Introduction
The genus Entamoeba is constituted by cosmopolitan parasites belonging to the phylum Amoebozoa with world distribution. This genus contains seven species: Entamoeba histolytica, Entamoeba dispar, Entamoeba moshkovskii, Entamoeba coli, Entamoeba poleki, Entamoeba bangladeshi and Entamoeba hartmanni [Reference Fotedar1, Reference Royer2]. Among these, the first three (E. histolytica, E. dispar and E. moshkovskii) species are morphologically identical and considered as Entamoeba complex. Of those, only E. histolytica is the causative agent of amoebiasis, a global expanded gastrointestinal disease [Reference Haque3, Reference Ximénez4]. Although, some recent studies suggested that E. moshkovskii could have potential pathogenic effect in human [Reference Fotedar5, Reference Shimokawa6], E. dispar is still considered commensal organism [Reference Ali, Clark and Petri7].
Amebic infection is most prevalent in developing countries located in tropical and subtropical zones and its prevalence is associated with climatic conditions, sanitary and socio-economic status [Reference Nowak, Mastalska and Loster8]. It is estimated that about 50 million people were affected by invasive amoebiasis, resulting in up to 100 000 deaths per annum [Reference Haque3]. In a comprehensive global burden of disease study 1990–2010, Murray et al. (2013) reported 32 persons per 100 000 (95% confidence interval 25–41) disability-adjusted life years for amoebiasis [Reference Murray9].
Due to different biochemical, genetic and pathogenic features of Entamoeba complex, the differentiation of three aforementioned species is avery important issue in the effective clinical management of patients. For example, an infection with non-pathogenic species could mistakenly be diagnosed as E. histolytica infection and patient be unnecessarily treated with metronidazole that is the drug of choice for invasive amoebiasis, but not effective for the non-invasive species [Reference Al-Areeqi10, Reference Wolfe11].
Iran is one of the largest developing countries in Middle-East area with highly diverse geography, climatic and sociodemographic conditions. According to the Statistical Centre of Iran, the number of its total population is 80.28 million and approximately one-third of the people live below the national poverty line [12]. During the past years, several microscopy-based studies have investigated the prevalence of amoebiasis in different population groups, although discriminating studies between species (using molecular methods) are relatively few. According to the results of these studies, amoebiasis should be considered as a public health problem in Iran. Nevertheless, there is no comprehensive study showing the reliable status of amoebiasis at the national level. In this study, we performed a systematic review and meta-analysis to achieve an overview regarding the prevalence of amoebiasis and/or Entamoeba complex in Iranian people and also identify the different species circulating among of them.
Methods
Search strategy and study selection
This systematic review and meta-analysis study was implemented in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Reference Moher13]. To assess the prevalence of Entamoeba complex in Iran, relevant studies were searched from five English language databases (PubMed, Web of Science, Science Direct, Scopus and Google Scholar) and four Persian language databases (Scientific Information Database, Iran-Medex, Iran Doc and MagIran) from 1 January 1995 to 30 September 2017. This study was performed using the following keywords: ‘Iran’, ‘Islamic Republic of Iran’, ‘intestinal parasite’ ‘amoebiasis’, ‘Entamoeba’, ‘Entamoeba histolytica’, ‘Entamoeba dispar’, ‘Entamoeba moshkovskii’, ‘E. histolytica’, ‘E. dispar’, ‘E. moshkovskii’, ‘Entamoeba complex’, ‘epidemiology’, ‘frequency’, ‘prevalence’ ‘molecular epidemiology’ and ‘PCR’ alone or combined together with ‘OR’ and/or ‘AND’. Reference lists of retrieved articles were explored for additional studies. We restricted our search to human subjects. After duplicate removal, the initial title and abstract screening were performed by two independent researchers (A.R. and A.T.). Only peer-reviewed original observational studies reporting the prevalence of Entamoeba complex using stool examination were included. Serological studies, conference papers, reviews and letters or correspondences were excluded.
Data extraction and study quality assessment
A data extraction form in an Excel sheet was designed by three investigators, A.R., A.T. and A.H. Full-text review was performed for all the selected included papers by two independent researchers (A.R. and A.T.) and information were extracted and sorted for the following variables: the first author's last name, publication year, implementation year, name of study region, design of study, type of studied population, mean age or age range of studied population, total sample size, number of infected subjects, number of E. histolytica or E. dispar or E. moshkovskii, in molecular studies. In cases of disagreement in data extraction, the consensus was achieved through discussion with a third researcher (A.H.). In order to evaluate the quality assessment of included studies, we used the JBI (Joanna Briggs Institute) Prevalence Critical Appraisal Tool [Reference Munn14]. We divided included studies to five population sub-groups based on types of participants recruited: (1) general population, (2) children, (3) immunocompromised patients, (4) patients with gastrointestinal disorders and (5) mentally retarded patients. In this classification, transplanted individuals, HIV positive patients, individuals undergoing hemodialysis or chemotherapy and individuals taking immunosuppressive drugs were considered as immunocompromised patients. Moreover, to evaluate the impact of poverty on the prevalence of infection, studied provinces were divided into developed, relatively developed and undeveloped areas according to Human Development Index (HDI) [Reference Sabermahani15].
