Policy Significance Statement
Neutralizing monoclonal antibodies (nMAbs) were an important part of the coronavirus disease-2019 (COVID-19) pandemic response. Delivering nMAbs required health systems to create new care pathways, capture information for reaching and treating patients most at-risk for severe disease, and leading partnerships for sharing scarce resources and best practices. Our work summarizes certain challenges that health systems encountered while treating patients with nMAbs to help health system stakeholders that are seeking to understand the real world safety and effectiveness of nMAbs and prepare for future pandemics. We also intend to inform policymakers seeking to apply such learnings to further prioritize patients for novel treatments, data infrastructure to facilitate the integration of clinical research with clinical care, and governance for decision-making.
1. Introduction
The coronavirus disease-2019 (COVID-19) pandemic compelled the rapid development of medical countermeasures (MCMs) to reduce the incidence of infection, severe illness, and death. Anti-spike neutralizing monoclonal antibodies (nMAbs) are MCMs that received emergency use authorizations (EUAs) from the US Food and Drug Administration for treatment of mild-to-moderate COVID-19 in nonhospitalized patients (FDA, 2021a, b, 2022a, b, c). Despite the potential benefits of treatment, the deployment of these novel MCMs at scale presented operational and logistical challenges for healthcare organizations (Goldstein and Walensky, Reference Goldstein and Walensky2020).
In this study, we present lessons learned, challenges, and promising practices from implementing nMAb treatment programs from four health systems. Although the four participating health systems varied in patient populations, clinical practices and workflows, policies and procedures, and other critical operational factors, there were similarities in lessons learned to enable their key decisions and quick pivots to improve care during the evolving pandemic. Lessons are summarized under three key themes developed from the data collected that serve as critical building blocks for health systems’ ability to deploy novel therapeutics: (1) clinical workflows, (2) data infrastructure and platforms, and (3) governance and policy. The critical building blocks comprise a holistic approach to delivery of nMAbs. While there are, as of June 2024, no nMAbs authorized or approved for intended use to treat COVID-19, these building blocks can facilitate future pandemic and public emergency response efforts and also inform rapid regulatory and policy decision-making and implementation related to MCMs to treat other disease conditions.
2. Methods
Health systems participating in this qualitative study were contributors to a real-world evidence (RWE) observational study launched by the US Department of Health and Human Services Administration for Strategic Preparedness and Response, in partnership with the Health Federally Funded Research and Development Center operated by The MITRE Corporation. Oversight for this study was provided by the institutional review board of The MITRE Corporation. In conjunction with the quantitative aim of the study to assess the treatment effectiveness and safety of nMAbs, qualitative assessments were conducted to gather data regarding the lessons learned, challenges, and promising practices resulting from health system implementation of nMAb programs (see Supplemental Information). The entire period of this qualitative assessment, including developing a structured questionnaire and interview instruments, conducting the assessments, and finalizing the results, was from October 2021 to June 2022. The questionnaires and interviews were completed from December 2021 to February 2022. The questionnaire included open-ended questions, which allowed flexibility for the respondents to detail important insights. Questions were segmented into four categories that aimed to explore nMAb treatment program operations: patient risk stratification, clinical workflows, data infrastructure and platforms, and governance and policy. From late 2021 through early 2022, biweekly discussions between the health systems, held as part of the larger RWE observational study, were used for shaping the content and segmentation of the questions.
The questionnaire was distributed via email to health system Principal Investigators of the RWE study and/or their delegates for completion. Respondents were given the option to complete the questionnaire in a Word document or through Qualtrics, an online survey platform. Follow-up interviews were conducted via a video conference platform with each questionnaire respondent to gather additional context around their responses. The interview team followed a scripted guide and consisted of a primary interviewer, secondary interviewer, and a note taker. Each interview was recorded, with consent being obtained prior to conducting the interview.
Inductive coding methods were applied using grounded theory approach, an inductive coding approach to describing phenomena, to qualitatively assess and categorize questionnaire and interview data (Chun Tie et al., Reference Chun Tie, Birks and Francis2019). To extract key lessons learned based on qualitative findings, one study team member led the initial qualitative coding of the participants’ questionnaire and interview responses, followed by independent qualitative coding by a second study team member for a comparison of data interpretations. A common codebook was developed and then used by each team member for independently coding data, which was followed by team deliberation to ensure consistent coding across responses. Any conflicts surrounding interpretation of the data were adjudicated by a third team member until >95% agreement was reached. Health system and health system representative names were anonymized for privacy purposes.
