INTRODUCTION
Leptospirosis is a bacterial zoonosis of ubiquitous distribution that significantly challenges livestock health and production worldwide. Cattle are maintenance hosts for serovar Hardjo, of which there are two strains: Hardjobovis (Leptospira borgpetersenii) and Hardjoprajitno (L. interrogans sensu stricto), which have genetic, epidemiological and pathogenic differences, but are indistinguishable by serology [Reference Bolin1]. Typically, cattle become infected by direct contact with contaminated urine or genital fluids of infected animals, or indirectly from a contaminated environment, but venereal and transplacental transmission may also occur [Reference Bolin1]. Bovine leptospirosis is associated with reproductive disease, including abortion, stillbirth, weak calf syndrome, infertility and milk drop syndrome [Reference Bolin1, Reference Guitian2]. Infected cattle can become chronic carriers excreting leptospires in the urine for up to 542 days [Reference Thiermann3], thereby serving as reservoirs for leptospiral transmission to other animals and to humans, especially those professionally exposed [Reference Benschop4].
Cattle herds can be protected from leptospirosis through the combined application of biosecurity measures, vaccination and selective chemoprophylaxis [Reference Bolin1]. Biosecurity measures are primarily aimed at minimizing the risk of introducing leptospires into a herd from outside sources and preventing the spread of infection within and outside the herd (biocontainment). Standard vaccination programmes for bovine leptospirosis typically start with calves aged 4–6 weeks and are based on the administration of two doses of vaccine, 4–6 weeks apart, followed by an annual (in closed herds) or semi-annual (in open herds) booster [Reference Bolin1]. Although development of an efficacious vaccine for bovine leptospirosis has been somewhat elusive [Reference Bolin5, Reference Bolin6], the currently available commercial vaccine formulated with chemically inactivated whole cultures of L. borgpetersenii serovar Hardjo strain Hardjobovis (Spirovac®, Pfizer, USA) has been shown to protect healthy cattle from reproductive disease, tissue colonization and urinary shedding when challenged 4 months after vaccination [Reference Bolin and Alt7]. According to the manufacturer's claim, such protection has been demonstrated to start 3 weeks after administration of the second dose of vaccine and to last for at least 12 months. Antibiotic treatment eliminates leptospires from tissues and stops leptospiruria, also providing a means of protection by limiting the degree of environmental load of leptospires until immunity is induced by vaccination [Reference Gerritsen8].
In Italy, bovine leptospirosis is a mandatorily notifiable disease. Outbreak detection is primarily based on syndromic surveillance, targeting cattle herds with a history of abortion or reproductive failure [9]. However, veterinary control programmes have a major constraint, as no commercial vaccine against bovine leptospirosis is currently licensed in Italy. The importation and use (under derogation) of vaccines licensed in other countries must be authorized by the Ministry of Health. The enforcement of appropriate control measures is further complicated by the ban on trade of Leptospira-seropositive animals, even after mandatory antibiotic treatment and discretionary vaccination. Paradoxically, as naturally acquired antibodies to leptospires can persist for months and, in some cases, years [Reference Bolin10], some persistently seropositive animals might even spend their entire productive life under sanitary restriction notwithstanding the absence of disease and leptospiruria, causing considerable economic losses and extra workload for the farmers and veterinary authorities.
Between 2007 and 2011, two abortion outbreaks of bovine leptospirosis were detected in northeastern Italy, an area particularly prone to leptospiral infections in humans and animals other than cattle [Reference Ciceroni11, Reference Ciceroni12]. These outbreaks provided a unique opportunity to apply and assess the value of an integrated outbreak management plan based on enhanced biosecurity measures, antibiotic treatment and vaccination in order to prevent further leptospiral circulation. The main aims of this study were to measure and characterize the vaccine-elicited immune response in terms of seropositivity rates and agglutinating antibody titres for serovar Hardjo developed in field conditions 3 and 24 weeks after vaccination, and to combine molecular, cultural and pathological analyses to supplement serological tests in substantiating that leptospiral circulation was effectively controlled.
METHODS
Outbreak description
The first outbreak (O1), partly described elsewhere [Reference Natale13], was detected in October 2007 in a dairy cattle farm in the province of Padua, northeastern Italy, showing a seroprevalence of 49% (n = 85). During the following 4 years, this outbreak could not be resolved, as there were still seropositive cows leading to fluctuating seroprevalence (Fig. 1), an indication of persistent leptospiral circulation probably maintained by either carriers that cyclically re-infected young stock or re-infection from pastures during the grazing season.
