Maternal Caregiving Ameliorates the Consequences of Prenatal Maternal Psychological Distress on Child Development
Fetal life is an exceptionally rapid period of neurological development, and is a time when the fetus is highly susceptible to both beneficial and harmful environmental influences (Barker, Reference Barker1998). Prenatal maternal psychological distress is linked to profound and lasting consequences for developmental trajectories and increases risk for subsequent mental health problems (Davis et al., Reference Davis, Glynn, Schetter, Hobel, Chicz-DeMet and Sandman2007; Davis & Sandman, Reference Davis and Sandman2010, Reference Davis and Sandman2012; Demers, Aran, Glynn, & Davis, Reference Demers, Aran, Glynn, Davis, Wazana, Oberlander and Szekel2021; Glynn et al., Reference Glynn, Howland, Sandman, Davis, Phelan, Baram and Stern2018; Van den Bergh et al., Reference Van den Bergh, Van Den Heuvel, Lahti, Braeken, Rooij, Entringer, Hoyer, Roseboom, Raikkonen, King and Schwab2017). The evidence that prenatal maternal stress and mental health has long-reaching implications for offspring psychopathology raises the need to investigate factors that can reduce or eliminate the consequences of prenatal adversity. High-quality parental caregiving is a likely factor that may increase resiliency in the face of prenatal adversity (Davis et al., Reference Davis, Hankin, Glynn, Head, Kim and Sandman2019; Kok et al., Reference Kok, Thijssen, Bakermans-Kranenburg, Jaddoe, Verhulst, White, van IJzendoorn and Tiemeier2015; NICHD Early Childcare Research Network, 1999). The current study addresses an important knowledge gap by investigating whether high-quality maternal caregiving during infancy ameliorates the consequences of prenatal maternal distress on child cognitive and emotional development, thereby shifting trajectories of risk towards more optimal mental health.
Fetal programming research illustrates that children exposed to maternal psychological distress (e.g., anxiety, depression, and stress) during pregnancy have poorer cognitive function and elevated negative emotionality during infancy, childhood, and adolescence (Blair, Glynn, Sandman, & Davis, Reference Blair, Glynn, Sandman and Davis2011; Glynn et al., Reference Glynn, Howland, Sandman, Davis, Phelan, Baram and Stern2018; Korja, Nolvi, Grant, & McMahon, Reference Korja, Nolvi, Grant and McMahon2017; Madigan et al., Reference Madigan, Oatley, Racine, Fearon, Schumacher, Akbari, Cooke and Tarabulsy2018; Mahrer et al., Reference Mahrer, Ramos, Guardino, Davis, Ramey, Shalowitz and Schetter2019; Sandman & Davis, Reference Sandman and Davis2010; Van den Bergh, Mulder, Mennes, & Glover, Reference Van den Bergh, Mulder, Mennes and Glover2005, Reference Van den Bergh, Van Den Heuvel, Lahti, Braeken, Rooij, Entringer, Hoyer, Roseboom, Raikkonen, King and Schwab2017). Importantly, these prospective and longitudinal studies document the predictive importance of prenatal distress on child outcomes, even after covarying potential confounders, including postnatal maternal psychological distress. Further, an independent line of research focusing on postnatal experiences reports that sensitive and responsive maternal care is associated with benefits for child cognitive and emotional development, as well as adult psychological health (Davis et al., Reference Davis, Stout, Molet, Vegetabile, Glynn, Sandman, Heins, Stern and Baram2017; Deans, Reference Deans2018; Fan et al., Reference Fan, Buka, Kosik, Chen, Wang and Eaton2014; Farrell et al., Reference Farrell, Waters, Young, Englund, Carlson, Roisman and Simpson2019; Malmberg et al., Reference Malmberg, Lewis, West, Murray, Sylva and Stein2016; Spinrad & Stifter, Reference Spinrad and Stifter2002). Maternal caregiving during the first year postpartum may be especially important, because this is a sensitive window for mother–infant relationship development (Ainsworth, Reference Ainsworth1979; Feldman, Reference Feldman2007).
Although the opportunity to assess the joint contributions of prenatal and postnatal experiences are rare in human research, and often are limited by a lack of objective assessment of maternal behavior (Sharp et al., Reference Sharp, Pickles, Meaney, Marshall, Tibu and Hill2012, Reference Sharp, Hill, Hellier and Pickles2015), experimental research with animals shows that high-quality maternal care can compensate for exposure to prenatal maternal stress. Specifically, the adverse effects of prenatal maternal stress on offspring cognition, emotion stress regulation, and brain development can be prevented with experimental manipulations of maternal quality of care (Bogoch, Biala, Linial, & Weinstock, Reference Bogoch, Biala, Linial and Weinstock2007; Lemaire, Lamarque, Le Moal, Piazza, & Abrous, Reference Lemaire, Lamarque, Le Moal, Piazza and Abrous2006; Raineki, Lucion, & Weinberg, Reference Raineki, Lucion and Weinberg2014; Wakshlak & Weinstock, Reference Wakshlak and Weinstock1990).
Several studies with clinical populations have tested the benefit of sensitive, responsive care on human infant development. Among mothers with an anxiety or a depression diagnosis during pregnancy, high-quality maternal care is associated with a reduction in the correlation between prenatal maternal mental health and offspring cortisol regulation at 4 months (Kaplan, Evans, & Monk, Reference Kaplan, Evans and Monk2008), on negative affect responses to still-face at 7 months (Grant, McMahon, Reilly, & Austin, Reference Grant, McMahon, Reilly and Austin2010a), and on Bayley cognitive development scores at 7 months (Grant, McMahon, Reilly, & Austin, Reference Grant, McMahon, Reilly and Austin2010b). In addition to the relatively small sample sizes of these studies (ranging from 47 to 84), limitations include reliance on assessment of a single diagnostic category (i.e., diagnoses of anxiety or depressive disorders; Grant et al., Reference Grant, McMahon, Reilly and Austin2010a, Reference Grant, McMahon, Reilly and Austin2010b; Kaplan et al., Reference Kaplan, Evans and Monk2008). Reliance on a single timepoint assessment as well as diagnostic cut offs in a single disorder may not fully capture fetal exposure to maternal psychological distress for several reasons. First, there is compelling evidence that maternal distress across the spectrum and including in the nonclinical range is linked to child outcomes (Davis & Sandman, Reference Davis and Sandman2012; Glynn et al., Reference Glynn, Howland, Sandman, Davis, Phelan, Baram and Stern2018; Kingston, Tough, & Whitfield, Reference Kingston, Tough and Whitfield2012; Krueger & Markon, Reference Krueger and Markon2006; Lee et al., Reference Lee, Lam, Sze Mun Lau, Chong, Chui and Fong2007). Second, women frequently experience comorbidity of stress and internalizing symptoms from multiple disorders, all of which may impact the fetus (Falah-Hassani, Shiri, & Dennis, Reference Falah-Hassani, Shiri and Dennis2016; Glynn et al., Reference Glynn, Howland, Sandman, Davis, Phelan, Baram and Stern2018). For these reasons, it is important to assess prenatal exposure to maternal anxiety, depression, and stress multiple times during gestation to create a reliable index of exposure to multiple types of maternal distress over the entire course of gestation and then evaluate the compensatory benefit of maternal care.
