Introduction
Home to a staggering 15,000 endemic species (Myers et al., Reference Myers, Mittermeier, Mittermeier, Fonseca and Kent2000), New Guinea has appealed to naturalists for centuries, with extreme diversity emerging as an unsurprisingly common theme to all their studies of the region. Descriptions of New Guinea demand an excessive use of superlative adjectives; it is both the largest and the highest tropical island (with Puncak Jaya reaching 4884 m) and is home to the world’s largest and smallest parrots, largest rat, smallest frog, tallest tropical trees and smallest species of Rhododendron L. Furthermore, the island boasts an intermingled tapestry of climate zones, topographical heterogeneity and geology. The complex relationships between these varied factors have resulted in a remarkable number of different ecological niches existing on New Guinea in a broad range of ecosystems including alpine meadows, cloud forests, tropical forests, mangroves and savannahs.
Described as a “keystone in Pacific botany” (van Steenis, Reference Van Steenis and van Steenis1950) due to its position at the critical junction between Asia and Australia, the island’s vegetation is heterogeneous, containing both Laurasian and Gondwanan elements as well as numerous neo-endemics. Much of the remarkable botanical diversity of the island today is underpinned by the complex relationships between these biogeographically distinct components (Takeuchi, Reference Takeuchi, Marshall and Beehler2007).
Specimen coverage of Papua New Guinea is reasonable, with 725 Begoniaceae collections. However, their collection localities are concentrated in more accessible regions, so considerable geographical and taxonomic gaps remain. A three-week collecting expedition targeting such a sampling gap in Begonia L., in the Telefomin District of Sandaun Province in July 2018, yielded 42 collections comprising at least 14 species, one of which we describe here as a new species.
Begonia sect. Oligandrae M.Hughes & W.N.Takeuchi was described to contain five anomalous species from New Guinea with distinctive horned fruit and a very reduced number of stamens (Hughes & Takeuchi, Reference Hughes and Takeuchi2015). Two of these species, Begonia brassii Merr. & L.M.Perry and B. oligandra Merr. & L.M.Perry, were described in 1943 from West Papua (Indonesia) (Merrill & Perry, Reference Merrill and Perry1943). The other three were described very recently following fieldwork in the highlands of Papua New Guinea: Begonia chambersiae W.N.Takeuchi (Takeuchi, Reference Takeuchi2012), B. pentandra W.N.Takeuchi (Hughes & Takeuchi, Reference Hughes and Takeuchi2015) and B. sandsiana W.N.Takeuchi (Takeuchi, Reference Takeuchi2013). The section is well defined morphologically by species having a reduced stamen number (4–9), symmetrical androecium, free tepals, and unequal wings on the fruit (one being enlarged, verruculose and extended apically towards the tepals, the other two being small and rounded). Images of the specimens cited are available from the Begonia Resource Centre (Hughes et al., Reference Hughes, Moonlight, Jara-Muñoz, Tebbitt, Wilson and Pullan2015).
Key to Begonia sect. Oligandrae
1a. Lamina divided almost to the midrib ___________________________________ 2
1b. Lamina not divided _________________________________________________ 4
2a. Lamina simple; stamens 4 _________________________________ B. chambersiae
2b. Lamina compound, formed of 3- to 6-lobed leaflets; stamens 6–9 ____________ 3
3a. Stems repent; leaflet lobes narrow (majority < 3 mm wide), lobe apices rounded _________________________________________________ B. oligandra
3b. Stems suberect; leaflet lobes broad (majority > 5 mm wide), lobe apices acute B. maguniana
4a. Lamina lanceolate, margin dentate _______________________________ B. brassii
4b. Lamina ovate to orbicular, margin ciliate ________________________________ 5
5a. Plant hairy, bracts ciliate ____________________________________ B. pentandra
5b. Plant glabrous, bracts glabrous _______________________________ B. sandsiana
Begonia maguniana H.P.Wilson sp. nov.