Data synthesis and statistical analysis
For the meta-analysis, we applied a random effects model to calculate pooled prevalence estimates with 95% confidence intervals using metaprop command in Stata software. Freeman-Tukey double arcsine transformation and score confidence intervals were applied to calculate the pooled prevalence in raw cell counts, for the individual studies [Reference Freeman and Tukey16–Reference Nyaga, Arbyn and Aerts18]. Heterogeneity between studies was assessed using the I 2 measure and the Cochran Q-statistic and an I 2 value above 75% indicates high heterogeneity [Reference Riahi and Mokhayeri19]. To address the sources of heterogeneity we separately performed meta-regression and subgroup analyses. Meta-regression was used for some predictors such as geographical latitude/longitude of different provinces and implementation years of studies. In addition, subgroup analysis was used for (HDI and type of participants. Assessing publication bias in prevalence studies is not routine and logical, because the main aim in these studies was only the estimate of prevalence and these studies did not examine the association between exposure an outcome (i.e. odds ratio, relative risk, etc.). Therefore, the extent of reported prevalence has no effect on the publication and in these studies, there was no publication bias. In all statistical analyses, the significance level was considered as P value < 0.05 and meta-analysis was done by using STATA version 13 (STATA Corp., College Station, Texas).
Retrieving sequence and phylogenetic tree
To show a cladistic relationship between the populations of Entamoeba spp. a maximum likelihood haplotype tree was drawn by MEGA 5.05 software. The sequences generated at 18S small subunit ribosomal RNA (18S rRNA) gene of E. histolytica (Accession nos: KX528457-KX528462) and E. moshkoskii (Accession no: AB520687) were directly retrieved from the GenBank database for FASTA format. The topology of the constructed tree was supported by bootstrap values of higher than 60%. Entamoeba bovis was considered as an out-group branch (Accession no: FN666250).