A recent study that addressed these limitations reported that high levels of maternal positive engagement at ages 2.5 to 5 years alleviated the cognitive delays associated with prenatal maternal distress and suggested that parental care may be an important target for intervention (Schechter et al., Reference Schechter, Brennan, Smith, Stowe, Newport and Johnson2017). The present study extends the findings by Schechter et al. (Reference Schechter, Brennan, Smith, Stowe, Newport and Johnson2017) in two important ways. First, by assessing maternal care during the first postnatal year (6 and 12 months), a sensitive window for attachment formation, this study assesses a potential target for early intervention. Second, the current study assesses both child cognitive function and negative emotionality, two important indices of early child development. Specifically, the current prospective research characterized maternal psychological distress (anxiety, depression, and perceived stress) across five gestational intervals and four postpartum time points. This approach was used to address the question: Does the quality of maternal care mitigate the link between prenatal exposure to maternal psychological distress and child cognitive function and negative emotionality?
Method
Participants
Study participants included 136 mothers and their children (79 male, 57 female) participating in a longitudinal study evaluating the role of early experiences on development. Pregnant women were recruited from a large university medical center in Southern California. At recruitment, inclusion criteria were: (a) adult (≥18 years of age), (b) English-speaking, and (c) intrauterine, singleton pregnancy. Exclusion criteria at recruitment were: (a) presence of uterine or cervical abnormalities, (b) conditions such as endocrine, hepatic, or renal disorders, or use of corticosteroid medication, and (c) self-reported abuse of tobacco, alcohol, or recreational drugs in the pregnancy. An additional postnatal inclusion criterion for the current study was gestational age of 34 weeks or greater at birth. Descriptive information for the sample is provided in Table 1. All mothers provided informed, written consent for themselves and their child as approved by the Institutional Review Board for Protection of Human Subjects.
Table 1. Sample characteristics
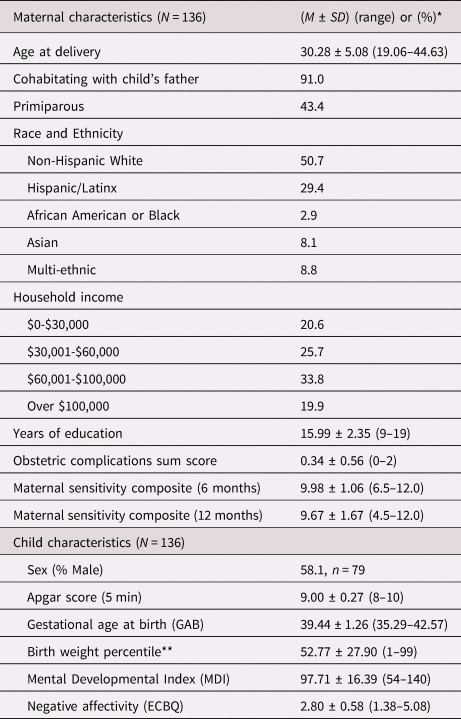
* Values presented as (means ± SD) or (%) where applicable.
** Birth weight percentile was calculated according to the infant's sex and gestational age at birth.
ECBQ = Early Childhood Behavior Questionnaire
Procedures
Measures of maternal psychological distress were collected at five time points prenatally and four time points postnatally. Maternal sensitivity was assessed at 6 and 12 months postpartum and child cognitive and emotional outcomes were evaluated at 2 years (see Figure 1 for study timeline). Gestational and child ages at each assessment are presented in the Appendix Table 1. Appendix Table 6 shows missing data for each measure.
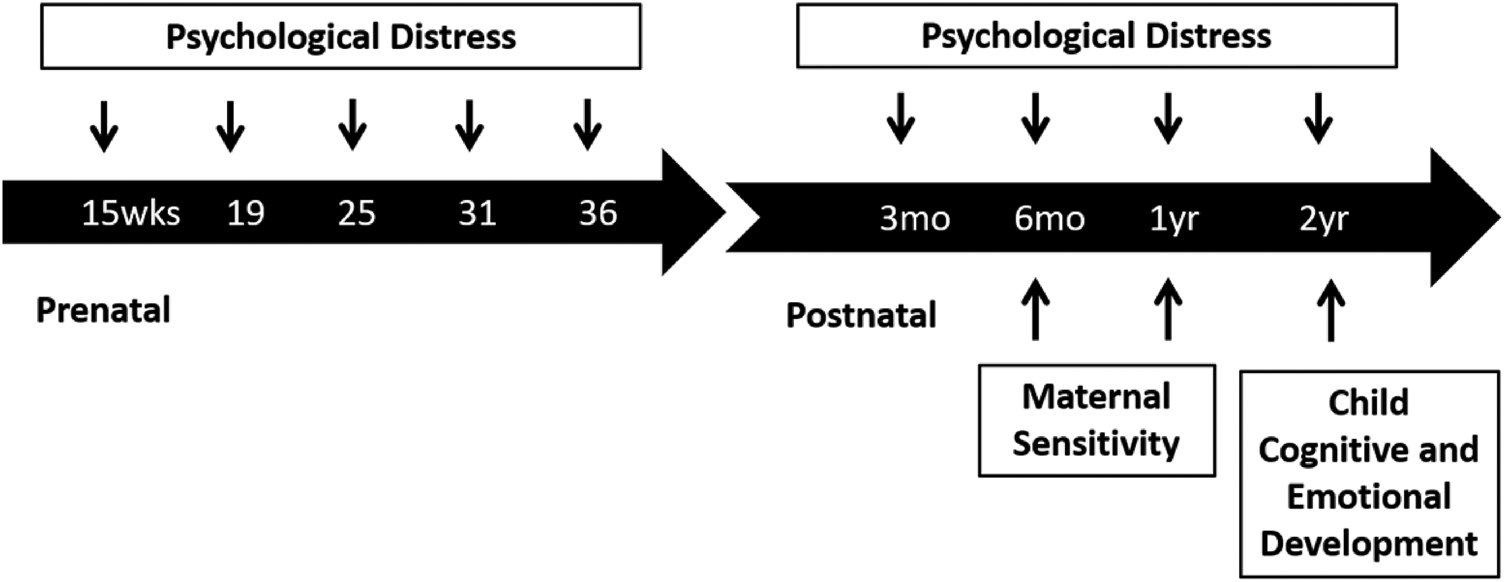
Figure 1. Study timeline.