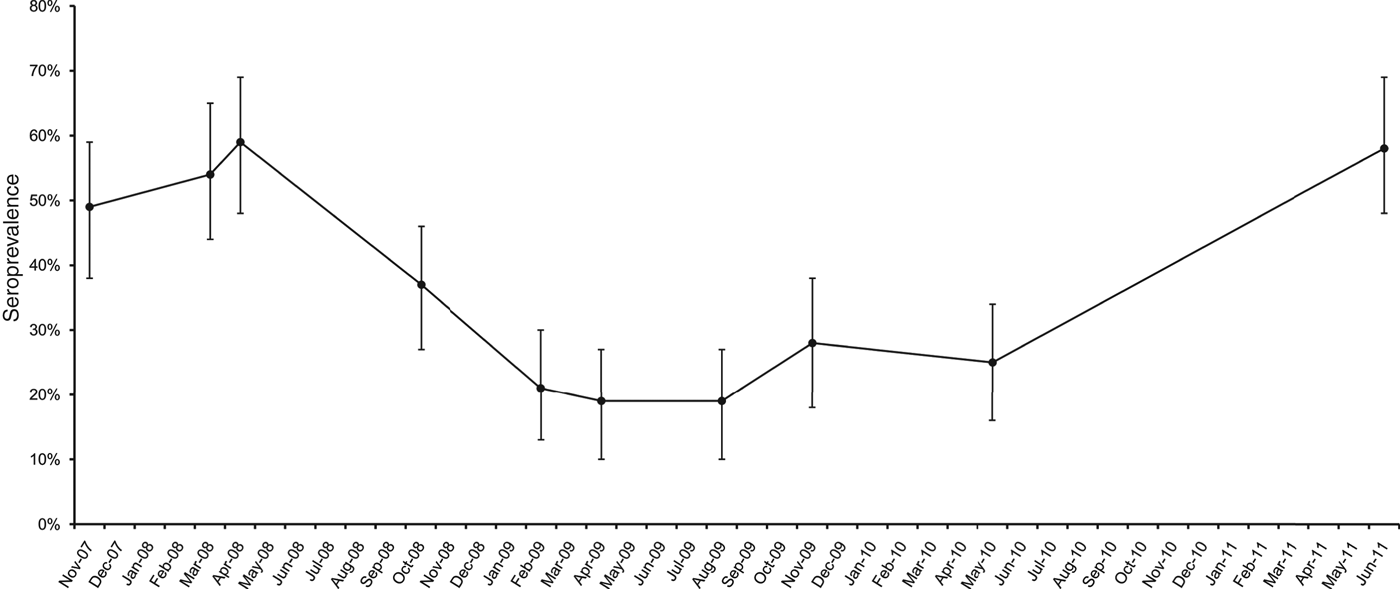
Fig. 1. Pre-vaccination seroprevalence for serovar Hardjo in a dairy cattle farm in Padua province, northeastern Italy. Error bars represent 95% confidence interval.
The second outbreak (O2) was detected in March 2011 in another dairy cattle farm in the province of Vicenza, at <20 km distance from the other affected farm, showing a seroprevalence of 57% (n = 324). According to the regional control programme in force for bovine abortion [9], the aborting cows and their aborted foetuses from both outbreaks were tested for brucellosis, bovine viral diarrhoea (BVD) and infectious bovine rhinotracheitis (IBR) viruses, neosporosis, chlamydiosis, Q fever and other common abortigenic bacteria (e.g. Campylobacter, Salmonella, etc.) in addition to leptospirosis. However, except for leptospirosis, none of these tests gave positive results [Reference Natale13].
Vaccination and serological testing
On November 2011, all animals aged >4 weeks present in O1 (n = 94) and O2 (n = 340) were vaccinated twice, 4 weeks apart, with Spirovac, according to the manufacturer's instructions. A preliminary serological screening of all animals was performed before vaccination. This allowed for the differentiation of seropositive (naturally infected) from seronegative (putatively non-infected) cows within the two affected farms. All animals were identified individually and seronegative cows were moved to a clean stable, away from seropositive cows. Seropositivity rates and vaccine-elicited antibody titres were measured in paired serum samples from putatively non-infected cows at 3 weeks (T1) and 24 weeks (T2) after administration of the second dose of vaccine.