Results
Study characteristics
Our systematic literature search yielded 945 studies, of which 862 had not eligibility to be included in the quantitative analysis based on inclusion and exclusion criteria. A flowchart illustrating of the study selection process is depicted in Figure 1. Finally, a total of 71 studies involving 330 937 Iranian people were included in the meta-analysis. Among these, 36 studies (n = 237 117) were in general population, 17 (n = 64 471) in patients with gastrointestinal disorders, eight (n = 27 398) in children only, six studies (n = 910) in immunocompromised patients and four (n = 1041) in mentally retarded patients. The studies were performed in all geographical area of Iran. The majority of studies have cross-sectional design and few (some studies related with immunocompromised and mentally retarded patients) have a case-control design. Main characteristics of the included studies have been embedded in Table 1. Among the included studies, 17 (containing 462 polymerase chain reaction (PCR)-positive isolates) performed molecular analysis on Entamoeba complex isolates for inter-species differentiation (Table 2).
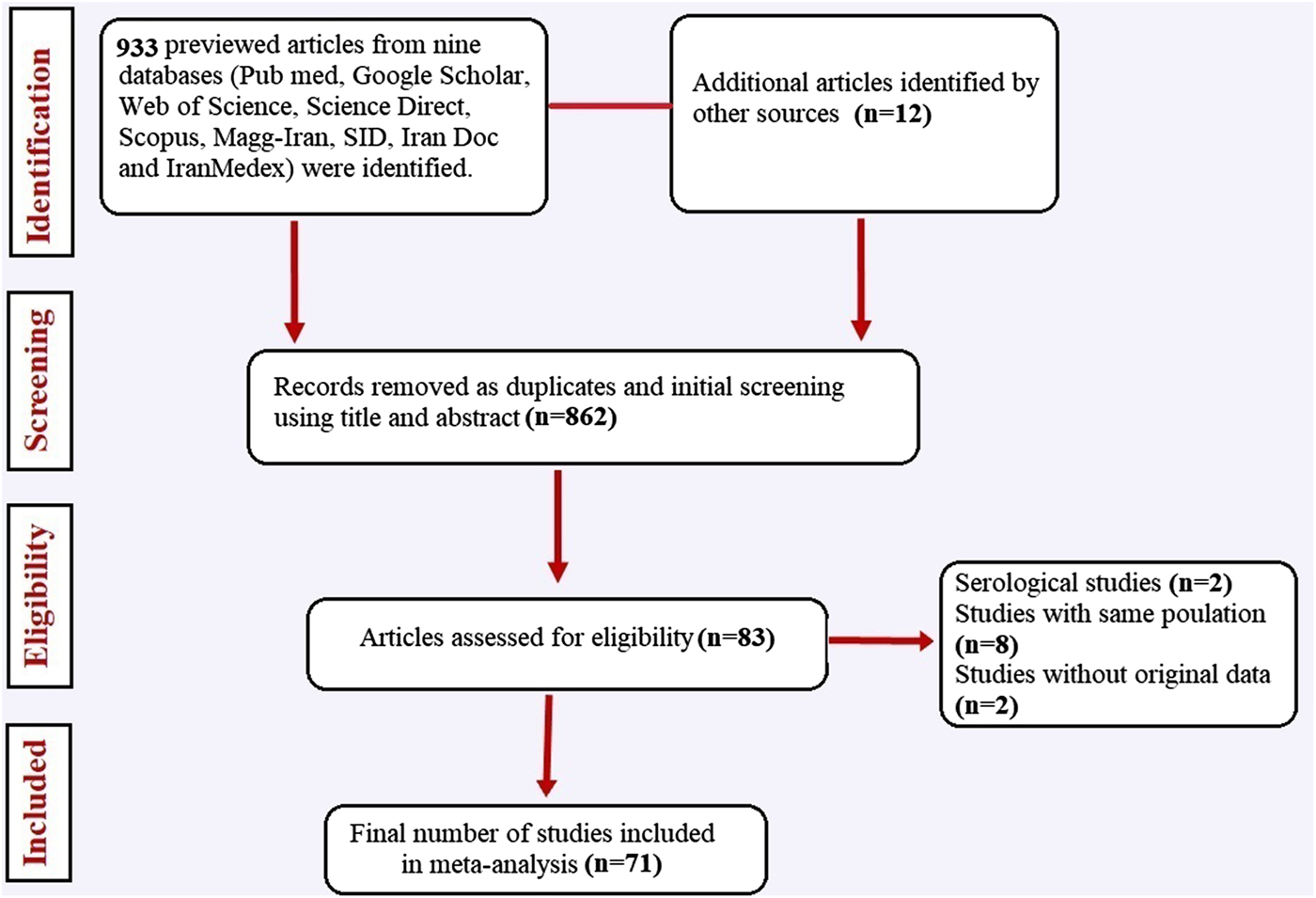
Fig. 1. Flow chart of the study selection process showing inclusion and exclusion of studies identified.
Table 1. Main characteristics of selected studies reporting the prevalence of Entamoeba complex in Iran
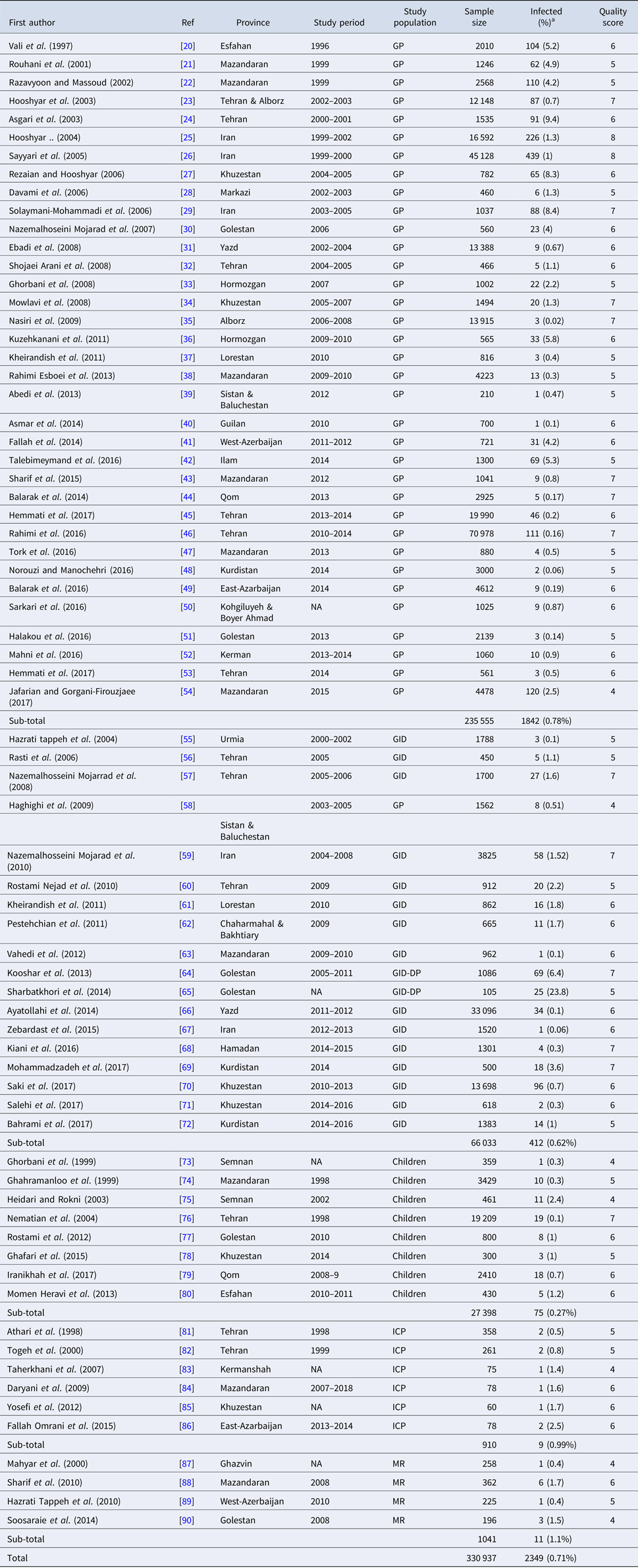
GP, general population; MR, mentally retarded patients; ICP, immunocompromised patients; GID, patients with gastrointestinal disorders; GID-DP, diarrheic patients with gastrointestinal disorders.