Measures
Maternal psychological distress
Maternal anxiety was assessed using the 10-item state anxiety subscale of the State-Trait Personality Inventory (STAI; Spielberger, Reference Spielberger1983). Maternal depressive symptoms were assessed using the nine-item short form of the Center for Epidemiologic Studies Depression Inventory (CES-D; Santor & Coyne, Reference Santor and Coyne1997). Generalized or nonspecific perceptions of stress were assessed using the 12-item version of Cohen's Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, Reference Cohen, Kamarck and Mermelstein1983). The internal consistencies of the three measures across time points were good (STAI α = .87–.90, CES-D α = .85–.88, PSS α = .87–.91 in the current sample). Mean levels of maternal depression, stress, and anxiety and distress composites at prenatal and postnatal time points are presented in the Appendix Table 2. The correlation between the three distress indicators and the pattern of association for anxiety, stress, and depression across pregnancy are presented in Appendix Tables 3–5.
Mean depression, perceived stress, and anxiety scores were standardized and averaged to create a composite prenatal distress and a composite postnatal distress score. This distress composite score was used as an index of prenatal exposure to multiple distress indicators, in order to provide a test of the ability of postnatal maternal sensitivity to compensate for prenatal maternal distress exposure. Prior research has indicated that the composite of anxiety, stress, and depression throughout pregnancy consistently predicts child outcomes (Glynn et al., Reference Glynn, Howland, Sandman, Davis, Phelan, Baram and Stern2018; Howland et al., Reference Howland, Sandman, Davis, Stern, Phelan, Baram and Glynn2020).
Quality of maternal care
Maternal care was characterized in the present investigation using a maternal sensitivity index developed by the NICHD Early Child Care Study comprising of three indicators (a composite of positive regard, sensitivity to nondistress, and intrusiveness reverse-scored) that is a potent predictor of numerous child development outcomes (NICHD Early Child Care Research Network, 1997, 1999, 2001).
Mothers were video-recorded interacting with their infants in a semi-structured 10-minute play episode in the lab at 6 and 12 months of age. During this play interaction, mothers were given a standard set of age-appropriate toys and told to play with their infants as they would at home. Maternal behavior was scored from video using a coding system developed for the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Study of Early Child Care and Youth Development (Glynn, Davis, Sandman, & Goldberg, Reference Glynn, Davis, Sandman and Goldberg2016; NICHD Early Child Care Research Network, 1999). Based on standard procedures, a composite rating of maternal sensitivity was created by summing 4-point ratings of sensitivity to nondistress, positive regard, and intrusiveness (reverse-scored). A composite maternal sensitivity score was calculated by averaging 6- and 12-month scores. All coders were blind to other data gathered on study participants. Twenty percent of sessions were selected at random, without coder knowledge, and coded again by a second independent coder to obtain an index of inter-rater reliability. Reliability for each of the subscales were: sensitivity to nondistress (90%), intrusiveness reverse-scored (90%), and positive regard (93%).
Child cognitive function
The Bayley Scales of Infant Development, second edition (BSID-II) Mental Developmental Index (MDI) was administered to assess child cognitive function at 2 years of age (Bayley, Reference Bayley1993). Examiners were directly supervised by a clinical psychologist. Interrater reliability, calculated on 15% of the assessments at each age, was 93%.
Child negative emotionality
Child negative emotionality at 2 years of age was assessed via maternal report on the Early Childhood Behavior Questionnaire (ECBQ; Putnam, Gartstein, & Rothbart, Reference Putnam, Gartstein and Rothbart2006). Prior research demonstrates that the ECBQ Negative Affectivity scale at 2 years has demonstrated inter-rater reliability and longitudinal stability (Putnam et al., Reference Putnam, Gartstein and Rothbart2006). Within the current sample, the Negative Affectivity scale had excellent internal consistency (α = .95).
Measurement of covariates
Sociodemographic characteristics
Maternal socioeconomic status (SES), age, cohabitation with child's father, and race and ethnicity were collected via maternal interview. Maternal SES was calculated as the sum of standardized numbers of year of maternal education and standardized household income.
Pregnancy and birth outcomes
Maternal obstetric complications, parity, infant sex, birth weight, and 5-minute Apgar score were abstracted from the medical record. Estimated date of delivery was calculated utilizing both early ultrasound measures and date of last menstrual period based on American College of Obstetricians and Gynecologists (ACOG) guidelines, and used to assess gestational age at birth (GAB; Committee on Obstetric Practice, the American Institute of Ultrasound in Medicine, and the Society for Maternal-Fetal Medicine, 2017). An obstetric complications score was calculated, indicating the presence or absence of any pregnancy-related complications, including: prenatal infection, pregnancy-induced hypertension, gestational diabetes, oligohydramnios, polyhydramnios, preterm labor, anemia, vaginal bleeding, or placenta previa (Hobel, Reference Hobel, Bolognese, Schwarz and Schneider1982).
Maternal intelligence
Maternal intelligence was assessed at a subsequent visit via the Perceptual Organization Index (POI) of the Wechsler Adult Intelligence Scale (WAIS-III) once postnatally. The POI was a proxy of general intelligence, because it is highly correlated with the general factor g of intelligence (r = .94) (Deary, Reference Deary2001). The WAIS and its subscales are widely used and valid measures of intelligence. Previous studies demonstrate the validity of the WAIS, as well as the reliability of the POI score (r = .93) (Wechsler, Coalson, & Raiford, Reference Wechsler, Coalson and Raiford1997). The WAIS-III was administered at a postnatal visit (M = 5.34, SD = 1.99 years postdelivery).
