be considered as a diagnostic tool in paediatric heart failure. For example, one of the benefits of NT-proBNP in paediatrics is aiding diagnosis of heart failure at initial presentation. BNP and NT-proBNP are both significantly elevated in paediatric patients with respiratory distress secondary to cardiac disease compared to respiratory distress secondary to respiratory disease and healthy controls. Reference Sezgin Evim, Ucar, Kilic and Colak17–Reference Koulouri, Acherman, Wong, Chan and Lewis19 Therefore, it could be used as a screening tool in the assessment of suspected heart failure in children who present with respiratory symptoms.
In CHD, brain naturetic peptide/NT-proBNP is proven to correlate to symptoms of heart failure through Ross and/or NYHA classification systems. Reference Şahin, Portakal and Karagöz13,Reference Sugimoto, Manabe and Nakau20,Reference Hauser, Demyanets and Rusai21 Importantly, NT-proBNP also correlates to objective measures of ventricular function on echocardiogram. Reference Lin, Zeng, Zhang and Meng22–Reference Maher, Reed and Cuadrado24 A higher BNP/NT-proBNP is associated with increased symptoms of heart failure and reduced EF suggesting an increased likelihood of adverse outcomes.
However, because of the small number of paediatric patients who have been studied, there is not a clear definitive brain naturetic peptide/NT-proBNP assay level that could be used as a reference value to diagnose heart failure in children with an underlying cardiac condition. As previously discussed, brain naturetic peptide/NT-proBNP reference ranges based on age and sex are useful but we must consider if reference ranges for children with CHD and other comorbidities can be developed.
Salem et al, in their paper published in 2021, suggested on-admission brain naturetic peptide at a cut-of-point 507.13 pg/ml has 95.5% sensitivity and 88% specificity for in-hospital mortality. Reference Salem, Saleh, Soliman and Abo-Haded25 Despite lack of assay cut-off values in congenital cardiac disease, this suggests brain naturetic peptide/NT-proBNP may be utilised as a prognostic indicator in known cardiac disease to aid earlier identification of patients most at risk of adverse outcomes.
Similarly, an elevated brain naturetic peptide/NT-proBNP preoperatively is associated with increased adverse events and/or increased circulatory support requirement post operatively. Reference Mori, Nakashima, Kaneko, Inoue and Murakami26,Reference Mir, Haun, Lilje, Läer and Weil27 One study of 86 patients with complex cardiac disease scheduled for biventricular repair, of which most had evidence of mild heart failure symptoms, had preoperative brain naturetic peptide levels measured before surgical intervention. They found that a preoperative brain naturetic peptide level >60.9pg/ml was associated with increased risk of an adverse event. Reference Mori, Nakashima, Kaneko, Inoue and Murakami26 This suggests brain naturetic peptide has the potential to identify higher risk patients for adverse outcomes even if they are clinically stable preoperatively.
However, postoperative brain naturetic peptide/NT-proBNP levels appear to be less useful in predicting adverse events and postoperative prognosis. This is likely because of the influence of acute cardiomyocyte injury causing an acute brain naturetic peptide/NT-proBNP assay level rise which will recover and is an inaccurate assessment of potential ventricular failure in this period. Reference Cantinotti28,Reference Butnariu, Iancu, Samaşca, Chira and Lupan29
Role of NT-proBNP in single ventricle physiology
Brain naturetic peptide/NT-proBNP is significantly elevated in patients with heart failure secondary to single ventricle physiology compared to children with similar anatomy who are not clinically in heart failure. Reference Lowenthal, Camacho and Lowenthal30–Reference Law, Ettedgui, Beerman, Maisel and Tofovic32 Studies have found that brain naturetic peptide/NT-proBNP baseline levels reduce with each subsequent stage of palliative surgery regardless of heart failure status. Reference Knirsch, Häusermann, Fasnacht, Hersberger, Gessler and Bauersfeld33 It has been suggested that once children have undergone their Fontan procedure, if they do not clinically have signs/symptoms of heart failure, there is no difference in their brain naturetic peptide/NT-proBNP levels compared to a healthy age matched control. Reference Lechner, Gitter and Mair34 Ghelani et al report a negative correlation between ejection fraction on cardiac MRI and NT-proBNP in Fontan patients in NYHA Class I-II. They suggested NT-proBNP >150 pg/ml may be used as an indicator of ventricular dysfunction. Reference Ghelani, Opotowsky and Harrild35 Similarly, a study of 133 Fontan patients with a median age of 13.2 years found that the 27% of patients who had an adverse cardiac outcome had significantly higher NT-proBNP levels at recruitment and their NT-proBNP level correlated with severity of event. Reference van den Bosch, Bossers and Kamphuis36
This highlights the potential role of NT-proBNP as a prognostic indicator in Fontan patients given that it is elevated prior to clinical symptoms or signs of ventricular dysfunction and correlates to cardiac MRI findings.