Studies are listed in order of year published.
a The percentages presented in this table are crude.
Table 2. Main characteristics of studies reporting molecular distinguish of Entamoeba complex

a The percentages presented in this table are crude.
Results of meta-analysis on prevalence using microscopic results
The pooled prevalence of Entamoeba infection among Iranian general population from 1995 to 2017 was 1% (95% CI 0.8–2.0%), however, there was significant heterogeneity in this meta-analysis (I 2 = 98.65%, P < 0.001) (Fig. 2 and Supplementary Fig. S1). A similar prevalence was observed among patients with gastrointestinal disorders, immunocompromised patients and mentally retarded patients 1% (95% CI 0.8–2.0%). The slowly lower prevalence was observed among children 1% (95% CI 0.0–1.0%) (Fig. 2). As shown in Figure 3 and Supplementary Figure S2, meta-regression analysis on implementation year demonstrated that it is a crucial source of heterogeneity (tau2 deceased from 0.0002 to 0) and prevalence of Entamoeba infection reduced during the time (1995–2017) (b = 0.009 P = 0.001 R 2 = 100%). Subgroup analysis regarding HDI demonstrated that prevalence of Entamoeba infection is higher in undeveloped provinces (2%, 95% CI 1–4%) compared with relatively developed (1%, 95% CI 1–3%) or developed provinces (1%, 95% CI 0–1%) (Supplementary Fig. S3). Meta-regression on the prevalence of Entamoeba infection in different provinces according to geographical latitude/longitude have not yielded any significant results (data not shown).
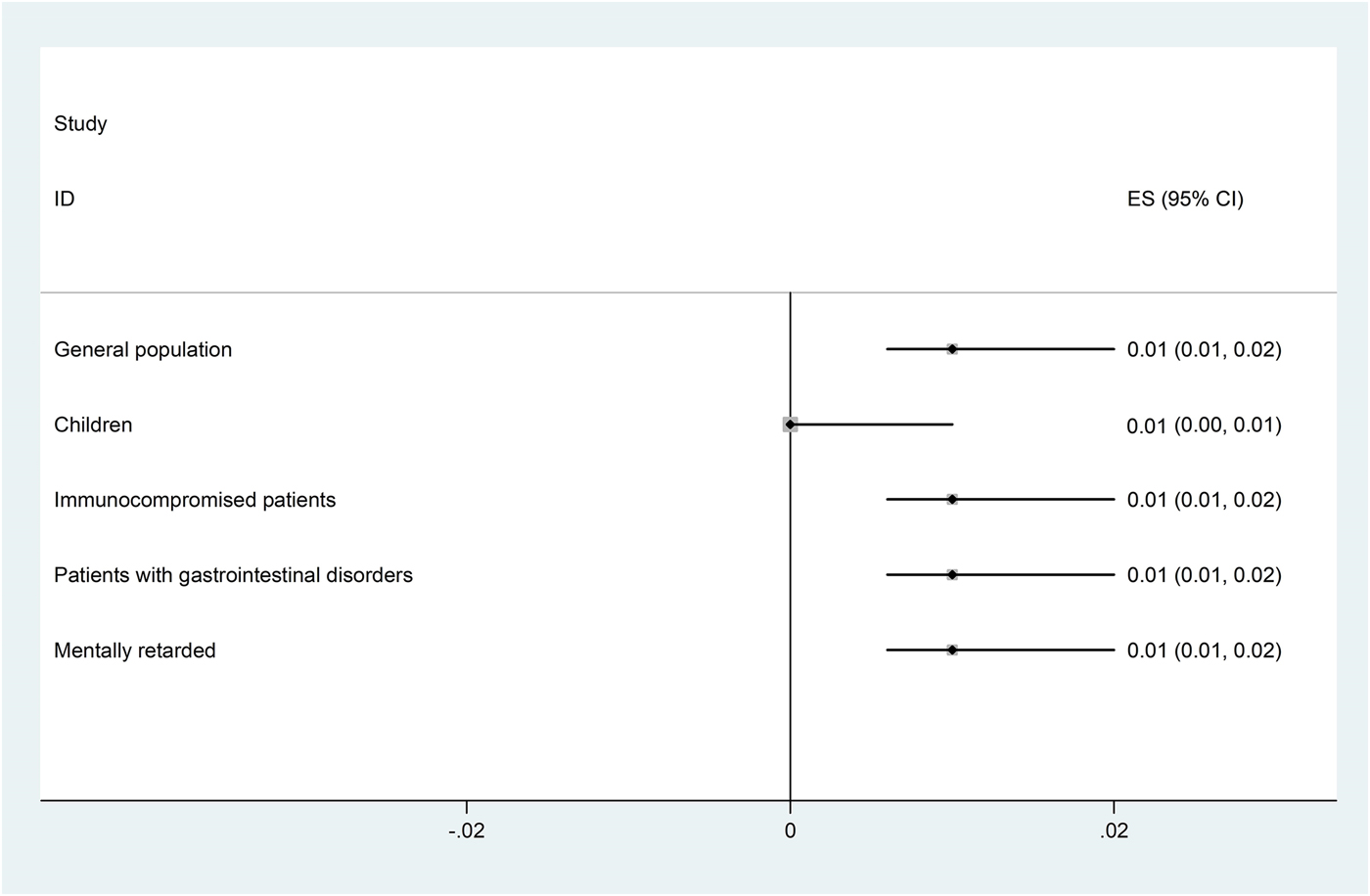
Fig. 2. Forest plot for random-effects meta-analysis on prevalence Entamoeba infection in different groups of Iranian population (using microscopic results).