Statistical analyses
Sociodemographic and obstetric covariates
Potential covariates were selected based on the literature (Blair et al., Reference Blair, Glynn, Sandman and Davis2011; Polanska et al., Reference Polanska, Krol, Merecz-Kot, Jurewicz, Makowiec-Dabrowska, Chiarotti, Calamandrei and Hanke2017) and included infant characteristics (biological sex at birth, gestational age at birth (GAB), birthweight percentile adjusted for sex, and 5-minute Apgar score), as well as obstetric (obstetric health-related factors, parity) and maternal characteristics (SES, age, cohabitation with infant's father, race and ethnicity, and the WAIS, an index of intelligence). All regression models included covariates associated (p < .10) with the child outcome. Thus, the following covariates were included in cognitive function analyses: maternal age, SES, parity, cohabitation with child's father, maternal index of intelligence (WAIS), sum of obstetric risk, ethnicity (Non-Hispanic White or Hispanic/Latinx), GAB, and child sex. Covariates included in negative emotionality analyses were maternal age, SES, cohabitation with child's father, maternal index of intelligence (WAIS), and ethnicity (Non-Hispanic White or Hispanic/Latinx). The correlations between all potential covariates, prenatal and postnatal distress, maternal sensitivity, and child outcomes are presented in Appendix Table 7.
Psychological distress, maternal care and child outcomes
Initial bivariate correlations were performed to test whether prenatal and postnatal maternal psychological distress were associated with child outcomes, cognitive function, and negative emotionality. The relations between the maternal sensitivity composite and child outcomes were also evaluated via bivariate correlation.
Moderation of prenatal psychological distress by maternal caregiving
Regression models tested the primary hypothesis that the maternal sensitivity composite moderates the relation between prenatal maternal psychological distress and child outcome. Continuous predictor variables were mean-centered in the regression model. Significant interactions were probed by calculating and plotting simple slopes. Finally, to test the potential contribution of postnatal maternal psychological distress, regression models included it as an additional covariate.
Secondary analyses: Assessment of sex differences, individual distress indicators, and timing and subscales of maternal care
Sex differences: Secondary analyses were conducted in order to explore sex differences as follows (a) whether there are sex differences in the association between prenatal distress and child outcomes, (b) sex differences in the association between the maternal sensitivity composite and child outcomes, and (c) whether the interaction between prenatal psychological distress and maternal sensitivity was moderated by child sex (three-way interaction).
Individual distress indicators: To test whether the maternal sensitivity composite moderated the effect of each of the three distress indicators, regression models were conducted separately for prenatal anxiety, stress, and depression (see Appendix Tables 7–8).
Timing and subscales of maternal care: To evaluate whether care at 6 or 12 months was a more important moderator of prenatal distress (timing) interaction models were conducted with maternal sensitivity separately at 6 and 12 months (see Appendix Tables 9-10). Finally, to test the role of the individual subscales that comprise the sensitivity composite (positive regard, sensitivity to nondistress, and intrusiveness reverse-scored) in moderating the effect of prenatal maternal distress, moderation analyses were conducted separately with each of the three subscales (Appendix Tables 11–12).
Results
Child developmental outcomes
Descriptive information for the cognitive and emotional outcomes is shown in Table 1. Poorer child cognitive function was associated with higher child negative emotionality (r = −.32, p < .001).
Maternal distress, maternal care, and child cognitive function
Elevated prenatal psychological distress composite was associated with poorer cognitive function (r = −.28, p = .001) and as shown in Appendix Table 5, the pattern of association was similar across the five gestational timepoints. Further, elevated postnatal psychological distress was associated with poorer child cognitive function at 2 years of age (r = −.26, p = .003). In contrast, a higher maternal sensitivity composite score was associated with enhanced child cognitive function (r = .47, p < .001).
Does postnatal care moderate the relation between prenatal psychological distress and child cognitive function?
The maternal sensitivity composite moderated the association between prenatal distress and child cognitive function even with the inclusion of covariates [b = 2.80, t(119) = 2.25, p = .027; see Table 2]. As shown in Figure 2, children exposed to elevated prenatal maternal distress and low maternal sensitivity exhibited the poorest cognitive performance, but children exposed to higher prenatal maternal distress who then received sensitive maternal care did not display deficits in cognitive function. This association remained when postnatal distress was additionally included as a covariate [b = 2.78, t(118) = 2.22, p = .028; see Appendix Table 13a].
Table 2. Regression model examining the association between prenatal maternal psychological distress, maternal care and child cognitive function
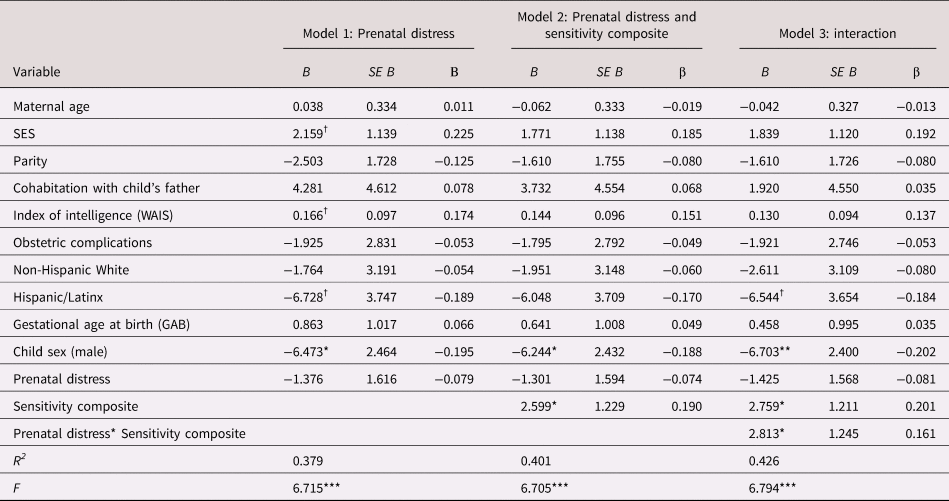
B = unstandardized coefficient; β = standardized coefficient; SES = socioeconomic status; WAIS = Wechsler Adult Intelligence Scale
† p < .10 *p < .05. **p < .01. ***p < .001.