3. Results
The four health systems were geographically located in the Midwest, South Central, Mountain, and Western regions of the United States. Respondents and/or respondent teams, all of whom were practicing clinicians, from three health systems initially completed the questionnaire, as one health system was unable to complete the questionnaire in the desired time window. Subsequently, all four health systems participated in individual interviews, allowing for comparative information to be collected from all of them, with one to two representatives participating from each health system. Responses to the questionnaire were obtained during the interview process from the one health system that was earlier unable to complete it, thereby completing the full data gathering process. The average duration of the interviews was 40 minutes and interviews ranged in recording time from 22 to 52 min. Table 1 contains a full summary of key lessons learned, based on the qualitative findings reported below.
Table 1. Lessons learned based on key qualitative findings
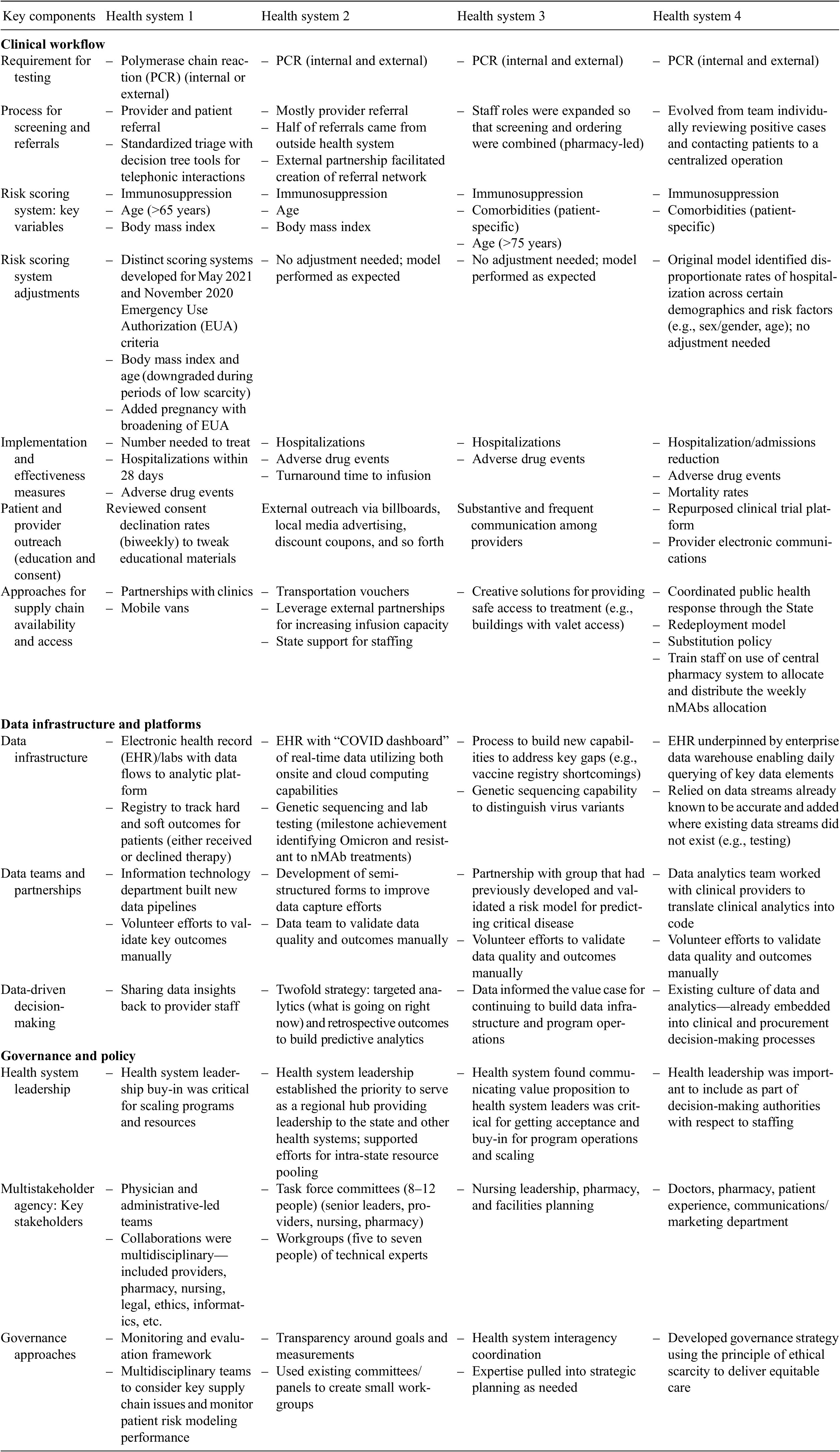
Results cover the following thematic areas identified during interviews: clinical workflows, data infrastructure and platforms, and governance and policy.