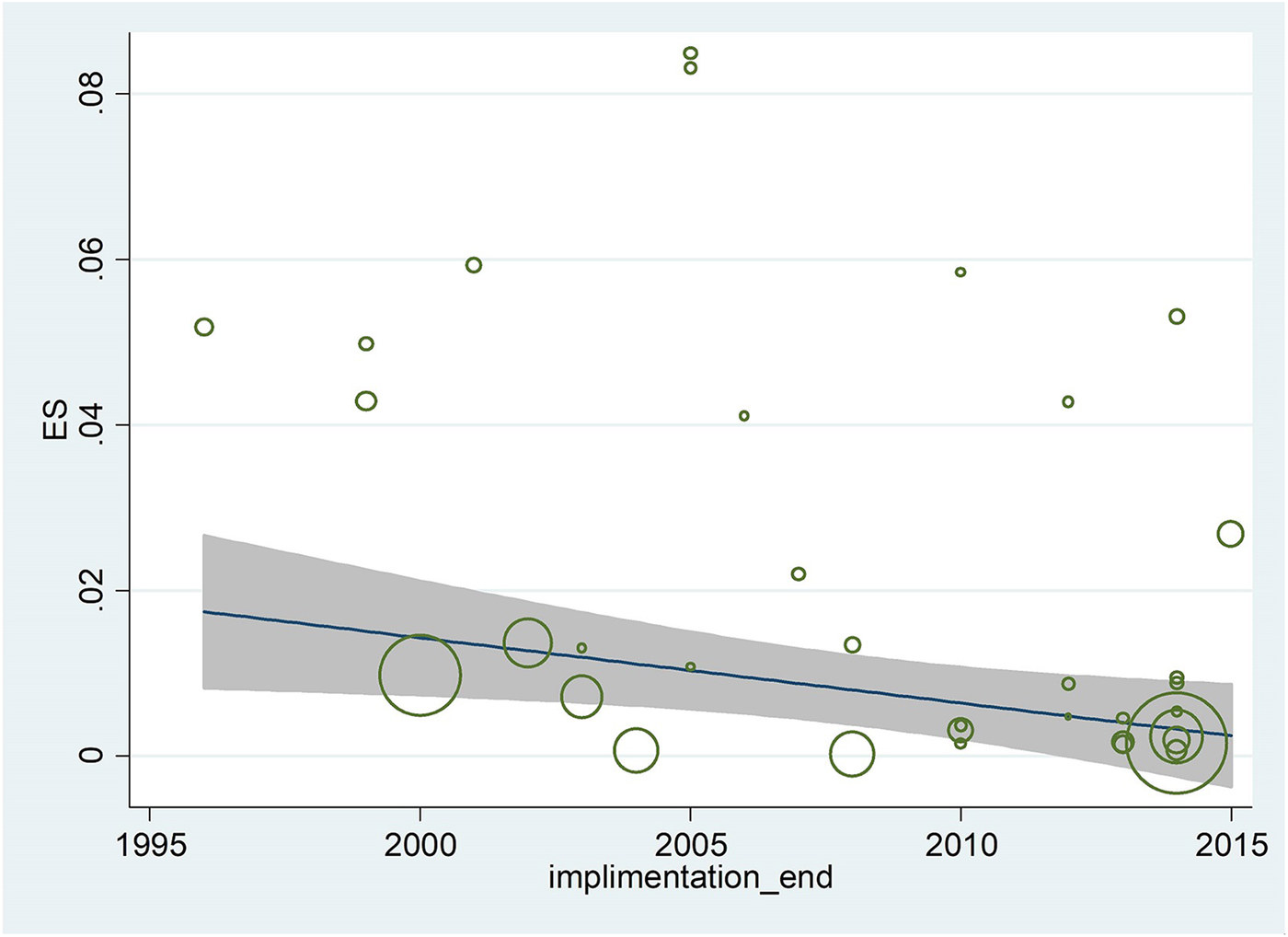
Fig. 3. Meta-regression regarding the effects of during time on the prevalence of Entamoeba infection in Iranian population.
Results of meta-analysis on molecular epidemiology
Seventeen studies involving 44 272 human subjects and 703 Entamoeba complex isolates have performed molecular analysis for inter-species differentiation. Out of 703 Entamoeba complex isolates, 462 isolates were successfully amplified and sequenced. Among these, 396, 55 and 11 isolates were identified as E. dispar E. histolytica and E. moshkovskii, respectively (Table 2). Meta-analysis demonstrated that 83% (95% CI 69–94%; I 2 = 87.8%) and 12% (95% CI 3–24%; I 2 = 85.9%) of isolates were E. dispar and E. histolytica, respectively (Fig. 4a, b). In subgroup analysis based on molecular results, in general population prevalence of E. dispar and E. histolytica were 91% (95% CI 80–99%) and 7% (95% CI 0–19%), while prevalence of these species in patients with gastrointestinal disorders were 75% (95% CI 45–96%) and 18% (95% CI 1–43%), respectively (Fig. 4a, b and Table 2). Related heterogeneities are shown in Figure 4a and b. Due to a low number of E. moshkovskii (11 positive isolates out of only six published articles; two (0.7%) in general population and nine (6%) in patients with gastrointestinal disorders) the prevalence of this Entamoeba based on meta-analysis was not calculable (Table 2).