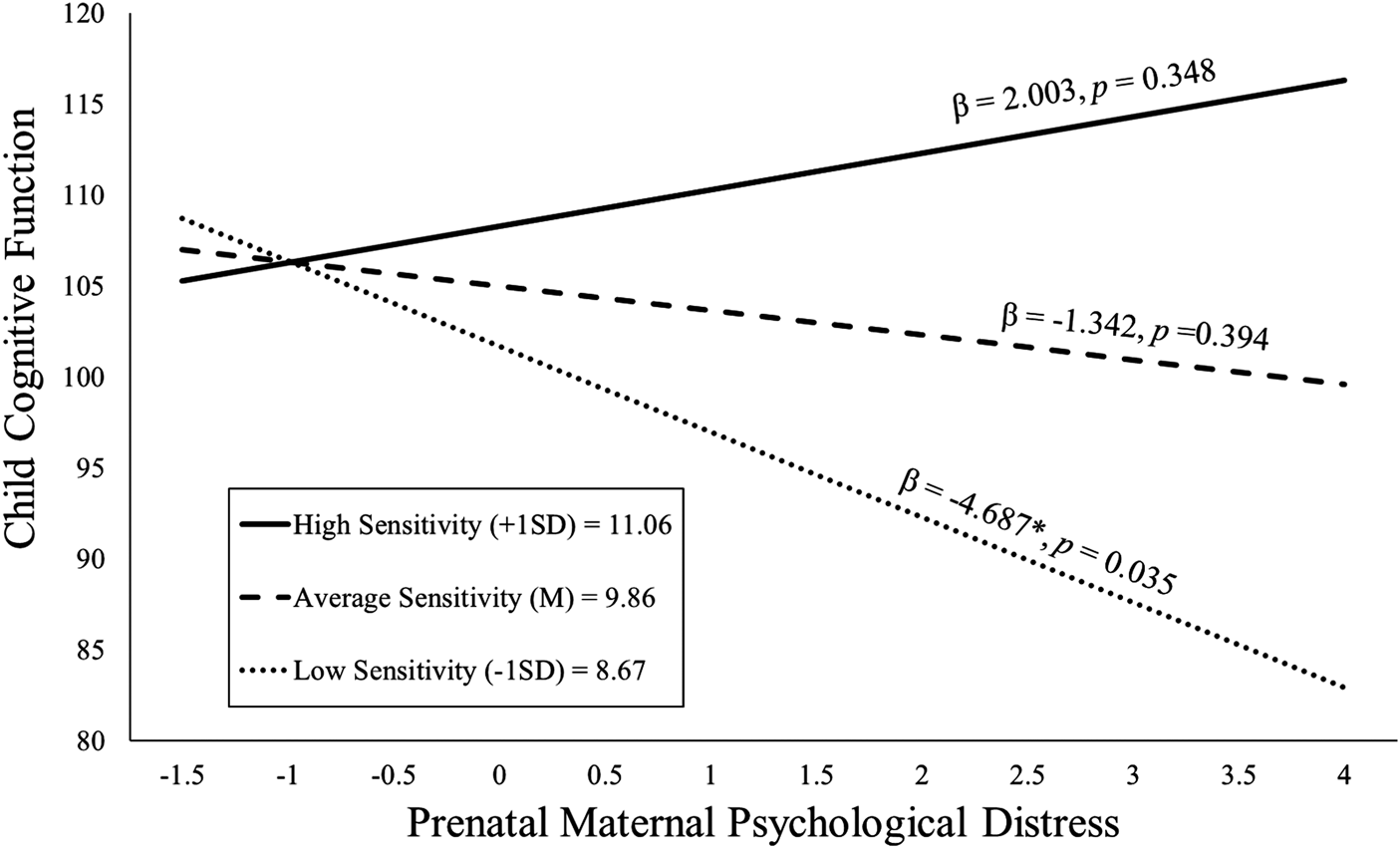
Figure 2. Maternal sensitivity composite was analyzed as a continuous variable using regression, but for illustrative purposes are depicted here as low (1 SD below the mean), average (at the mean), and high (1 SD above the mean) levels of maternal sensitivity. Prenatal maternal distress (on the x-axis) is the standardized composite of anxiety, depressive symptoms, and perceived stress scores. Children exposed to elevated prenatal maternal distress did not exhibit impaired cognitive function at age 2 if they received higher quality maternal caregiving.
Cognitive function secondary analyses: Assessment of sex differences, individual distress indicators, and timing of maternal care
Sex differences: Secondary analyses revealed that child sex did not moderate the effects of prenatal psychological distress (β = 0.070) or maternal sensitivity (β = 0.002) on child cognitive function; additionally, the three-way interaction of child sex, prenatal distress, and maternal sensitivity was nonsignificant (β = 0.072) (ps > .52).
Individual distress indicators: The moderating effect of sensitivity on prenatal anxiety (β = 0.143), stress (β = 0.166), and depression (β = 0.114) examined separately yielded similar effect sizes (see Appendix Table 7).
Timing and subscales of maternal care: The moderating role of maternal sensitivity on child cognitive function was similar when maternal sensitivity was examined separately at 6 months (β = 0.181) and 12 months (β = 0.158) (Appendix Table 9). In addition, the moderating effect of the individual subscales that comprise the sensitivity composite, including positive regard (β = 0.154), sensitivity to nondistress (β = 0.151), and intrusiveness reverse-scored (β = 0.022), revealed similar effect sizes (Appendix Table 11).
Maternal distress, maternal care, and child negative emotionality
Elevated prenatal maternal psychological distress was associated with higher child negative emotionality at 2 years of age (r = .28, p = .002), and as shown in Appendix Table 5, the pattern of association was similar across the five gestational timepoints. Elevated postnatal maternal psychological distress (r = .25, p = .005) was associated with higher child negative emotionality at 2 years of age. A higher maternal sensitivity composite score was associated with lower child negative emotionality (r = -.24, p = .008).
Does postnatal care moderate the relation between prenatal psychological distress and child negative emotionality?
As shown in Figure 3, children exposed to elevated prenatal distress and who received high-quality maternal caregiving exhibited low negative emotionality even after consideration of covariates [b = −0.11, t(109) = −2.01, p = .047; see Table 3]. With the addition of postnatal maternal distress as a covariate this association remained statistically significant [b = −0.11, t(108) = −2.03, p = .045; see Appendix Table 13b].
