Major depressive disorder (MDD) and depressive episodes in the course of bipolar disorders (BD) are a major public health concern. In MDD, the remission rate in response to classical, monoaminergic antidepressants is 40–60% in clinical trials, and 20–40% in naturalistic settings. Numbers are even lower for bipolar depression. The persistence of depressive symptoms in MDD and BD have been associated with increased risk of relapse, social, and occupational impairments, cognitive deficits, and increased suicide risk.
The benefits of combining psychotherapy with classical antidepressants are a matter of debate. Meta-analyses have consistently found small benefits from combination treatment over treatment with an antidepressant or psychotherapy alone. However, it remains unclear whether these benefits are clinically relevant.Reference Craighead and Dunlop1, Reference Cuijpers, van Straten and Warmerdam2
All types of psychotherapies involve learning and behavioral change. One likely source of synergies between pharmacotherapy and psychotherapy is the neuroplasticity-enhancing effect of psychotropics that enables lasting cognitive restructuring and behavioral change. The lack of strong synergies between classical antidepressants and psychotherapy in MDD may be due to the molecular effects of classical antidepressant drugs on neuroplasticity. These drugs modulate synapses, but they do not substantially influence synaptogenesis. They also increase brain-derived neurotrophic factor (BDNF). However, for activity-dependent formation and plasticity, BDNF release has to work in concert with activation of synaptogenesis.Reference Duman, Aghajanian and Sanacora3
There has been considerable excitement about the discovery of ketamine’s antidepressant effect when given at a sub-anesthetic dose. Ketamine has a clearly different therapeutic mechanism than classical antidepressants, which is of clinical importance. For example, the onset of action is more rapid, it effectively reduces suicidal risk, and it is effective in patients who frequently do not respond to monoaminergic antidepressants, for example, patients with bipolar depression and depressed patients who also suffer from obesity.
Ketamine is an antagonist at the N-methyl-D-aspartate (NMDA) receptor. However, the therapeutic mechanisms are way more complex than this receptor blockade. Particularly, ketamine leads to fast changes in synaptic function and plasticity that go well beyond effects on neuroplasticity of classical antidepressant compounds. Ketamine increases mTORC1 signaling, synaptic number, and function. These changes persist over a week and even longer and correlate with the mitigation of depressive symptoms.Reference Duman, Aghajanian and Sanacora3
As a result, ketamine may turn out to have the capacity to considerably enhance the effects of psychotherapy in treatment-resistant unipolar and bipolar depression. In fact, such an enhancing effect has the potential to become an important clinical indication for ketamine. The transient nature of ketamine’s antidepressant effects and the potentially severe side effects of a long-term treatment with repeated applications has raised concerns about the use of this new antidepressant in clinical settings. As a result, the identification of strategies to enhance the sustainability of ketamine’s initial strong antidepressant effect has become an important research question in the field.Reference Costi, Soleimani and Glasgow4
There is preliminary evidence that cognitive behavioral therapy (CBT) extends the duration of ketamine’s antidepressant effects.Reference Krystal, Abdallah and Sanacora5
Ketamine alters activity in brain circuits related to reward and motivation.Reference Krystal, Abdallah and Sanacora5 In a clinical study on MDD and BD, patients with at least moderate anhedonia at baseline were found to be 55% more likely to respond to IV ketamine.Reference Thomas, Baker and Lind6 Moreover, ketamine’s pronounced pro-hedonic effects have been associated with ketamine’s antisuicidal effects.Reference Ballard, Wills and Lally7 These findings are interesting in the context of behavioral models of depression that emphasize low positive reinforcement as a causal factor of depressed states.
The main goal of behavioral activation, a highly effective form of psychotherapy for depression, is to increase environmental reinforcement. Subjective experience of environmental reward, rather than objective reward, mediates the relationship between avoidance and mitigation of depression. As a consequence, this approach works only if the human reward pathways are responsive and plastic. Therefore, in MDD and BP with pronounced anhedonia, the combination of ketamine and behavioral activation appears to be a highly promising approach. The behavioral change resulting from such a combination therapy may last much longer than ketamine’s transient antidepressant effects.
Several studies have demonstrated that inflammation is a predictor of poor response to CBT.Reference Lopresti8 Unspecific effects such as cognitive deficits related to memory, attention, learning, and fatigue may underlie this relationship. In addition, inflammation has specific effects on social cognition and behaviors, such as feelings of social disconnection, impaired social cognitive processing, and increased rejection sensitivity. These symptoms may have had an evolutionary survival function. However, in modern times, they can reduce the effectiveness of psychosocial interventions, particularly of interpersonal psychotherapies. Thus, ketamine has the potential to enhance the effects of interpersonal and psychodynamic therapies by its anti-inflammatory effects.
Attentional and mnemonic biases toward negative information and emotions are one of the most consistent neuropsychological findings in MDD that may even play a causal role in the development of depressive disorders.Reference Hasler, Drevets and Manji9 In our own, we found in unpublished data that ketamine reduced pessimism, which is consistent with the finding that ketamine blocks lateral habenula bursting.Reference Yang, Cui and Sang10 The combination of ketamine with cognitive bias modification therapy may prolong ketamine’s positive neuropsychological effect.