4. Clinical workflows
Establishing efficient clinical workflows was critical to establish the novel care pathway for delivering nMAb infusion programs. Workflows across health systems consisted of the following key steps: testing and referral processes, patient stratification, protocol and guideline development, education and consent, and delivering nMAb therapies. While these steps occurred in the same sequence across health systems, they differed in how they were implemented. Lessons learned focused on the innovations developed for implementing these pathways, along with the infrastructure and staffing models to address both logistical and regulatory challenges in providing care for patients with a highly contagious disease.
4.1. Testing
All four health systems required documentation of a positive COVID-19 diagnostic test prior to receiving nMAb treatment, consistent with the criteria in the nMAbs’ EUAs. Three of the four health systems would repeat COVID-19 laboratory diagnostic testing for all referred patients unable to provide satisfactory documentation of their diagnostic test results, and for most of the reporting period, this confirmatory testing was a polymerase chain reaction (PCR) test. One of the health systems performed confirmatory PCR diagnostic tests on all referred patients. One health system developed a comprehensive user-friendly approach for facilitating prospective nMAb patients’ fulfillment of requisite criteria for receipt of nMAbs. This health system utilized four entry points: a testing site, an urgent-care clinic, an emergency room, and completion of an online survey followed by accessing a self-collection site, in order to collect demographic, symptom, and medical history information, as well as to provide COVID-19 diagnostic testing, if needed. Each entry point was accessible to patients who did not belong to the health system but who sought access to nMAb therapies. Patients could also fax positive test results to administrative hubs serving as a coordination point for referrals. When tested within a health system’s network, patients with a qualifying COVID-19 diagnostic test result were automatically screened for nMAb eligibility using patient information in the electronic health records (EHRs) for treatment referral. Each eligible case was reviewed by healthcare team members, with an automated pull of the patient risk profile—via a screening scoring system—which reduced the burden for both internal and external referrals.
4.2. Referrals
Each health system identified the referral process as one of the primary barriers to the efficient delivery of nMAb therapies, given the multitude of ways patients were identified for or requested nMAb therapy. Referrals came for patients both within and outside the health systems. From the early onset of the pandemic, centralized tools and decision trees were used to help triage patient referrals and outside requests, ensuring that access to nMAbs was equitable for all patients. These central scheduling hubs had dedicated clinical and administrative staff. Clinical staff supported the reviews for incoming referrals, which included reviewing medical records to identify and prioritize patients based on their risk profiling strategies. Administrative staff received and processed referrals and queued potentially eligible patients. Multidisciplinary teams, including clinicians, reviewed referrals, particularly when demand for nMAb therapies exceeded supply. These teams helped to consistently and equitably apply risk criteria, from developed stratification strategies, to identify patients for treatment when nMAbs were scarce (Sakata et al., Reference Sakata, Brunisholz, Andersen, Davie, Srivastava and Webb2022).
4.3. Patient risk stratification
Given the broad eligibility criteria included in the EUA, limited supply of nMAb treatments during some periods of the pandemic when demand was high, and limited clinical evidence on which patients may benefit the most from nMAb treatments, there was a need for health systems to develop strategies to prioritize which referred patients from within and outside the health system should be treated. The necessity of patient prioritization for nMAb treatments was particularly urgent during the Delta- and Omicron-driven increases in COVID-19 case counts.
To handle the changing supply and demand for nMAbs, health systems in this study developed internal strategies for risk profiling (Chow et al., Reference Chow, Glavis-Bloom and Soun2020; Berry et al., Reference Berry, Liebl, Pauline and Brownewell2021; Razonable et al., Reference Razonable, Ganesh and Bierle2022; Webb et al., Reference Webb, Levin and Grisel2022). Although the approaches slightly varied in how patients were prioritized, all health systems stated that risk stratification strategies were critical. Risk scoring allowed health systems to identify subpopulations within the nMAb-eligible patients who were considered at higher risk for severe COVID-19. For one health system, its state’s Standard of Care Committee determined that nMAbs were scarce and applied scarcity ethics to determine the fairest way to target patients most likely to clinically benefit from nMAbs. Therefore, a lottery system to determine who received treatment was not allowed and the health system utilized internal data to develop a patient risk score.