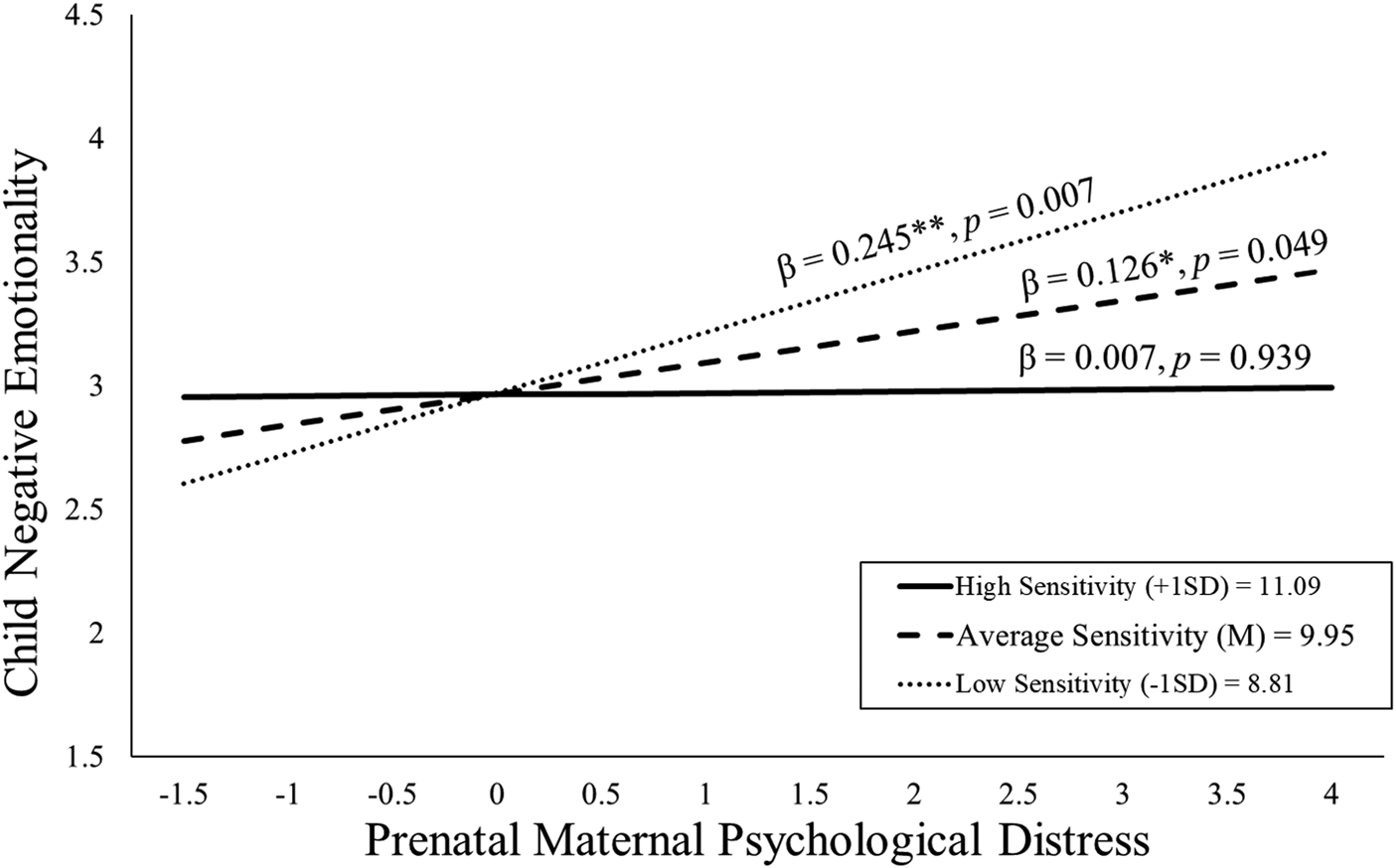
Figure 3. Maternal sensitivity composite was analyzed as a continuous variable using regression, but for illustrative purposes are depicted here as low (1 SD below the mean), average (at the mean), and high (1 SD above the mean) levels of maternal sensitivity. Prenatal maternal distress (on the x-axis) is the standardized composite of anxiety, depressive symptoms, and perceived stress scores. Children exposed to elevated prenatal maternal distress did not exhibit high negative emotionality at age 2 if they received higher quality maternal caregiving.
Table 3. Regression model examining the association between prenatal maternal psychological distress, maternal care and child negative emotionality
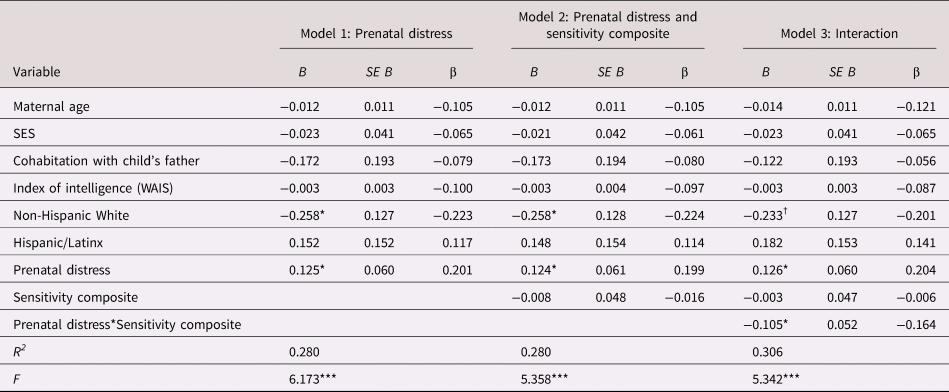
B = unstandardized coefficient; β = standardized coefficient; SES = socioeconomic stress; WAIS = Wechsler Adult Intelligence Scale
† p < .10 *p < .05. **p < .01. ***p < .001.
Negative emotionality secondary analyses: Assessment of sex differences, individual distress indicators, and timing of maternal care
Sex differences: Secondary analyses revealed that child sex did not moderate the effects of prenatal psychological distress (β = 0.065) or maternal sensitivity (β = −0.133) on child negative emotionality; additionally, the three-way interaction of child sex, prenatal distress, and maternal sensitivity was nonsignificant (β = 0.104) (ps > .29).
Individual distress indicators: The moderating effect of sensitivity on prenatal anxiety (β =−0.135), stress (β = −0.136), and depression (β =−0.165) examined separately yielded similar effect sizes (see Appendix Table 8).