Role of NT-proBNP in cardiomyopathy
A longitudinal study of paediatric patients with dilated cardiomyopathy found that NT-proBNP was useful in identifying the patients at recruitment who were most likely to deteriorate over time. In addition, within the group that would deteriorate, it was able to distinguish between those who would experience adverse events (heart transplant or death) and those who did not. Reference Meulen, Boer and Marchie Sarvaas37
Studies show higher brain naturetic peptide/NT-proBNP levels in children with heart failure secondary to dilated cardiomyopathy compared to CHD. This may be partly because of the increased prevalence of heart failure in the dilated cardiomyopathy subgroup and the associated systolic impairment which causes elevated brain naturetic peptide/NT-proBNP compared to the effect of volume overload as seen in the CHD subgroups. Reference Hauser, Demyanets and Rusai21,Reference Lin, Zeng, Zhang and Meng22,Reference Westerlind, Wåhlander, Lindstedt, Lundberg and Holmgren38
Role of NT-proBNP in haemodynamically significant shunts
In infants and young children, septal defects causing pulmonary overperfusion may result in symptomatic heart failure. NT-proBNP has been shown to positively correlate to shunt size in atrial and ventricular septal defects and to fall following closure correlating to reduced symptoms of heart failure. Reference Schoen, Zimmermann and Kittner39–Reference Cura, Argun and Koçer44 NT-proBNP has also been reported to be positively correlated with symptoms of heart failure and in-hospital mortality in haemodynamically significant shunts. Reference Paneitz, Zhou and Yanek45 Clinically, NT-proBNP may be used to aid risk stratification and identification of children with more haemodynamically significant left to right shunts in known septal defects.
Correlation to imaging
An advantage of NT-proBNP would be if it could identify cardiac dysfunction or improvement before evident on imaging. Yet few studies have monitored serial brain naturetic peptide/NT-proBNP levels in children with heart failure and compared them with cardiac function on echocardiogram. One study of 30 children which identified elevated NT-proBNP levels in children with heart failure secondary to dilated cardiomyopathy showed there was a significant difference in NT-proBNP levels following 1 week of medical heart failure therapy despite a non-significant change in echo findings. Reference Narin, Hekimoglu and Baykan46 Another small study which used tissue doppler echocardiogram imaging in children with cardiomyopathy secondary to ectopic atrial tachycardia concluded that tissue doppler imaging correlates better to elevated NT-proBNP levels than standard 2D echocardiogram parameters in terms of identifying early diastolic dysfunction. Reference Ge, Li, Tang, Zhang, Liu and Li47 This highlights the potential role of brain naturetic peptide/NT-proBNP in identifying myocardial changes and function before evident on 2D-echocardiography and the importance of larger scale research in this area.
Atrial naturetic peptide
Atrial naturetic peptide/MR-proANP has shown similar results in paediatric heart failure diagnosis and prognosis as brain naturetic peptide/NT-proBNP. Atrial naturetic peptide appears to be significantly elevated in patients with heart failure compared to healthy controls, and increased in a stepwise manner to reflect increasing Ross or NYHA class of heart failure. Studies show that as patients received treatment and their NYHA score improved, their atrial naturetic peptide assay level reduced in keeping with similar studies focusing on brain naturetic peptide. Reference Yeh, Hsu, Dai, Liou, Chen and Wu48–Reference Kader51 Atrial naturetic peptide values were found to be highest amongst children with cardiomyopathy compared to CHD. Reference Kotby, Taman, Sedky, Habeeb, El-Hadidi and Yosseif50 When atrial naturetic peptide and brain naturetic peptide are compared directly in assessment of paediatric heart failure, the changes noted in brain naturetic peptide/NT-proBNP are reflected by atrial naturetic peptide/MR-proANP. Reference Hauser, Demyanets and Rusai21,Reference Ohuchi, Takasugi and Ohashi23,Reference Suda, Matsumura and Matsumoto52–Reference Holmgren, Westerlind, Lundberg and Wahlander54 This supports the belief that naturetic peptides have a role in paediatric heart failure diagnosis and monitoring but that brain naturetic peptide/NT-proBNP and atrial naturetic peptide/MR-proANP perfom similarly and there is therefore little merit in pursuing both as a biomarker of heart failure.