When distribution constraints intensified—most often due to the emergence of a treatment-resistant variant that narrowed the field of effective nMAb treatments—health systems adjusted the risk score cutoffs and thresholds to select from the eligible population a subset of patients to match the available supply of nMAbs. One health system mentioned downgrading weights on certain variables when patient prioritization was not as critical, as case counts lowered and there was less demand for nMAb treatment. However, amid the Delta (summer and fall 2021) and Omicron (winter 2021–2022) surges, the weights of those variables were re-upgraded to make the risk scoring system more selective. Generally, it was found that the most useful risk stratification strategies allowed for thresholds or variable weights to be adjusted to match the available supply when the supply–demand balance changed and the mismatch became more acute.
In implementing scoring systems, a few key variables were identified as being critical for matching those who would benefit the most from nMAb treatment and included immunosuppression, age (typically over 65 years), and body mass index (Chow et al., Reference Chow, Glavis-Bloom and Soun2020Berry et al., Reference Berry, Liebl, Pauline and Brownewell2021; ; Razonable et al., Reference Razonable, Ganesh and Bierle2022; Webb et al., Reference Webb, Levin and Grisel2022). Health systems monitored and evaluated the effectiveness of their risk profiling strategies using continuous feedback cycles of information to support better planning and improve program success. Assessment of risk profiling strategies was carried out with real-time reviews of clinical outcomes and data-driven reports that monitored metrics such as hospitalizations and intensive care unit admissions. Adverse drug event reporting was another outcome tracked. With nMAbs authorized under emergency use, health systems felt the need to assess the emerging safety profile. However, adverse events (e.g., allergic or nonallergic infusion-related reactions) were found to be a less reliable indicator due to data lag from infusion centers, variable data quality, and difficulty correlating exposure to outcome.
4.4. Education and consent
Another key component of the process was improving awareness of newly established nMAb programs. To increase awareness among providers, most health systems leveraged existing communication mechanisms, such as “All Hands” emails, or leveraged education seminars, such as “Grand Rounds,” to discuss current treatment guidelines. In addition, existing clinical trial tools were repurposed to facilitate and streamline nMAbs outreach to providers, as well as patient recruitment, education, and consent. To raise patient awareness, community-based outreach efforts included media campaigns (e.g., newspaper, television, radio, or billboard advertisements) to promote the availability of nMAbs as a treatment option.
Although these education efforts facilitated dialogue between providers and patients, health systems had a consent process for patients to receive nMAb therapy. Consent rates were utilized as a metric of outreach effectiveness. Specifically, health systems monitored declination rates to determine the effectiveness of the outreach materials. When a patient declined therapy, health system protocols included immediate follow-up to determine reasons for declination and, when appropriate, use this feedback to update consent and educational materials. Because the EUA required patient education and consenting prior to infusion, the time spent for these activities allowed for concurrent confirmation of the eligibility criteria (symptom, onset of infection, risk-profile).
4.5. Innovations for delivery of nMAb treatments
Identifying the physical infrastructure for clinical workflows was an area where health systems had to make tradeoffs and develop innovative solutions. Novel ideas were needed when designing physical spaces to deliver nMAb infusions, given the varying access needs for care among diverse patient populations and the need to ensure safe conditions for healthcare providers and patients. Health systems utilized mobile clinical facilities to provide nMAb treatments to patients living in long-term care facilities, rural areas, and other locations lacking easy access to infusion sites offering nMAbs. Existing buildings and spaces were also repurposed as nMAb infusion sites, including the conversion of valet entrances unused due to pandemic restrictions, as another strategy to expand availability of safe infusion sites for nMAbs.
4.6. Staffing
Underpinning each of these aspects of clinical workflow was the ability to scale operations. A key component of scalability was having sufficient staff support to implement each workflow step of the program. Hospital systems had to accommodate rapid increases in COVID-19 patients requiring hospitalization or other care, while maintaining routine operations, in the setting of staffing shortfalls due to COVID-19 morbidity among health system personnel. As a result, there were labor shortfalls that hindered the delivery of nMAb therapies. Labor shortfalls were felt across each step of the clinical workflow. Innovative labor solutions that were developed included redeployment of nonclinical staff from areas experiencing a sudden steep decline in patient volume (e.g., outpatient dermatology) to areas where they were most needed; substitution of clinical staff from other specialties to care for COVID-19 patients (e.g., training nurses to administer nMAbs); and partnerships between health systems to float available staff to areas of high need within a region or locality.