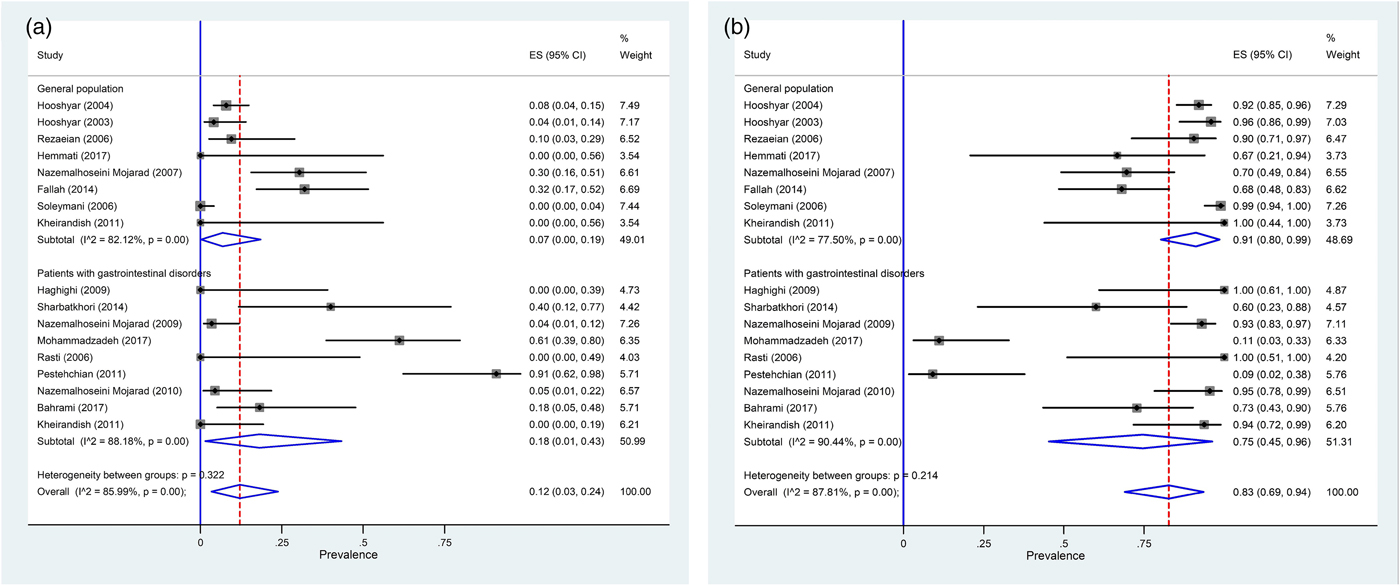
Fig. 4. Forest plot for random-effects meta-analysis on molecular prevalence Entamoeba histolytica (a) and Entamoeba dispar (b) among Iranian general population and patients with gastrointestinal disorders.
Sequence and phylogenetic analyses
The different clades of identified Entamoeba spp. is given in Figure 5 based on the 18S rRNA gene. Cladistic phylogenetic tree indicated the E. dispar clade has a sister relationship with E. histolytica clade in comparison with E. moshkowskii.