Serum samples were taken and processed for the presence of leptospiral antibodies using the micro-agglutination test (MAT) [Reference Bolin10]. Live cultures of the following nine serovars, prevalent in the areas in question [Reference Ciceroni11, Reference Ciceroni12, Reference Ebani14], and checked for purity, mobility and agglutination power [Reference Zuerner15] were used as antigens: Bratislava (strain Riccio 2, serogroup Australis), Canicola (strain Alarik, serogroup Canicola), Grippotyphosa (strain Moska V, serogroup Grippotyphosa), Copenhageni (strain Wijnberg, serogroup Icterohaemorrhagiae), Icterohaemorrhagiae (strain Ictero 1, serogroup Icterohaemorrhagiae), Pomona (strain Pomona, serogroup Pomona), Hardjo (strain Hardjoprajitno, serogroup Sejroe), Tarassovi (strain Mitis, serogroup Tarassovi) and Ballum (strain Swart, serogroup Bataviae). Sera with antibody titres ⩾1:100 were considered positive. Twofold serial dilutions were successively tested to determine the endpoint titre.
Antibiotic treatment
Before vaccination, all non-lactating cows were treated with oxytetracycline (Panterramicina®, Pfizer, USA), 50 cc/cow per day for 5 days, according to the manufacturer's instructions. Treatment in lactating cows was postponed in the forthcoming dry period to save market milk.
Demonstration of leptospires in urine and kidneys
Urine samples of infected cows were collected in O1 and O2, before and after antibiotic treatment and vaccination (Table 1), and were processed according to standardized methods for leptospiral culture on Ellinghausen–MacCullough–Johnson–Harris (EMJH) semi-solid medium with 5-fluorouracil as a selective agent [Reference Bolin10] and for real-time PCR detection of bacterial DNA [Reference Smythe16], which targets a fragment of 87 bp corresponding to a portion of the gene encoding the 16S rDNA expressed in vivo by leptospires. A volume of 2 ml urine from each sample was centrifuged at 12 000 g for 20 min and the supernatant was poured out. After addition of poly-A carrier to increase efficiency of nucleic acid recovery, DNA extraction was performed using a commercially available kit (High Pure PCR Template Preparation kit, Roche Diagnostics, Germany). According to manufacturer's instructions, a sample was considered positive when, after amplification, a FAM fluorescence signal was detected by the 7900HT fast real-time PCR system within a cycle threshold value of 38.
Table 1. Number of urine and kidney samples taken from cows housed in the two Leptospira-affected dairy farms in Padua (O1) and Vicenza (O2) provinces, northeastern Italy, that were tested for the presence of leptospires by real-time PCR before and after antibiotic treatment and vaccination
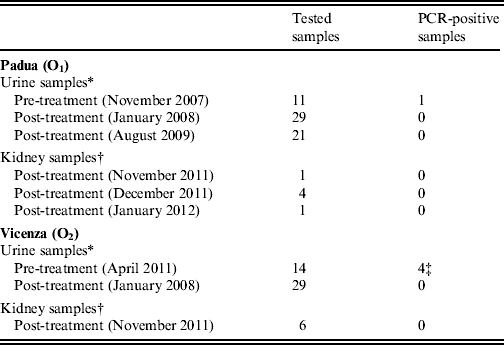
* Each of the samples was taken individually from different animals that were naturally seropositive to infection with serovar Hardjo.
† Each of the samples refers to both kidneys taken at necropsy of 12 cows sent to abattoir, nine of which were naturally seropositive to serovar Hardjo (six from O1, three from O2) and three (from O2) were seronegative, i.e. putatively non-infected.
‡ Two of which borderline positive.
Gross pathology of kidneys was investigated at necropsy of nine naturally seropositive (six from O1, three from O2) and three putatively non-infected (from O2) cows sent to abattoir at different times after antibiotic treatment and vaccination (Table 1). Kidney samples were collected at necropsy and processed for histopathology as described previously [Reference Bolin5]. Kidney samples were also processed for leptospiral culture [Reference Bolin10] and real-time PCR detection of bacterial DNA [Reference Smythe16].
Biosecurity measures
In both farms, enhanced biosecurity measures were implemented, including new on-farm pest control and extra environmental sanitization programmes, removal of piles of discarded material (scrapheap), mowing perimeter areas, fencing-off cattle pastures from those grazed by other herds or by different livestock (especially sheep and pigs) or wildlife, as well as limiting access to potentially contaminated (water) sources, use of catch basins to prevent run-off and leaching from stockpiled manure, separate housing for naturally infected animals (confinement rearing), maintenance of a closed herd (ban on animal movements), and administration of high-quality vitamin and mineral supplements for preventing pica (depraved appetite), particularly drinking of slurry and urine (sometimes drunk directly from other animals), which was frequently observed among heifers.
Statistical analysis
Differences in seropositivity rates between time periods, farms and age groups at first vaccination (calves aged <12 months, heifers aged 12–35 months, and adult cows aged >36 months) were tested using χ 2 or Fisher's exact tests, while those in post-vaccination antibody titres were tested using Mann–Whitney or Kruskal–Wallis tests, as appropriate. