Published online by Cambridge University Press: 01 January 2024
Feroxyhyte (δ′-FeOOH) is a relatively uncommon Fe oxide mineral and one of the few phases in the system Fe2O3-H2O for which thermodynamic properties are not known. In natural occurrences, it is always fine-grained, although samples with larger particle sizes and better crystallinity (labeled as δ-FeOOH) can be prepared in the laboratory. This contribution presents a thermochemical study on a series of feroxyhyte samples. One is fine-grained and poorly crystalline, similar to natural materials, while the other three are of better crystallinity. The enthalpy of formation of feroxyhyte at 298.15 K is −547.4±1.3kJ mol−1 for the poorly crystalline sample (surface area 88 m2/g), and −550.6±1.4, −550.9±1.3, and −552.6±1.2 kJ mol−1 for the samples with better crystallinity. The entropy of feroxyhyte can be estimated only crudely, because it is influenced to a great extent by its magnetic properties, particle size, and structural disorder. The S298o$S_{298}^{\rm{o}}$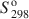 of feroxyhyte is estimated here to be 65±5 J K−1 mol−1. The Gibbs free energy of the reaction feroxyhyte → hematite + liquid water is −7.4 to −12.6 kJ mol−1 at 298.15 K. The Gibbs free energy of formation (ΔGfo${\rm{\Delta }}G_{\rm{f}}^{\rm{o}}$
of feroxyhyte is estimated here to be 65±5 J K−1 mol−1. The Gibbs free energy of the reaction feroxyhyte → hematite + liquid water is −7.4 to −12.6 kJ mol−1 at 298.15 K. The Gibbs free energy of formation (ΔGfo${\rm{\Delta }}G_{\rm{f}}^{\rm{o}}$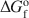 ) of the fine-grained, poorly crystalline feroxyhyte is −478.1±2.0 kJ mol−1 at 298.15 K. Since this sample is closest in its physical properties to natural feroxyhyte, this ΔGfo${\rm{\Delta }}G_{\rm{f}}^{\rm{o}}$
) of the fine-grained, poorly crystalline feroxyhyte is −478.1±2.0 kJ mol−1 at 298.15 K. Since this sample is closest in its physical properties to natural feroxyhyte, this ΔGfo${\rm{\Delta }}G_{\rm{f}}^{\rm{o}}$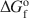 value should be used in thermodynamic modeling related to processes involving naturally occurring feroxyhyte. In terms of Gibbs free energy and enthalpy, feroxyhyte is very similar to lepidocrocite and maghemite, and, like these two phases, has no thermodynamic stability field in the system Fe2O3-H2O, except possibly at the nanoscale.
value should be used in thermodynamic modeling related to processes involving naturally occurring feroxyhyte. In terms of Gibbs free energy and enthalpy, feroxyhyte is very similar to lepidocrocite and maghemite, and, like these two phases, has no thermodynamic stability field in the system Fe2O3-H2O, except possibly at the nanoscale.
To send this article to your Kindle, first ensure no-reply@cambridge.org is added to your Approved Personal Document E-mail List under your Personal Document Settings on the Manage Your Content and Devices page of your Amazon account. Then enter the ‘name’ part of your Kindle email address below. Find out more about sending to your Kindle. Find out more about saving to your Kindle.
Note you can select to save to either the @free.kindle.com or @kindle.com variations. ‘@free.kindle.com’ emails are free but can only be saved to your device when it is connected to wi-fi. ‘@kindle.com’ emails can be delivered even when you are not connected to wi-fi, but note that service fees apply.
Find out more about the Kindle Personal Document Service.
To save this article to your Dropbox account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Dropbox account. Find out more about saving content to Dropbox.
To save this article to your Google Drive account, please select one or more formats and confirm that you agree to abide by our usage policies. If this is the first time you used this feature, you will be asked to authorise Cambridge Core to connect with your Google Drive account. Find out more about saving content to Google Drive.