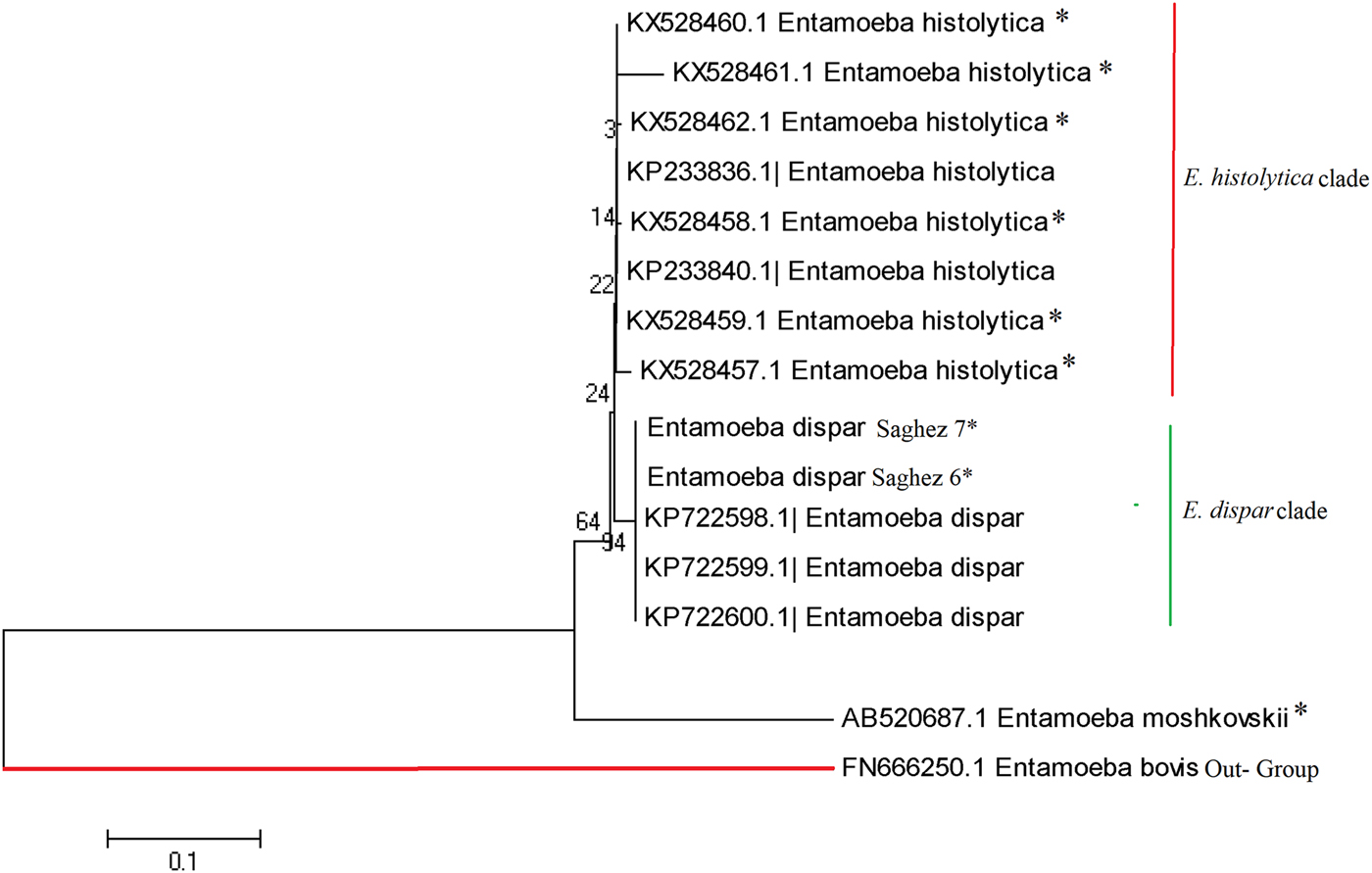
Fig. 5. Phylogenetic analysis of 5S nucleotide sequences of Entamoeba complex isolates recovered from different part of Iran.
Discussion
It is critically important to understand the prevalence of amoebiasis and distributing and molecular epidemiology of Entamoeba complex in developing countries located at tropical and sub-tropical countries. This systematic review and meta-analysis study, based on approximately 331 000 human subjects, 462 PCR-positive Entamoeba complex isolates, resulting from 71 studies, covering 25 provinces in Iran, enables us to judge reliable the prevalence and molecular epidemiology of Entamoeba complex at the national level. Results of our study showed that, nationally, one percent of Iranian people were infected by Entamoeba complex. Prevalence reported here (1%) is much lower than those reported from Ghana (39.8%), South Africa (27%), Mexico (21%), Brazil (21%), India (19%), Malaysia (18.6%) and also lower than reports from Middle East countries, including Yemen (59%), United Arab Emirates (30%), Turkey (2.5%) and Lebanon (2.3%) [Reference Al-Areeqi10, Reference Anuar91–Reference Verweij99]. The lower prevalence of Entamoeba complex in Iran could be explained by prevailing of dry climate in most parts of Iran and also higher sanitary status, which had a significant improvement in the three last decades. Our results showed a decrease in the prevalence of Entamoeba complex during the time, indicating that improvement of sanitary status and implementation of health educational programs in the three last decades in Iran was effective to reduce the intestinal parasites including Entamoeba complex. In this present study, we observed the similar prevalence of Entamoeba complex in different population groups. Although in contrast with our results, some previous studies demonstrated that Entamoeba infection is more prevalent in immunocompromised or mentally retarded patients and one of the major health problems in this individuals [Reference Hung100–Reference Tachibana104]. This could be explained by the fact that such individuals are suppressed in their immune responses and unable to provide adequate personal hygiene, poor environmental sanitations existing in mentally retarded institutions that is due to lack of toilet training and also direct person-to-person transmission of Entamoeba complex [Reference Sharif88, Reference Moran101]. Another result from the present study was a higher prevalence of Entamoeba infection in undeveloped provinces. In agreement with our results, it is well known that poverty, overcrowding, poor socioeconomic conditions, impoverished sanitation and hygiene conditions, as well as illiteracy and malnutrition, are main factors contributing to the high prevalence of Entamoeba infection [Reference Al-Areeqi10, Reference Lim105, Reference Ngui106].