Troponin
Troponins are cardiac-specific proteins which make up thin filaments, which along with thick filaments, form the sarcomere which is the contractile apparatus of cardiomyocytes. Reference Hammarsten, Mair, Möckel, Lindahl and Jaffe55 When cardiomyocytes are damaged, troponin is released into the blood stream and can be detected almost immediately following an ischaemic or hypoxic event. Reference Torre and Jarolim56 Transient levels are also detected in chronic conditions which are understood to be secondary to necrosis. Chronic kidney disease is associated with increased serum troponin levels in the adult population due to reduced renal clearance and continuous myocardial damage from exposure to uraemic and other renally cleared toxins. Reference Chesnaye, Szummer and Bárány57
Troponin I and troponin T are established highly sensitive biomarkers of acute myocardial injury and are used widely in the diagnosis and monitoring of acute coronary syndrome in the adult population. Reference Torre and Jarolim56,Reference Kontos and Turlington58 Troponin I and troponin T perform similarly in adult studies assessing acute myocardial events Reference Welsh59 and are both used in the paediatric studies in this review.
In paediatric patients, ischaemic cardiac disease is rare and caused by inflammatory and structural abnormalities rather than atherosclerosis of the coronary arteries. Therefore, troponin has a role in aiding diagnosis of suspected inflammatory conditions, e.g. pericarditis or myocarditis, but the role in heart failure is less clear. Although troponin is not recommended as a biomarker for diagnosis of heart failure in adults in NICE guidelines, studies in adults suggest that increased troponin T levels are associated with greater risk of developing heart failure. Models using both BNP and troponin T are better at predicting future adverse events than BNP alone as they can identify evidence of myocyte damage in addition to myocyte stretch. Reference Torre and Jarolim56
Bohn et al performed troponin T measurements using the Roche assay on 598 serum samples from healthy children aged 0–19 years. They found that troponin T levels initially increased from 0 to 6 months of age and then decline steadily after 1 year old until adulthood. Reference Bohn, Higgins, Kavsak, Hoffman and Adeli60 The gender-specific 99th percentiles from 1 to <19 years were 14 ng/L for males and 11 ng/L for females. They proposed reference ranges for healthy children without structural cardiac disease based on these results (see Table 2). Reference Bohn, Higgins, Kavsak, Hoffman and Adeli60
Table 2. Proposed 99th percentile upper limit cut-off for normal ranges in healthy children for troponin using Abbott and Roche assays
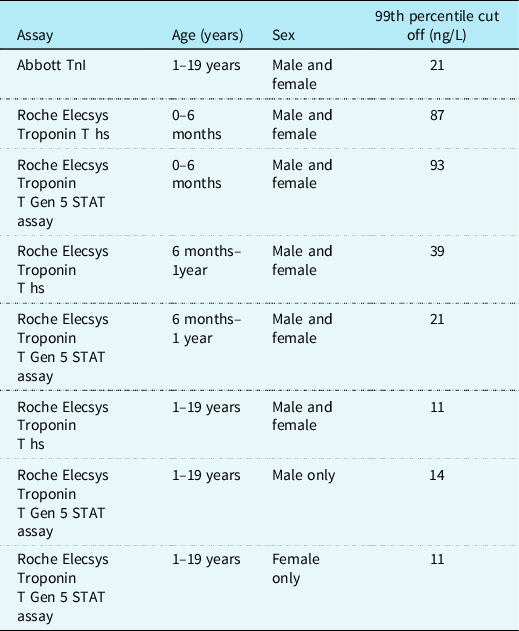
It appears that children with CHD have a higher baseline troponin level than healthy children regardless of heart failure status. Reference Uner, Doğan, Ay and Acar61 Studies of children with heart failure secondary to biventricular physiology have found a correlation between troponin levels and clinical and/or echocardiogram evidence of heart failure, yet when compared to BNP, troponin remains diagnostically and prognostically inferior. Reference Salem, Saleh, Soliman and Abo-Haded25,Reference Zhou, Zhou and Tian62 A small study of single ventricle patients which compared those with clinical evidence of heart failure to those without found that cTnI was unable to distinguish clinical heart failure in this cohort. Reference Shah, Feraco, Harmon, Tacy, Fineman and Bernstein31
Studies have also focused on troponin in children with evidence of pressure or volume overload. They describe that a rise in troponin levels is evident before changes on conventional or 2D-STE derived strain echocardiogram Reference Sugimoto, Ota, Kajihama, Nakau, Manabe and Kajino63,Reference Kotby, Abd Al Aziz, Husseiny and Al-Fahham64 and that troponin levels were more elevated in the children with pressure overload syndromes compared to volume overload. Reference Eerola, Jokinen, Savukoski, Pettersson, Poutanen and Pihkala65 Whilst this suggests a potential role for troponin in identifying myocardial damage before evident on echocardiogram, it isn't clear if this has clinical impact.
Troponins have been measured in the perioperative period to assess short and long-term outcomes for children with CHD who undergo surgery. Studies suggest that preoperative troponin levels can be used as an adverse outcome indicator, Reference Mori, Nakashima, Kaneko, Inoue and Murakami26 and patients with higher troponin levels are at an increased risk of adverse outcomes in the immediate postoperative period. Serial monitoring of postoperative troponin levels found that troponin levels correlated to degree of myocardial damage and were significantly higher in the immediate postoperative period, and in patients requiring increased levels of respiratory and inotropic support. Reference Immer, Stocker and Seiler66–Reference Hammer, Loeff and Reichenspurner71 It may be that a degree of this troponin rise is because of surgical manipulation on the myocardium.
Similarly, the available studies were unable to find a correlation between immediate postoperative troponin levels for children with CHD and long-term outcomes. This would suggest a degree of myocardial remodelling in children, and that even patients with severe ischaemic injury may recover some function if they survive the immediate postoperative period. Reference Pérez-Navero, Merino-Cejas and Ibarra de la Rosa72–Reference Bottio, Vida, Padalino, Gerosa and Stellin74
MR-proADM
Adrenomedullin is a peptide hormone secreted by endothelial and vascular smooth muscle cells. There are adrenomedullin receptor and binding sites throughout the body, but they are particularly concentrated in the heart and lungs. MR pro-ADM is the stable inactive prohormone which is enzymatically converted to the biologically active adrenomedullin-amide and mid-regional pro-adrenomedullin (MR-proAM). Reference Nishikimi and Nakagawa75
Adrenomedullin’s roles involve vasodilation and maintenance of endothelial integrity to reduce vascular leakage. Studies demonstrate high levels of circulating levels of adrenomedullin in adult patients with volume overload secondary to sepsis and acute heart failure. There are currently small clinical trials studying the effect of adrenomedullin as a novel therapy in adult congestive heart failure using Adrecizumab a humanised, monoclonal, non-neutralizing antibody against the N-terminus of adrenomedullin. Reference Holmgren, Westerlind, Lundberg and Wahlander54 There is promising evidence that it may improve haemodynamic and hormonal outcomes for adult patients with volume overload.
There are few paediatric studies which study the role of MR pro-ADM in diagnosis and prognosis of heart failure. It appears to have a limited role, performing less well than NT pro-BNP or MR-proANP in children with heart failure secondary to CHD or cardiomyopathy. Reference Hauser, Demyanets and Rusai21 However, one small study of 53 patients found it was significantly raised in children with failing Fontan circulation, correlated to NYHA score, and was more reliable than NT pro-BNP in identifying failing Fontan circulation. Reference Kaiser, Abdul-Khaliq, Wilkens, Herrmann and Raedle-Hurst76 MR pro-ADM may have a role in monitoring heart failure in the Fontan population given venous return relies on peripheral muscle pump function in this cohort.
MR pro-ADM has also been studied in the perioperative period. One small study, focusing primarily on vasoactive inotropic scores following cardiac surgery, found that including postop MR pro-ADM levels did not aid predictive value to their score. Reference Pérez-Navero, Merino-Cejas and Ibarra de la Rosa72 However, other studies concluded that both pre and postoperative MR pro-ADM levels correlated to length of mechanical ventilation and PICU stay. Reference Pérez-Navero, de la Torre-Aguilar and Ibarra de la Rosa77,Reference Szekely, Vijay, Sharp, Bando and Brown78 One study of postoperative MR pro-ADM levels suggested a cut-off value of MR pro-ADM of 1.223nmol/L may indicate higher likelihood of mechanical ventilation requirement post operatively. Reference Bobillo-Perez, Jordan, Corniero, Balaguer, Sole-Ribalta, Esteban, Esteban, Cambra and Passino79
sST2
Soluble suppression of tumorigenicity-2 is a circulating form of suppression of tumorigenicity-2glycoprotein. ST2L is a receptor for interleukin-33 and the interaction of interleukin-33 and ST2L is cardioprotective by reducing fibrosis and cardiomyocyte hypertrophy in experimental models. When soluble suppression of tumorigenicity-2 is released in response to cardiomyocyte stretch, it binds with interleukin-33, ST2L is blocked and the interleukin-33/suppression of tumorigenicity-2 system disrupted, resulting in elimination of the cardioprotective effects and associated inflammation. Elevated soluble suppression of tumorigenicity-2 serum levels are associated with increased heart failure and mortality in adult patients with heart disease. Reference Bi, Garg and Yates80–Reference Weinberg, Shimpo, Hurwitz, Tominaga, Rouleau and Lee83
There are a limited number of studies focusing primarily on paediatric heart failure and serum levels of Soluble suppression of tumorigenicity-2 and not all results are consistent.
In a small study comparing biomarker levels in children and adults pre and post ventricular assist device insertion, it was found that Soluble suppression of tumorigenicity-2 levels were significantly higher in children with heart failure than healthy children with no underlying CHD. They also found that soluble suppression of tumorigenicity-2 levels were higher in children compared to adults at all timepoints. Reference Ragusa, Prontera and Di Molfetta84 However, a prospective study of participants with heart failure secondary to CHD and cardiomyopathy which compared to healthy controls found that soluble suppression of tumorigenicity-2 was unable to show a significant difference between patients with heart failure and healthy controls. Reference Hauser, Demyanets and Rusai21
A longitudinal study of patients with Fontan circulation found that higher baseline soluble suppression of tumorigenicity-2 levels were associated with increased likelihood of adverse events, but no cut-off values were proposed. Reference van den Bosch, Bossers and Kamphuis36 Ghelani et al found suppression of tumorigenicity-2 negatively correlated to ejection fraction in clinically stable Fontan patients, but it was not statistically significant and performed worse than NT-proBNP and troponin. Reference Ghelani, Opotowsky and Harrild35
Soluble suppression of tumorigenicity-2 may have a role in diagnosis and monitoring of paediatric heart failure, but given the difference in pathophysiology compared to adults, this may be limited and warrants further studies before routine use.
Galectin-3
Galectin-3 is a member of the galectins which are evolutionarily conserved proteins with the ability to bind β-galactosides through characteristic carbohydrate-recognition domains. Reference Amin, Amin and Wijaya85,Reference Díaz-Alvarez and Ortega86 Galectin-3 is highly expressed in myeloid, epithelial, endothelial cells, and fibroblasts. It can be located both intracellularly in the cytoplasm, nucleus, and membranes and in the extracellular matrix.