5. Data infrastructure and platforms
Timely, accurate, and actionable information was critical for providing optimal care and reaching patients most at risk of severe disease. Information and insights, however, depended on capturing granular data related to diagnosis, treatment, and outcomes; curating that data; and turning insights into updated clinical and public health decisions. This required building a data flow that allowed for near-real-time data ingestion from EHRs, performing data cleaning and quality checks, and making transformations of these datasets into an analysis-ready state.
5.1. Developing and utilizing RWE
Adaptation of nMAb programs was contingent on decision-makers having reliable and up-to-date information. Health systems provided numerous examples of how clinical data were analyzed to inform decision-making about the management of clinical workflows. One health system used turnaround time from referral to infusion as a metric to monitor program implementation efficiency. Turnaround time was reported at least weekly to leadership and operations teams. Infusion capacity assessment required combining knowledge of physical locations, staffing, and nMAb supply to meet the needs of as many patients as possible. Another health system developed customizable data dashboards to illustrate and visualize relevant information utilizing both local and cloud computing solutions. One health system invested significant resources into genomic surveillance capabilities by adding additional sequencing equipment. Prioritizing genomic surveillance provided new capabilities to identify emerging variants in near-real time. This health system tracked Omicron prevalence and disseminated that information to leadership, which enabled evidence-based decision-making to stop nMAb treatments that were not effective against the Omicron variant.
5.2. Improving data quality for at-scale RWE generation
The availability and use of RWE necessitates a baseline level of data quality that directly corresponds to its utility for decision-making. In other words, data are actionable only to the extent it is trusted and categorized as “fit for use.” Trust hinges on understanding, with a high degree of certainty, the underlying processes used to capture and curate raw data and transform it into analysis-ready datasets for analytics.
Missing data were identified as a key issue at most participating health systems. There was often missing data when patients were referred by local healthcare providers for nMAb treatment (e.g., missing results of a positive COVID-19 test, unreliable COVID-19 vaccination data). Even within health systems, some data elements were not reliably captured. For example, the EUA for nMAbs specified that treatment be initiated within 10 days of symptom onset; however, COVID-19 symptoms and onset date were not reliability captured because these data elements did not have standard fields within EHR systems. Symptoms and other data elements had to be captured either manually or through additional data extraction and coding, which sometimes resulted in incomplete, inaccurate, or missing data.
Another mechanism to improve data quality and capture missing data were linkage between EHR systems and other real-world data (RWD) sources. Three of the four health systems linked patient data from state immunization registries to EHR data to gather information on vaccinations received outside of their network. Due to the emerging evidence on protection provided by vaccines, vaccination status was a critical piece of information as health systems attempted to understand the real-world effectiveness of nMAbs. Two health systems were tightly integrated with their state vaccination registries, with periodic, completely automated refreshes of all patient vaccination records into the EHR. One health system had the ability to perform bulk pulls of patient vaccination statuses from a state vaccination registry. One health system relied primarily on providers to document patient vaccinations, based on face-to-face encounters with patients (vaccination data captured in provider notes or possibly as structured data elements); in this instance, there was no systematic interface with a state vaccination registry. Through the quantitative RWE study, it was found that the rates of vaccination were lower in this health system’s population than in the other health systems, suggesting the possibility of a higher proportion of patients with missing vaccination information.
6. Governance and policy
Health systems reported that the COVID-19 pandemic presented a historic challenge as they faced rising numbers of COVID-19 cases, rapidly emerging treatment options, changing guidelines, and an evolving threat from new variants. Addressing these challenges required consistent leadership, as well as the sharing of scarce resources and lessons learned through participation in multi-stakeholder partnerships. Health systems also came to recognize that short-term efforts to bolster the clinical workflow, data infrastructure, and data platforms were not sufficient for long-term sustainability. Recognition from clinical leadership was to transition the care pathway into a more conventional system comparable to what was done for similar therapeutics. Creative use of staffing, space, and data systems bolstered investment in novel care pathways by ensuring outcomes were shared across teams.