Timing and subscales of maternal care: The moderating role of maternal sensitivity on child negative emotionality was similar when maternal sensitivity was examined separately at 6 (β = −0.162) and 12 months (β = −0.176) (Appendix Table 10). In addition, the moderating effect of the individual subscales that comprise the sensitivity composite, including positive regard (β = −0.083), sensitivity to nondistress (β = −0.151), and intrusiveness reverse-scored (β = −0.166), revealed similar effect sizes (Appendix Table 12).
Discussion
Consistent with the fetal programing literature, we show that prenatal exposure to maternal psychological distress (anxiety, depression, and perceived stress) is associated with child cognitive and emotional vulnerabilities (Buss, Davis, Muftuler, Head, & Sandman, Reference Buss, Davis, Muftuler, Head and Sandman2010; Davis et al., Reference Davis, Glynn, Schetter, Hobel, Chicz-DeMet and Sandman2007, Reference Davis, Hankin, Glynn, Head, Kim and Sandman2019; Davis & Sandman, Reference Davis and Sandman2010; Glynn et al., Reference Glynn, Howland, Sandman, Davis, Phelan, Baram and Stern2018; Kingston et al., Reference Kingston, Tough and Whitfield2012; Van den Bergh et al., Reference Van den Bergh, Van Den Heuvel, Lahti, Braeken, Rooij, Entringer, Hoyer, Roseboom, Raikkonen, King and Schwab2017). Further, our findings are consistent with decades of research that have established the importance of parental care during sensitive periods, such as the first postnatal year, for promoting optimal developmental outcomes (Ainsworth, Reference Ainsworth1979). The present study provides new evidence that high-quality maternal care during the first postnatal year ameliorates the negative cognitive and emotional outcomes that follow exposure to prenatal maternal distress. These findings remained after considering potential confounding factors including SES, postnatal maternal distress, and maternal intelligence scores. These data indicate that consequences of fetal programming are malleable and that prenatal and postnatal experiences synergistically impact child development. Specifically, we find that high-quality caregiving can compensate for the impact of prenatal maternal distress by altering developmental trajectories and improving child mental health.
The present study addresses a key issue in the fetal programming literature by demonstrating that the consequences of prenatal exposures are modifiable by postnatal experiences. Our data are consistent with experimental rodent models that report manipulations of maternal care compensate for prenatal adversity (Bogoch et al., Reference Bogoch, Biala, Linial and Weinstock2007; Lemaire et al., Reference Lemaire, Lamarque, Le Moal, Piazza and Abrous2006; Raineki et al., Reference Raineki, Lucion and Weinberg2014; Wakshlak & Weinstock, Reference Wakshlak and Weinstock1990) as well as studies showing that infants with a secure attachment relationship to their mother do not show behavioral problems following prenatal stress (Ali et al., Reference Ali, Letourneau, Benzies, Ntanda, Dewey, Campbell and Giesbrecht2020; Bergman, Sarkar, Glover, & O'Connor, Reference Bergman, Sarkar, Glover and O'Connor2008). There are several important contributions of our project to the relatively small human literature evaluating whether maternal behavior towards her infant mitigates the consequences of prenatal stress (Grant et al., Reference Grant, McMahon, Reilly and Austin2010a, Reference Grant, McMahon, Reilly and Austin2010b; Kaplan et al., Reference Kaplan, Evans and Monk2008; Schechter et al., Reference Schechter, Brennan, Smith, Stowe, Newport and Johnson2017; Sharp et al., Reference Sharp, Pickles, Meaney, Marshall, Tibu and Hill2012, Reference Sharp, Hill, Hellier and Pickles2015). First, maternal distress was assessed repeatedly throughout gestation and we have shown previously that this composite measure of anxiety, stress, and depression symptoms predicts both cognitive and emotional outcomes through childhood and adolescence (Glynn et al., Reference Glynn, Howland, Sandman, Davis, Phelan, Baram and Stern2018; Howland et al., Reference Howland, Sandman, Davis, Stern, Phelan, Baram and Glynn2020). Second, we assessed a composite of maternal sensitivity twice during the first postnatal year, a sensitive window for attachment formation (Ainsworth, Reference Ainsworth1979). Thus, our design is a rigorous test of the hypothesis that high-quality maternal care can offset the impact of prenatal distress exposure after covarying for confounding factors including postnatal maternal distress. Third, we characterize both child cognitive and emotional function. Finally, these data provide evidence that infancy may be a sensitive window when maternal care can mitigate the consequences of prenatal maternal distress, thereby identifying a plausible target for early intervention following prenatal adversity.
Maternal care that is warm, sensitive, and responsive to infant's signals (Ainsworth, Blehar, Waters, & Wall, Reference Ainsworth, Blehar, Waters and Wall1978), may play a particularly important role in child neurodevelopment and long-term developmental outcomes (Malmberg et al., Reference Malmberg, Lewis, West, Murray, Sylva and Stein2016; Spinrad & Stifter, Reference Spinrad and Stifter2002; Wang et al., Reference Wang, Zhang, Wee, Lee, Poh, Chong, Tan, Gluckman, Yap, Fortier, Rifkin-Graboi and Qiu2019). Thus, it is highly plausible that high-quality maternal care during the first postnatal year may alter neurodevelopmental trajectories following prenatal exposures towards more optimal outcomes. We show that maternal care at 6 and 12 months similarly moderated the association between prenatal maternal distress and child outcomes, suggesting that care throughout the first postnatal year may be important for ameliorating the impact of prenatal distress (Appendix Tables 9–10). The maternal sensitivity composite in the current study measured positive regard, sensitivity to nondistress, and intrusiveness reverse-scored, key aspects of maternal behavior that influence development. Findings suggest that all of these components of maternal sensitivity captured by this measure contribute to positive child development following prenatal maternal distress (see Appendix Tables 11–12). Future research could consider other aspects of positive parenting and paternal parenting behaviors that may ameliorate the impact of prenatal maternal distress.