Galectin-3 is a multifunctional protein which affects cell growth, differentiation, apoptosis, angiogenesis, inflammation, tumour growth, and fibrogenesis. It is widely distributed in tissues throughout the human body and although its baseline expression levels vary between organs, it is inducible by cardiac injury. Because of its expression in activated macrophages and damaged cardiomyocytes, it induces fibroblast proliferation and collagen deposition which eventually leads to reduced cardiac function. Reference Suthahar, Meijers, Silljé, Ho, Liu and de Boer87,Reference Zhong, Qian, Chen and Song88
Studies of children with heart failure suggest that galectin-3 levels are positively correlated to both clinical signs of heart failure and ejection fraction on echocardiogram. One study of children experiencing congestive heart failure secondary to left to right shunt physiology, highlighted that there was positive correlation between Ross score and galectin-3 levels. Galactin-3 showed a better diagnostic value than Ross heart failure classification score in early diagnosis of heart failure in this cohort. Reference Saleh, Khattab, Rizk, Salem and Abo-Haded89 Similarly, in a cross-sectional study of children and adults with heart failure, NYHA score was positively correlated with galectin-3 levels, and galectin-3 was negatively correlated to ejection fraction and positively correlated with NT-proBNP. Reference Gocer, Günday and Ünal90
However, galectin-3’s role in determining long-term prognosis is uncertain as some studies did not find significant correlation between baseline galectin-3 levels and long-term outcomes and mortality. Reference van den Bosch, Bossers and Kamphuis36,Reference Saleh, Khattab, Rizk, Salem and Abo-Haded89,Reference Frogoudaki, Pantelakis and Bistola91 Similarly, a longitudinal multicentre study conducted of 133 patients post Fontan found that whilst NT-proBNP and sST2 correlated with patient outcomes, galectin-3 did not. Reference Lipshultz, Law and Asante-Korang6 This is contraindicated by a study of adult Fontan patients which concluded that galectin-3 is elevated in Fontan patients compared to healthy controls, and that there is an independent association between elevated galectin-3 and adverse outcomes, but they did not directly compare to NT-proBNP levels. Reference Opotowsky, Baraona and Owumi92
Galectin-3 may have a role in monitoring response to medical management of heart failure. One study noted that galectin-3 levels for children with heart failure were lower in those treated with spironolactone, presumably because of improved cardiac function. A study of adults and children with heart failure suggested that adults with lower galectin-3 levels treated with statins had a better response to those with higher levels and galectin-3 could be used to better guide medical management in early heart failure. Reference Gocer, Günday and Ünal90,Reference Kotby, Youssef, Elmaraghy and El Sharkawy93
The current evidence suggests that galectin-3 may have a role in diagnosis and monitoring of heart failure in children and could guide medical management. It may be superior to sST2 despite both being associated with myocardial fibrosis. Reference Bi, Garg and Yates80 However, further large studies which compare galectin-3 levels and NT-proBNP in established paediatric heart failure are needed to consider if it has any role above that established by NT-proBNP.
Growth differentiation factor-15
Growth differentiation factor-15, also known as macrophage inhibitory cytokine, is a member of the growth factor beta cytokine superfamily. In normal physiological state, it is expressed in low amounts in many tissues except for placental and prostate tissue in which it is found in higher concentrations. In heart tissue, it is found to rapidly increase with inflammation and ischaemia. It is also noted to increase with advancing age which is likely due to oxidative stress. High levels of circulating growth differentiation factor-15 have been associated with cardiovascular disease, cancer, renal dysfunction, and diabetes mellitus. Reference Arkoumani, Papadopoulou-Marketou, Nicolaides, Kanaka-Gantenbein, Tentolouris and Papassotiriou94 Elevated growth differentiation factor-15 has been associated with children experiencing failure to thrive Reference Wang, Liu, McDonald and Lupino95 and is significantly higher in children with failure to thrive and CHD compared to CHD alone. Reference Paneitz, Zhou and Yanek96