Begonia maguniana is closest to B. oligandra Merr. & L.M.Perry but differs in its habit, being a larger (15–40 cm tall), more upright plant forming dense clumps of erect to suberect stems, whereas B. oligandra is low growing (5–30 cm tall) with suberect to repent stems that root at the nodes. Begonia maguniana has striking red stems and some red colouring on abaxial leaf surfaces; occasionally, this is reduced to a red flush on a green stem at the nodes and at the point of attachment between leaf lamina and petiole. The leaves of Begonia oligandra are finely divided, with prominent venation giving the appearance of a filmy fern to sterile plants, whereas the leaves of B. maguniana are more reminiscent of Anemone nemorosa L. – Type: Papua New Guinea, Sandaun Province, Telefomin District, Telefomin, 1775 m, 23 vi 2018, Wilson, Hughes, & Paul ELAE7 (holo E [E00914141], iso LAE). Fig. 1 .

Fig. 1. Begonia maguniana H.P.Wilson, sp. nov. A, Habit; B, female flower; C, female flower and ovary; D, male flower; E, ripe fruit; F, leaf lamina (upper surface). A, B and F, Wilson, Hughes, & Paul ELAE7; C, Wilson, Hughes, & Paul ELAE106; D, Wilson, Hughes, & Paul ELAE101; E, Wilson, Hughes, & Paul ELAE30. (Photographs: A, B and E, Mark Hughes; C, D and F, Hannah Wilson.)
Erect tuberous caulescent herb 15–40 cm tall, forming clumps with up to c.50 stems. Stems red, succulent, c.5 mm wide, flattened on one side (above petiole attachment), internodes (1.5–)3–7 cm long, glabrous to villose, hairs simple, 1–2 mm long. Stipules caducous, ovate lanceolate, keeled, glabrous, 6 × 4 mm, tip shortly cuspidate. Leaves: petioles red, succulent, (0.5–)1.5–3 cm, subglabrous to villose, hairs simple, 1–2 mm long; lamina compound, leaflets 5, occasionally fewer, total size 2.5 × 2 cm to 6 × 4.5 cm, apical leaflet with pinnate venation, leaflets lobed, dark green above with occasional hairs just inside the margin, red or pale green below with hairs on the main veins; margin irregularly serrate with hairs at the tips of the serrations. Inflorescences bisexual drepanium, 2–5 cm long, protandrous, with 2 or 3 male flowers and a single terminal female flower; primary peduncle glabrous, c.2–4 cm long; bracts conspicuous, white, ovate, glabrous, 10 × 8 mm. Male flowers: pedicel white, glabrous, 6–12 mm long; tepals 4, white with pale green at the base, outer two broadly ovate, 12 × 10 mm, inner two elliptic, 10 × 5 mm; androecium on a raised red torus, stamens 9 in three whorls of 3, filaments white, 2 mm long, anthers 1.5 mm in diameter, ovate orbicular, white, dehiscing all around the perimeter, pollen white. Female flowers: pedicel white, 2–5 mm long, glabrous; ovary 3-locular, placentation axile, 2 placental lamellae per locule, ovary green, glabrous, with one horn-like wing, slightly verruculose, and two rounded succulent wings, all wings clustered to upper side of the ovary such that wing ridges all point upwards during flowering; tepals 3, white, forming a bell with a slightly flared entrance, outer two ovate, 12 × 9 mm, inner tepal elliptic, 8 × 4 mm; styles 3, free, pale green, deeply forked, linear, 4 mm long, stigmatic surface slightly spiral along the linear forks. Fruit remains green when ripe, held with the larger wing downwards, dehiscing through membranous valves inside the two round fleshy wings.