6.1. Support from senior leaders
Given the competing demands for limited resources, it was important that senior leaders within each health system supported the development of nMAb programs and understood the challenges. Engagement with senior leaders also provided high visibility to the project, creating an urgency to find solutions amid the ongoing public health emergency. In developing these programs, the pilot phase was cost-intensive and did not necessarily provide an immediate return on investment. Because the return on investment might not be immediate, it is important to have health system leadership buy in to support the start-up phase.
6.2. Partnerships
Partnerships occurred within health systems and with external partners. Internally, collaborations consisting of multidisciplinary teams that included nursing and pharmacy expertise were particularly important to ensure that eligible patients were identified and received treatments quickly. Health systems leveraged existing clinical committees or new task forces that combined experts representing different perspectives including providers, pharmacy, nursing, legal/ethics, informatics/data analysts, and healthcare administration/leadership. Key outputs from these groups included developing an evidence-based strategy, an implementation plan, and a plan to monitor and evaluate the implementation program.
Partnerships with neighboring health systems and facilities were also established to broaden outreach to vulnerable populations and center equitable distributions of treatments as a key organizing principle. State and regional partnerships were essential in striving toward equitable distribution of nMAbs. One health system developed strong collaborative relationships across the state government and health system partners to pool resources, creating a statewide coalition for nMAb treatments. Health systems noted the capability to quickly deliver nMAb treatments was likely a key factor for receiving state-based allocations of nMAb therapies; fundamental to this capability was establishing key partnerships to collect, distribute, and monitor the effectiveness of delivered treatments.
6.3. Hubs for learning
As the nMAb treatment program and associated delivery platforms matured, health systems identified opportunities to provide support to their partner health systems and regions. One health system, with support from senior leadership, positioned itself as a regional hub allowing the main campus to provide services for patients while providing certain services at satellite sites. As a regional hub, there was an opportunity to share expertise, lessons learned, resources, and services with other health systems that did not have the same capabilities. While there was limited success setting up a referral network and satellite facilities, logistical capability was lacking; an important lesson was to formalize agreements ahead of time with partner organizations.
7. Discussion
Through the synthesis of experiences from four participating health systems, high-level building blocks were identified as necessary components for generating adaptable health care in response to public health emergencies and creating a holistic approach to delivery of a novel treatment. Health systems must be sufficiently agile to quickly scale programs and resources in times of uncertainty—a key example from the clinical workflow considerations was around the development, assessment, and refinement of risk stratification scoring systems. These weighted, multivariate scoring systems provided an efficient means of decision support, allowing for point-of-care discrimination of subclasses of patients more likely to benefit from treatment within a broader category of eligible patients, such as the immunocompromised. These scoring systems served the purpose of directing treatment to those at the very highest risk of severe COVID-19 and death during times of constrained supply. Another major health system, not included in this study, successfully deployed a reserve system of nMAbs for ensuring equitable distribution based on state-issued guidance for allocation due to scarcity (Rubin et al., Reference Rubin, Dryden-Peterson and Hammond2021). A broader framework for equitable delivery of nMAbs may seek to pair risk-stratification systems with other mechanisms, including a reserve system of nMAbs or centralized access platform (Leider et al., Reference Leider, Lim and DeBruin2023), for ensuring equitable distribution.
Each health system did approach their risk stratification system with different weighting of variables and mechanisms for adjusting to constraining supply of nMAbs. Further research into how these differences impact other decisions involved in steps of the clinical workflow including staffing, as well as demands on the data platforms would be helpful for health systems seeking to implement a similar approach. Additionally, the real-world outcomes and influence of social determinants of health have both been reported for the larger real-world observational study (Ambrose et al., Reference Ambrose, Amin and Anderson2023a, Reference Ambrose, Amin and Andersonb). The inequitable uptake of nMAbs is further enforced by research conducted in another large healthcare system (Wu et al., Reference Wu, Kumar and Moore2022). Any association of the effectiveness of the overall care delivery program with the outcomes and influence of social determinants of health would also be useful priors for other health systems, particularly to monitor equitable distribution. Comparison of strategies in other health systems that were less optimal may also be undertaken. Further work may also identify inequities arising from retesting patients that may have created bias in referrals, which could contribute to nMAbs not reaching populations most at-risk for severe disease.