In adult heart failure studies, growth differentiation factor-15 levels correlate to NYHA class and can differentiate between clinical heart failure staging. This is regardless of whether ejection fraction is reduced or preserved which may suggest a role above that of NT-proBNP which tends to be lower in patients with preserved EF compared to reduced EF in heart failure. Reference Wollert, Kempf and Wallentin97
A study of young adult Fontan patients which assessed growth differentiation factor-15 levels in relation to NYHA score, echo findings and NT-proBNP levels concluded that growth differentiation factor-15 was more useful than NT-proBNP in this cohort at identifying reduced ejection fraction and deteriorating NYHA class. They found that NT-proBNP was only weakly correlated to ejection fraction and that neither NT-proBNP nor growth differentiation factor-15 were significantly related to diastolic dysfunction measures. They have suggested a growth differentiation factor-15 level of 613 pg/ml to predict EF < 50% with a sensitivity of 90% and specificity of 85.7%. Reference Raedle-Hurst, Koenigstein, Gruenhage, Raedle, Herrmann and Abdul-Khaliq98 This was supported by a similar study focusing on growth differentiation factor-15 levels in adult CHD patients with evidence of heart failure. They found growth differentiation factor-15 levels correlated to NT-proBNP levels, lower exercise tolerance and increasing NYHA class. They commented that participants with single ventricle physiology had higher growth differentiation factor-15 levels than those with biventricular physiology. Reference Norozi, Buchhorn and Yasin99
In children with heart failure secondary to biventricular CHD physiology, growth differentiation factor-15 levels were increased with increasing Ross classification and severity of heart failure. Elevated growth differentiation factor-15 levels can be used to accurately identify participants at highest risk of adverse cardiac events or death. Reference van den Bosch, Bossers and Kamphuis36,Reference Zhou, Zhang, Zhang, Zhou, Zhou and Zhang100,Reference Khayal, Nemr, Serogy and Zoair101 Growth differentiation factor-15 levels correlated positively with NT-proBNP levels, and one study highlighted that when used in combination with NT-proBNP and hsTnT it could be used to identify those participants at highest risk of adverse events compared to using NT-proBNP alone. Reference Baggen, van den Bosch and Eindhoven102
However, as only some of the studies directly compared diagnostic accuracy of NT-proBNP and growth differentiation factor-15 in paediatric heart failure, it would appear that NT-proBNP remains more sensitive and specific for those with heart failure and biventricular physiology. Reference Hauser, Demyanets and Rusai21
Conclusion
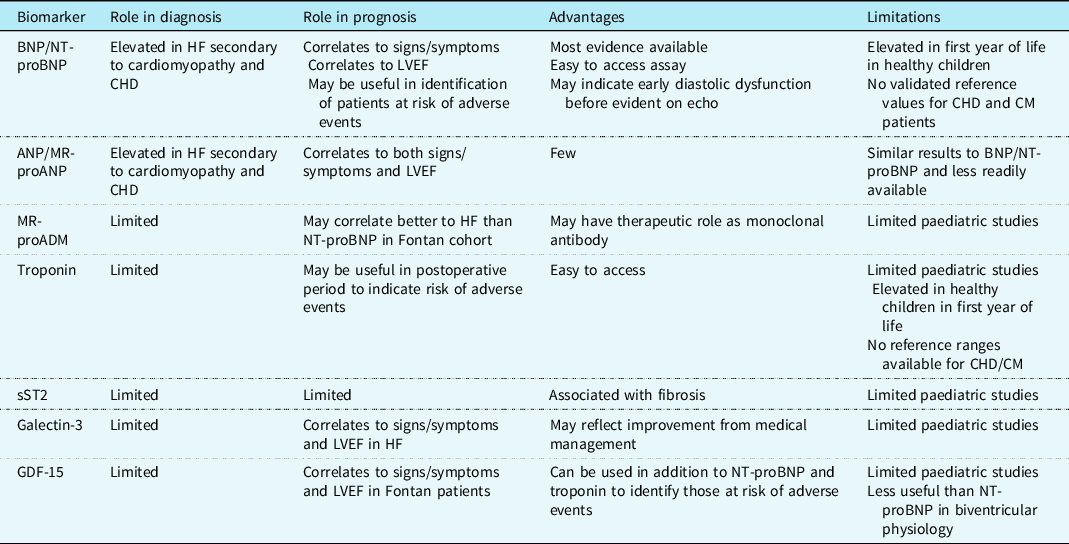
Paediatric heart failure is a complex physiological state which is influenced by underlying cardiac condition, age of patient, and surgical and/or medical management. The current literature has not identified one sole biomarker which is useful in diagnosing and monitoring heart failure in all affected children (see Table 3). There is evidence that NT-proBNP correlates closely to echocardiogram findings and functional status for children with biventricular physiology. Yet, the literature has described a range of diagnostic NT-proBNP cut-off values in this group, so it would require caution for use as a screening tool. It appears to be less reliable in cardiomyopathy and single ventricle physiology and therefore larger longitudinal paediatric studies are required to incorporate these patient groups and determine if biomarkers which reflect changes in systolic function and pressure overload are more suitable than NT-proBNP.
Table 3. Summary of cardiac biomarkers and their current role in paediatric heart failure assessment
Acknowledgements
None.
Financial support
Dr Claire McGinn is funded in her current PhD fellowship through the Royal Belfast Hospital for Sick Children (RBHSC) charitable funds.
Conflicts of interest
None.