Regarding the molecular epidemiology of Entamoeba complex in Iran, our result indicated that 396 (83%), 55 (12%) and 11 (2.4%) of the 480 PCR-positive isolates were E. dispar, E. histolytica and E. moshkovskii, respectively. In Yemen and United Arab Emirates, two southern neighbours of Iran, E. histolytica, E. dispar and E. moshkovskii were identified in 44.2%, 34.4% and 39.9% of the 276 PCR-positive products [Reference Al-Areeqi10] and 13.3%, 6.7% and 3.3% of the 120 samples, respectively [Reference ElBakri92]. Results from these studies suggest that in southern neighbour countries of Iran, where hot-humid climate prevails, E. histolytica is more prevalent than E. dispar. While in Turkey, a northwestern neighbour of Iran, study by Kurt et al., 2008 reported that E. histolytica and E. dispar were identified in 23.7% and 52.5% of the 59 PCR-positive products and in a study by Dagci et al., 2007 all obtained isolates were E. dispar, suggesting that E. dispar is the predominant species in Turkey, where hot- or cold-dry climate prevails [Reference Kurt93, Reference Dagci107]. In our study, E. dispar and E. moshkovskii have highest and lowest prevalence in Iranian people. Similar to our results, Anuar et al. [Reference Anuar91] in Malaysia reported that E. dispar was the most prevalent species (13.4%), followed by E. histolytica (3.2%) and E. moshkovskii (1.0%). While in Australia and Colombia E. moshkovskii had a similar prevalence to E. dispar [Reference Fotedar108, Reference López109]. The prevalence E. histolytica, E. dispar and E. moshkovskii were 5.6%, 70.8% and 61.8% in Australia and 0.55%, 23.2% and 25.4% in Colombia, respectively [Reference Fotedar108, Reference López109]. Molecular results observed in the present study highlight higher prevalence of E. histolytica in patients with gastrointestinal disorders (18%) compared with general population (7%) and contrariwise pattern for E. dispar that had a higher prevalence in general population (91%) than patients with gastrointestinal disorders (75%). This result is corroborated by previous studies in different part of world, indicating that E. dispar is responsible for asymptomatic amoebic infection and higher E. histolytica burden is associated with diarrhoeal or gastrointestinal symptoms [Reference Anuar91, Reference Ngobeni95, Reference Gilchrist110–Reference Taniuchi112]. An interesting result in our study is a higher prevalence of E. moshkovskii in patients with gastrointestinal disorders (6%) than the general population (0.7%). This result is in agreement with some recent studies suggesting that E. moshkovskii could have potential pathogenic effect in humans [Reference Fotedar5, Reference Ali113, Reference Yakoob114]. In line with these studies, Shimokawa et al. [Reference Shimokawa6], demonstrated that E. moshkovskii is associated with diarrhoea in infants and causes diarrhea and colitis in mice.
The Cladistic phylogenetic tree disclosed that the E. moshkowskii has a greater genetic variability (a distinct branch with distance scale 20%) than E. histolytica/E. dispar clades (Fig. 5). Mohammadzadeh et al.[Reference Mohammadzadeh69] have recently identified a new mutant of E. moshkovskii in a dysentery fecal sample from Saghez city, Kurdistan province, Northwest Iran. This indicates that occurrence of single nucleotide polymorphism can potentially play a pivotal role on pathogenicity rate of E. moshkovskii in clinical isolates. However, more studies with a higher case number are required on ethnic population from different geographic regions of Iran, in order to verify this assumption.
The strengths of this study included the large number of included studies, very large and diverse baseline population, covering different provinces and geographical areas of Iran, rigorous methodology, presentation of pooled data to highlight differences within and between different population groups, determination of molecular epidemiology regarding to high number of Entamoeba isolates and genetic characterisation of Entamoeba species. Moreover, this study is likely limited by significant heterogeneity existing between studies, lacking data for some few provinces and also underestimation the true prevalence, due to the different proficiency of the experimenter in included studies.
In conclusion, despite these limitations, this systematic review and meta-analysis study provides a comprehensive overview of the prevalence and molecular epidemiology of amoebiasis in Iran. We have found that there is the low burden of Entamoeba complex infection (1%) in Iran, although lower developed areas were more influenced. Moreover, our results have shown that less- or non-phatogenic Entamoeba isolate (E. dispar) is the predominant specie in Iran. The decline in prevalence of Entamoeba complex observed across time is likely due to growing standards of living and improved hygiene status in Iran in last years. These data should be taken into consideration by the health authorities of the country and, given the very low incidence of amoebiasis, proper diagnosis and treatment of patients should be done correctly. We suggest additional investigations to further clarify the prevalence of amoebiasis in Iran based on both epidemiological and molecular studies, to guide the development of appropriate public health interventions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818001863
Acknowledgements
The authors would like to thank Dr Vahid Fallah Omrani, for his assistance during the preparation of this manuscript.
Author contributions
A.R., S.M.R., A.T, A.S, M.E.D, M.M and A.H. conceived the study; A.R., A.T. and A.H initially searched the literature; A.R. A.T, A.S and M.M. collected all data; A.R. S.M.R, A.T and A.H., assessed the included articles; A.R., A.H., M.E.D and S.M.R. analysed and interpreted the data; A.R, A.S and A.H drafted the manuscript; and all authors commented on the drafts of the manuscript and approved the final draft of the paper.
Conflict interest
The authors declare that there is no conflict of interests regarding the publication of this paper.