Unfortunately, we do not yet know the full amount of possible diverse effects of low-dose ketamine, as administered in MDD and BP, on cognition. However, there is consistent preclinical evidence that ketamine has a complex effect on memory, including disruption of fear memory consolidation and strengthening the formation of extinction memory.Reference Fattore, Piva and Zanda11 These data suggest that ketamine may have the potential to enhance exposure-based therapies in traumatized depressed patients and those with phobias.
Regarding neuroplastic effects, there may be important similarities between ketamine and other putative antidepressants that increase glutamate release, including serotonergic hallucinogens such as LSD and psilocybin.Reference Krystal, Abdallah and Sanacora5 At the clinical level, serotonergic hallucinogens have the potential to induce long-term changes in certain personality traits. In particular, there is preliminary evidence that psilocybin increased the Big Five personality trait Openness. This therapeutic change lasted over 1 year.Reference MacLean, Johnson and Griffiths12 Carl Rogers, the highly influential founder of humanistic psychotherapy, considered openness as a core feature of therapeutic change and the fully functionally patient: “He is able to experience all of his feelings, and is afraid of none of his feelings. He is his own sifter of evidence, but he is open to evidence from all sources…because of the awareness of himself which flows freely in and through his experience, he is a fully functioning person.”Reference Rogers13 If ketamine has similar effects on openness as hallucinogens, it may have the potential to become an important enhancer of humanistic psychotherapies that are popular among therapists and patients in naturalistic settings.
Recently, we demonstrated that ketamine can change body feelings and induce subjective lightness and floating,Reference Stocker, Hasler and Hartmann14 which suggests a link between ketamine, body therapy, and mindfulness-based psychotherapies.
Depression is frequently associated with social anhedonia, increased rejection sensitivity, impaired social communication, and lack of empathy.Reference Kupferberg, Bicks and Hasler15 In our clinical experience, ketamine rapidly improved social functioning in depressed patients. As a consequence, it facilitated to build up a therapeutic relationship, which is the basis of almost all psychotherapies.
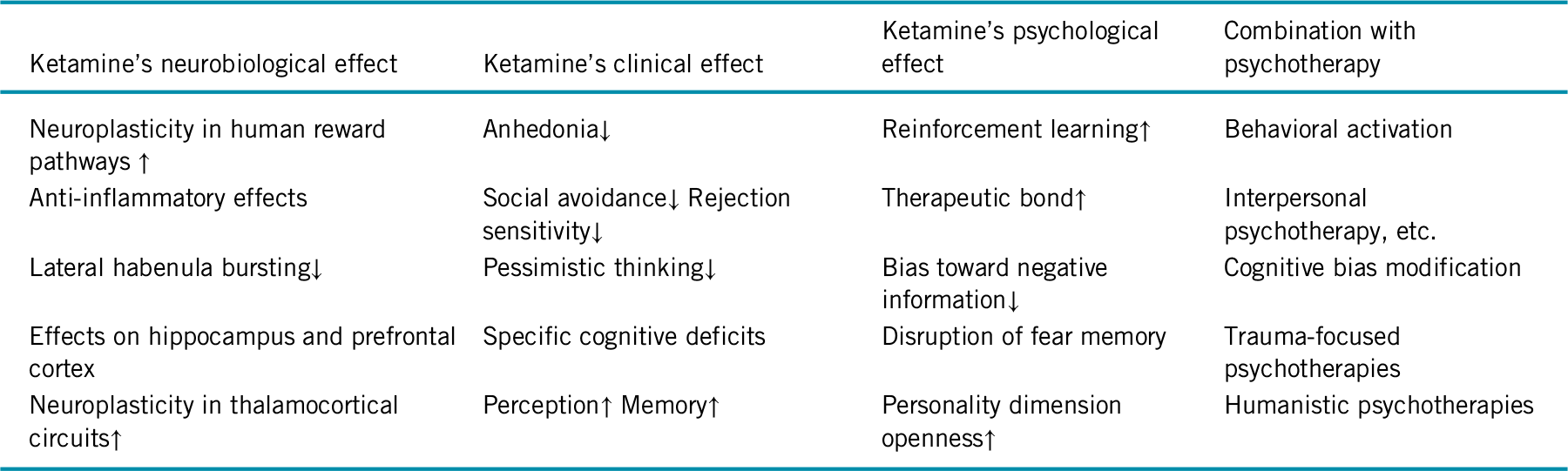
In sum, the transient nature of ketamine’s antidepressant effects in concert with growing evidence on its neuroplasticity-related therapeutic mechanisms encourages studies on psychosocial interventions to prolong its efficacy. Table 1 summarizes the related hypotheses described in this editorial. Research on ketamine’s effect on cognition will base such efforts on empirically driven hypotheses.
TABLE 1. Hypotheses on specific ways to combine ketamine and psychotherapy
Disclosures
Gregor Hasler has received support from Swiss National Foundation, University of Bern, Switzerland, and University of Fribourg, Switzerland.