Real-time monitoring of programs, policies, and processes helped improve understanding of how best to scale. Each health system built its capabilities from existing data and physical infrastructure, emphasizing the importance of maintaining a warm-base capability for scaling up data and physical infrastructure in response to demand. Data infrastructure and platforms across health systems were challenged by missing data. The extent of time and resources needed to address data quality issues can be mitigated by codeveloping processes and data model specifications with partners. Engaging data partners early in planning can provide better information about variable health system data standards, which in turn can better inform what must be addressed by data requirements and help set consistent expectations for data capture, sharing, and use. It is important to include interoperable data elements across EHRs to more efficiently standardize and curate data and establish privacy protections that are accepted across institutions but allow data sharing and access by the necessary analytic staff, and standard, validated, tested mechanisms for the efficient transfer and storage of data. Participating health systems recognized a national effort could spur more development and use of RWD to generate more robust RWE for public health.
A common benefit realized across health systems from implementing nMAb programs was the networking among diverse multidisciplinary teams and clinical staff. Rapidly standing up a novel care delivery program is inherently a multidisciplinary effort that requires input from a range of sources across the health system. The COVID-19 pandemic required staff to actively seek out collaborating partners and maintain frequent communications. A teamwork approach to the clinical workflows worked well within these major health systems: coordinated efforts from physicians, advance practice providers, pharmacists, nursing, and allied health staff—all had independent roles that complemented each other, reduced redundancies, and improved the work flows. If another pandemic were to occur, each health system representative noted that they would know who to contact for key resources and other inputs, and be committed to a set of governing principles to maintain and continue partnerships. A prior study has reported that many providers were not knowledgeable and unlikely to refer patients to receive nMAbs (Kwan et al., Reference Kwan, Sobczak and Beaty2022). Both the complexity of referral processes and missing knowledge on a positive test were cited as major impediments for clinicians to refer patients (Hamer et al., Reference Hamer, Alasmar, Kwan, Wynia, Ginde and DeCamp2022). Therefore, greater efforts are needed to build off the partnerships that resulted in efficient clinical workflows developed by these four health systems. Building off existing collaborative relationships can also be utilized to assess how key components of one health system’s nMAb program may interest and benefit another health system or partnering organization. Advanced agreements to align on staffing, physical space, data sharing, and other processes would facilitate scaling up future novel care delivery programs. For example, health systems reported that sufficient physical space was a unique consideration for planning infused nMAb treatment.
The most important components for replicating nMAb treatment programs in other health systems revolve around key governance considerations. The governance and policy key considerations enable implementation of key considerations for the clinical workflow and data infrastructure and platform building blocks. These include engaging senior leaders to develop the overall vision, guiding principles, and clinical workflow strategy; working with multidisciplinary teams with frequent touchpoints; and building out external partnerships in advance to lay the groundwork for a broad referral base where eligible patients can be efficiently routed to the appropriate location for treatment. In addition, developing key data infrastructure that can support data-driven processes is crucial to help inform program development and share insights to important stakeholders on the value of the high-quality data being captured and methods to incentivize collection and data storage. For future efforts, sites indicated an interest in documenting patient declination of treatment in a more discrete manner so that longer-term treatment patterns and outcomes could be monitored. There may also be room to explore governance and policy considerations in advance of the need for establishment of a novel care delivery pathway for an nMAb or other treatment program. Examination of governance and policy considerations could then facilitate future-proofing clinical workflows and data infrastructure and platforms to nimbly adapt for a future treatment program.
All health system representatives indicated a strong desire to maintain the governance mechanisms, data infrastructure, physical infrastructure, including infusion centers, and processes developed for nMAb treatment delivery for future acute medical needs, including other COVID-19 therapeutics in the development pipeline that may require rapid scale-up in the event of loss of efficacy of existing treatments due to resistant mutations in emerging variant strains or other factors. The key components established as part of the clinical workflow allowed for the creative use of staffing, space, and other resources to adjust processes based on logistical constrains and variability in demand. Data infrastructure and platform key components were needed to maintain the efficiency of the clinical workflow and allow for patients outside the health system to be treated. The governance and policy building blocks were instrumental for ensuring the impact of the nMAb treatment delivery was broadcast to all levels of the health system and ensured continued investment.
Based on the lessons learned and promising practices established during the COVID-19 pandemic, it is likely that during a future pandemic or public health emergency, nMAbs and other treatments could again be made available as MCMs, requiring rapid expansion of capacity and mobilization of resources. Many of the real-world insights provided in this article, and other documented lessons, (Lambrou et al., Reference Lambrou, Redd and Stewart2022; Kip et al., Reference Kip, McCreary and Collins2023) could be leveraged and used by other health systems, irrespective of size, geographical location, healthcare setting, or other factors. The readiness of a health system, along with regulatory and other environmental contexts around the logistical constraints and demand, could also affect the implementation and adoption of a treatment. Furthermore, health systems that have built a warm base capacity to quickly scale treatment implementation will be better positioned to conduct continuous process improvement as learnings inform better tailored responses to treatment context.