There are a number of potential mechanisms by which prenatal maternal psychological distress may have consequences for child cognitive and emotional development, including alterations to fetal neurodevelopment (Sandman, Class, Glynn, & Davis, Reference Sandman, Class, Glynn, Davis and Rosenfeld2015; Sandman, Glynn, Davis, Kisilevsky, & Reissland, Reference Sandman, Glynn, Davis, Kisilevsky and Reissland2016; Schuurmans & Kurrasch, Reference Schuurmans and Kurrasch2013; Wu et al., Reference Wu, Lu, Jacobs, Pradhan, Kapse, Zhao, Niforatos-Andescavage, Vezina, du Plessis and Limperopoulos2020). Animal studies demonstrate changes in offspring brain structure following prenatal stress exposure, such as reduced hippocampal volume and neurogenesis (Bogoch et al., Reference Bogoch, Biala, Linial and Weinstock2007; Charil, Laplante, Vaillancourt, & King, Reference Charil, Laplante, Vaillancourt and King2010). Children exposed to prenatal maternal psychological distress show reduced gray matter volume and thickness in frontal, temporal, and limbic areas, as well as reduced total gray matter density (Adamson, Letourneau, & Lebel, Reference Adamson, Letourneau and Lebel2018; Buss et al., Reference Buss, Davis, Muftuler, Head and Sandman2010; Davis et al., Reference Davis, Hankin, Glynn, Head, Kim and Sandman2019; Demers et al., Reference Demers, Aran, Glynn, Davis, Wazana, Oberlander and Szekel2021; Sandman, Buss, Head, & Davis, Reference Sandman, Buss, Head and Davis2015). Cross-species studies provide mechanistic evidence that dendritic atrophy may be a pathway by which prenatal maternal distress disrupts offspring brain development (Curran, Sandman, Davis, Glynn, & Baram, Reference Curran, Sandman, Davis, Glynn and Baram2017; Sandman et al., Reference Sandman, Curran, Davis, Glynn, Head and Baram2018). These neural systems impacted by prenatal exposures may be modifiable by high-quality postnatal maternal care. The first year postpartum continues to be a sensitive window of heightened neuroplasticity and rapid neural growth (Gee, Reference Gee, Rutherford and Mayes2016; Gilmore, Knickmeyer, & Gao, Reference Gilmore, Knickmeyer and Gao2018; Knickmeyer et al., Reference Knickmeyer, Gouttard, Kang, Evans, Wilber, Smith, Hamer, Lin, Gerig and Gilmore2008) and maternal care may modify developmental trajectories. Consistent with this possibility, high-quality maternal care is associated with enhanced child hippocampal volume growth; this growth trajectory is further associated with improved child emotion regulation (Luby, Barch, Whalen, Tillman, & Belden, Reference Luby, Barch, Whalen, Tillman and Belden2017) as well as with greater child gray matter volume at 8 years (Kok et al., Reference Kok, Thijssen, Bakermans-Kranenburg, Jaddoe, Verhulst, White, van IJzendoorn and Tiemeier2015). Experimental animal and cross species research provide strong evidence that maternal care directly impacts neural circuits underlying cognitive and emotional vulnerabilities that are impacted by prenatal maternal distress (Gee, Reference Gee, Rutherford and Mayes2016; Granger et al., Reference Granger, Glynn, Sandman, Small, Obenaus, Keator and Davis2021; Liu, Diori, Day, Francis, & Meaney, Reference Liu, Diori, Day, Francis and Meaney2000; Rao et al., Reference Rao, Betancourt, Giannetta, Brodsky, Korczykowski, Avants, Gee, Wang, Hurt, Detre and Farah2010).
The current study has several limitations. Although the use of a community sample highlights the importance of subclinical variation in maternal psychological symptoms of distress, the range of observed maternal psychological distress and maternal sensitivity is constrained. Because of this, the current study may underestimate the associations among prenatal distress, maternal sensitivity, and child outcomes. We also cannot completely disentangle the role of genetic influences. However, evidence from experimental animal research consistently reports the effects of maternal care on offspring outcomes are independent from effects of genetics, and provides experimental evidence that maternal care can compensate for prenatal exposures (Francis, Diorio, Liu, & Meaney, Reference Francis, Diorio, Liu and Meaney1999; Liu et al., Reference Liu, Diori, Day, Francis and Meaney2000).
Decades of research have confirmed the prenatal period as a time of enhanced responsivity to environmental input, when maternal psychological distress can have a profound influence on child development (Bush et al., Reference Bush, Jones-Mason, Coccia, Caron, Alkon, Thomas, Coleman-Phox, Wadhwa, Laraia, Adler and Epel2017; Davis et al., Reference Davis, Glynn, Schetter, Hobel, Chicz-DeMet and Sandman2007, Reference Davis, Hankin, Swales and Hoffman2018; Davis & Sandman, Reference Davis and Sandman2010, Reference Davis and Sandman2012; Doyle et al., Reference Doyle, Werner, Feng, Lee, Altemus, Isler and Monk2015; Van den Bergh et al., Reference Van den Bergh, Van Den Heuvel, Lahti, Braeken, Rooij, Entringer, Hoyer, Roseboom, Raikkonen, King and Schwab2017; Vehmeijer, Guxens, Duijts, & Marroun, Reference Vehmeijer, Guxens, Duijts and Marroun2019). Few studies have directly assessed processes that promote resilience following prenatal adversity (Atzl, Grande, Davis, & Narayan, Reference Atzl, Grande, Davis and Narayan2019; Davis & Narayan, Reference Davis and Narayan2020; D'Anna-Hernandez & Rivera, Reference D'Anna-Hernandez and Rivera2014; Røsand, Slinning, Eberhard-Gran, Røysamb, & Tambs, Reference Røsand, Slinning, Eberhard-Gran, Røysamb and Tambs2011). Our findings, coupled now with those from Schechter et al. (Reference Schechter, Brennan, Smith, Stowe, Newport and Johnson2017), provide strong support for postnatal prevention and intervention efforts to reduce the consequences of prenatal adversity. Efficacious interventions exist to support the transition to parenthood, and the development of positive parenting skills (Bick & Dozier, Reference Bick and Dozier2010; Eshel, Daelmans, Mello, & Martines, Reference Eshel, Daelmans, Mello and Martines2006; Nillni, Mehralizade, Mayer, & Milanovic, Reference Nillni, Mehralizade, Mayer and Milanovic2018). Thus, interventions to promote maternal psychological health and sensitive, responsive caregiving has enduring benefits that could thus mitigate the life-long cognitive and emotional consequences of prenatal psychological distress.
Supplementary Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579421000286
Acknowledgments
We wish to thank the families who participated in this project. The assistance of Megan Faulkner, Natalie Hernandez, and Kendra Leak of the Women and Children's Health and Well-Being Project, Department of Psychiatry & Human Behavior, University of California and the Department of Psychology, Chapman University is gratefully acknowledged.
Funding Statement
This research was supported by the National Institutes of Health [R01 HD065823; P50 MH096889; R03 MH86062; R01 HD51852; R01 NS041298].
Conflicts of Interest
None.