Additional specimens examined. Papua New Guinea. Sandaun Province: Telefomin District. Folongonom, 2100 m, 24 iv 1975, Barker LAE67484 (LAE); Star Mts, 2000 m, 8 iv 1975, Croft & Leiean 65767 and 65787 (LAE); Telefomin, 1750 m, 13 iv 2017, Magun & Poulsen LAE90444 (E, LAE); Star Mts, 2250 m, 11 v 1975, Veldkamp 6689 (LAE); Feranmin, 1699 m, 25 vi 2018, Wilson, Hughes, & Paul ELAE30 (E, LAE); Busilmin, 1707 m, 8 vii 2018, Wilson, Hughes, & Paul, ELAE101 (E, LAE); Busilmin, 1834 m, 9 vii 2018, Wilson, Hughes, & Paul ELAE106 (E, LAE).
Indonesia. Papua Province: Kabupaten Lanny Jaya, Nobaga, 2547 m, 10 i 2004, Danet 4285 (LYJB); Tenmasigin, Orion Mts, 1800 m, 22 v 1959, Kalkman 4016 (A).
Distribution and ecology. Endemic to the Central Range of New Guinea (Fig. 2). Locally common along streams, lithophytic on mossy limestone boulders or growing among small rocks in dry stream beds, occasionally terrestrial. Grows sympatrically with Begonia oligandra and B. vinkii Sands in some places, with no evidence of hybridisation seen in the field (although see notes regarding Danet 4285).
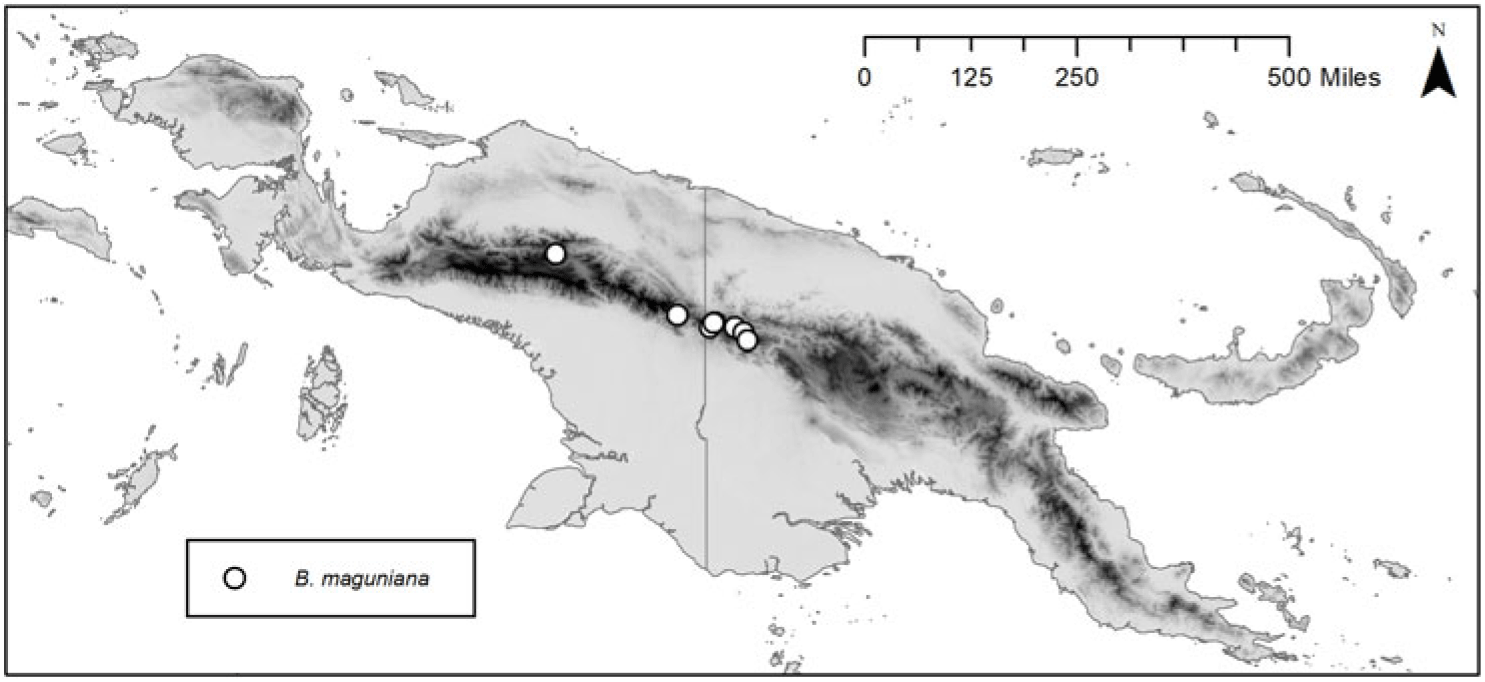
Fig. 2. Distribution of Begonia maguniana H.P.Wilson, based on collection localities of herbarium specimens.