In addition, the findings presented in this study can be applied by health systems for distribution of novel therapeutics beyond nMAbs and future pandemics. The response to the COVID-19 pandemic provided a unique opportunity to assess the deployment of an nMAb, unlike with the response to prior coronavirus outbreaks, that is, severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome. Continuation and expansion of existing collaborations and infrastructure will enable continued generation of high-quality RWE on the impact—including the safety and effectiveness—of MCMs including vaccines, drugs for preventing and treating severe and chronic conditions, devices, and other products to help inform timely treatment, regulatory, and policy decision-making and implementation. High-quality RWE can facilitate the right patient receiving the right intervention at the right time (Califf, Reference Califf2023).
The findings from this qualitative assessment have far-reaching policy implications and may be considered in future policymaking efforts by both public and private industry. For example, the promising practices used for patient risk stratification could inform how health systems, state health officials, clinicians, and other stakeholders can best ensure that, despite logistical challenges, those most at risk for severe disease are targeted and treated. Insights gathered from clinical workflow establishment and enhancements can provide the basis for how to better integrate clinical research with clinical care through innovative payment models that facilitate rapid evidence generation. Governance and policy practices used by health systems can inform future memorandums of understanding for data and resource sharing. Infrastructure and data platform learnings identify the need for an interoperable, nationwide, centralized data platform that helps share information with standardization and rigor. The ability to respond to COVID-19 and future pandemics requires long-term investment in readiness to implement nMAbs and other MCMs.
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/dap.2024.77.
Data availability statement
The survey and interview script used to conduct the qualitative assessment are included in the Supplemental Information section. Requests for de-identified data should be directed to the corresponding author.
Acknowledgements
This work was performed by the mAb Real-World Evidence Collaborative. Authors are listed alphabetically by last name. The authors would like to acknowledge Mark McClellan and Morgan Romine from the Duke-Margolis Institute for Health Policy for their contributions toward the development of this article.
Author contribution
Conceptualization: A.A., B.C., N.A., R.H.S., and J.Y. Methodology: R.H.S. Data curation: A.A., B.C., and M.D. Data visualization: A.A., B.C., M.D., and R.H.S. Project administration: B.A., N.A., and J.Y. Writing original draft: A.A. and B.C. Writing reviewing and editing: All authors.
Funding statement
This study was supported wholly or in part with federal funds from the Administration for Strategic Preparedness and Response, Biomedical Advanced Research and Development Authority, under Contract Number 75FCMC18D0047, Task Order 75A50121F80012, awarded to The MITRE Corporation. The views expressed are solely those of the authors and do not necessarily represent those of the US Department of Health and Human Services.
Competing interest
Dr Amin reported receiving grants from the University of California, Irvine, during the conduct of the study; grants from NIH/NIAID, NeuroRx Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, Alexion; and personal fees from Pfizer, Salix, Alexion, AstraZeneca, Bayer, Ferring, Seres, Spero, Eli Lilly, Nova Nordisk, Gilead, Renibus, GSK, Dexcom, Reprieve, HeartRite, Aseptiscope outside the submitted work. Dr Chow reported receiving personal fees from Canon Medical Systems outside the submitted work. Dr Drews reported receiving grants from The MITRE Corporation during the conduct of the study and outside the submitted work. Dr Hendricks-Sturrup serves as an independent Director of the Board at Public Responsibility in Medicine & Research. Dr O’Horo reported receiving grants from The MITRE Corporation during the conduct of the study; grants from nference; and personal fees from Bates College outside the submitted work. Dr Razonable reported receiving grants from Gilead Sciences, Regeneron Pharmaceuticals, and Roche and personal fees from Novartis outside the submitted work. Dr Webb reported receiving grants from Gilead Sciences and Regeneron Pharmaceuticals outside the submitted work. Mark B. McClellan, MD, PhD, is an independent director on the boards of Johnson & Johnson, Cigna, Alignment Healthcare, and PrognomIQ; co-chairs the Guiding Committee for the Health Care Payment Learning and Action Network; and receives fees for serving as an advisor for Arsenal Capital Partners, Blackstone Life Sciences, and MITRE. No other disclosures were reported.




Comments
No Comments have been published for this article.