Rheological measurements were used to evaluate the particle-particle associations of Na-rich montmorillonite in suspensions, under various electrolyte concentrations. A 2% free electrolyte clay suspension showed pseudoplastic flow behavior and had a high apparent viscosity, attributed at low shear rates to the high volume fraction of the suspended clay platelets, the flexibility of the platelets, and the presence of edge-to-edge association. The breaking of edge-to-edge associations and the progressive orientation of the individual platelets in the direction of flow contribute to the reduction in viscosity with increasing shear rate.
The compression of the diffuse double layer at a NaCl concentration of 10 mEq L-1 contributes to the free movement of the individual platelets, even at low shear rates. The flow behavior changed from pseudoplastic to plastic at an NaCl concentration of 100 mEq L-1. At this electrolyte concentration, face-to-face associations of specific junction points at certain areas of the planar surface are probably occurring.
The apparent viscosity of the clay suspension for the two particle-size ranges (<2 and <0.02 μm) at all shear rates converged to a minimum value of 4.5 mPa s at NaCl concentrations of 10–20 mEq L-1. On both sides of the minimum, the lower the shear rate, the greater the slope. The apparent viscosity of a 2% suspension of Na-rich montmorillonite <0.02 μm particles, however, was significantly greater than that observed for a suspension of <2 u,m particles. This high apparent viscosity is attributed to the increase in edge surface area and the number of clay particles in a unit volume.
We suggest that edge-to-edge association between Na-rich montmorillonite platelets prevails when the NaCl concentration is below the electrolyte critical concentration, for which the apparent viscosity of the suspension is at its minimum value, whereas face-to-face association prevails at NaCl concentrations above this critical value.

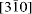 directions (orthohexagonal indexing), the four groups of the standard polytypes can be distinguished. Imaging along the [100], [110] and
directions (orthohexagonal indexing), the four groups of the standard polytypes can be distinguished. Imaging along the [100], [110] and 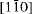 directions allows determination of the polytypes in each group. The polytypic sequences of groups A and C are intergrown at the monolayer level in cronstedtite from Lostwithiel, England, which is a new insight if compared with previous suggestions that layer stackings characteristic of different groups do not occur together. The HRTEM images also revealed the relationship between the layer polarity and the morphology of the cronstedtite crystals, where the tetrahedral sheet side points towards the top of the truncated pyramidal shape of the crystal.
directions allows determination of the polytypes in each group. The polytypic sequences of groups A and C are intergrown at the monolayer level in cronstedtite from Lostwithiel, England, which is a new insight if compared with previous suggestions that layer stackings characteristic of different groups do not occur together. The HRTEM images also revealed the relationship between the layer polarity and the morphology of the cronstedtite crystals, where the tetrahedral sheet side points towards the top of the truncated pyramidal shape of the crystal.