Etymology. Begonia maguniana is named after Thomas Magun (LAE Herbarium, Papua New Guinea), who, with Axel Poulsen, collected this species in Telefomin in April 2017.
In the field, at first sight the plant is reminiscent of the European wood anemone, Anemone nemorosa. As the capsule matures, the larger ovary wing develops and the pedicel elongates and curves, resulting in the capsule being held upside down at maturity (Fig. 3), such that the two carpels with smaller wings are now uppermost. The tissue at the top of these carpels then disintegrates, leaving behind a papery flap that lifts to allow the seed to be dispersed. The capsules are not persistent, and seed is dispersed while the capsule is still green. The anthers in Begonia maguniana are unusual in forming a continuous band, giving the stamen a lollipop-like appearance. The collection from the far west of the range (Danet 4285) has dimorphic leaves, with the lower leaves not as lobed, and appearing more similar to those of Begonia brassii, whereas the upper leaves are typical of B. maguniana. Further work is needed to assess whether this is due to hybridisation or part of the normal morphological variation of this species.
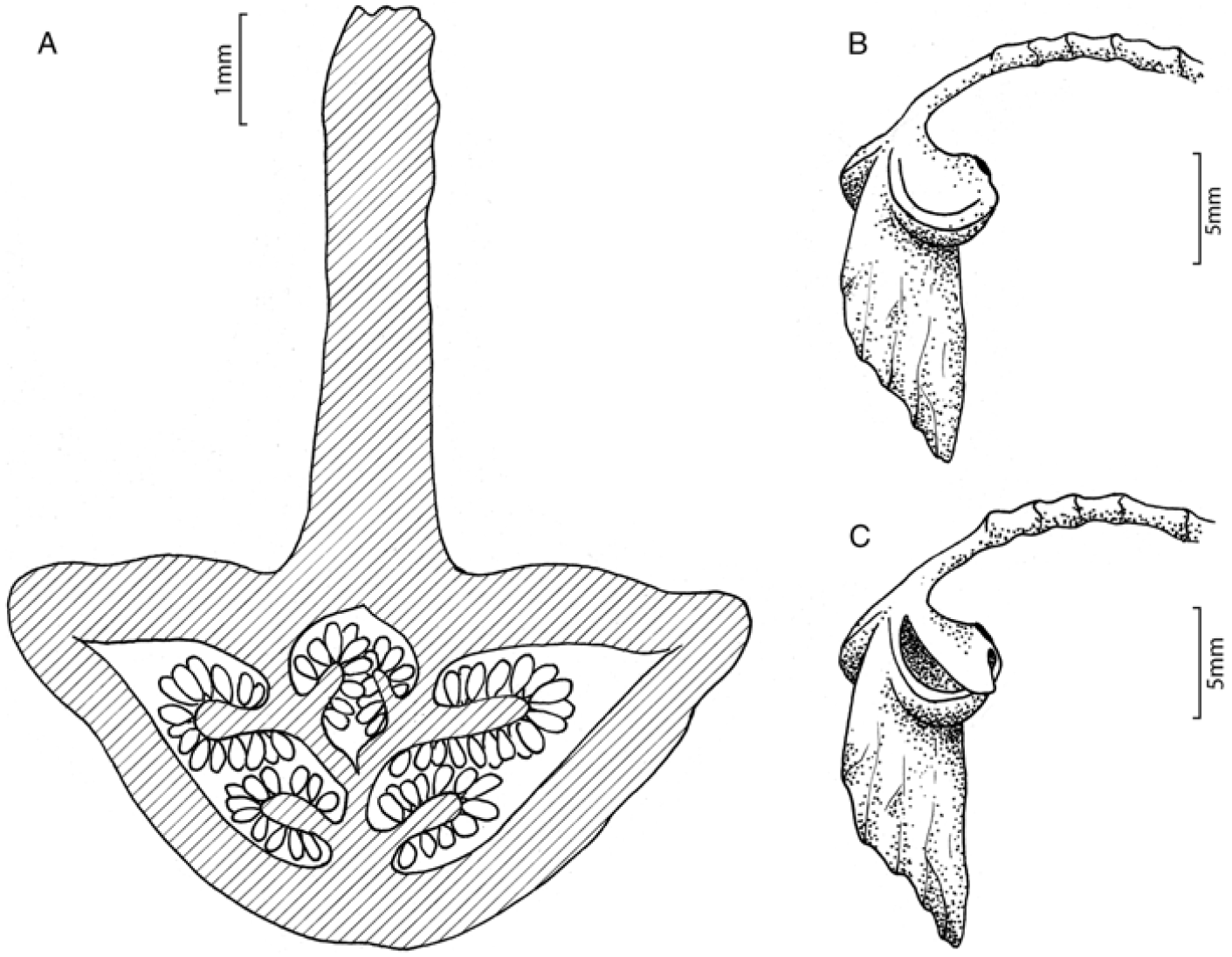
Fig. 3. Begonia maguniana H.P.Wilson. A, Transverse section through ovary, showing the three locules, each with two placentae; B, almost mature capsule showing line of dehiscence; C, mature capsule with dehisced ovary. (Drawings by Hannah P. Wilson.)
Proposed IUCN status. Without the isolated westernmost specimen (Danet 4285), the extent of occurrence (EOO) is 1255 km2 and the area of occupancy (AOO) is 28 km2; with this specimen, the EOO is 4879 km2 and the AOO is 32 km2. Either way, these figures suggest that Begonia maguniana should be regarded as Endangered (EN). There is no way to adapt EOO and AOO to take into account species with a naturally small distribution, as is common in Begonia. This species is thriving; it is present in numerous locations, with reasonably large populations where it occurs, and grows along trail sides (an indication that it can tolerate some disturbance). Furthermore, the montane forests of New Guinea are not under threat from logging, owing to their inaccessibility, so we consider an IUCN category of Least Concern to be appropriate (IUCN Standards and Petitions Subcommittee, 2017).
Acknowledgements
We are very grateful to the Ministry of Aviation Fellowship, particularly Siobhain and Ryan Cole, for their superb support in terms of travel logistics and for providing accommodation in Telefomin. We thank Rosalia and Lucas from Telefomin, Mr Ungip from Feranmin, Gideon from Tekin, and Patrick, Charles and Jaffen from Busilmin for their assistance and companionship in the field. The expedition was supported by the Papua New Guinea Forest Research Institute and the Papua New Guinea National Research Institute, and we particularly thank Dr Martin Golman and Georgia Kaipu for their assistance. We also thank the staff at LAE for their support, particularly Robert Kiapranis and Thomas Magun for hosting us and for assistance with permits. Funding for the expedition came from the Davis Expedition Fund, the Merlin Trust, the James and Eve Bennett Trust and the Friends of the Royal Botanic Garden Edinburgh. The Royal Botanic Garden Edinburgh is supported by the Scottish Government’s Rural and Environment Science and Analytical Services Division. This paper is part of the first author’s Ph.D. research, supported by the M.L. MacIntyre Begonia Trust.




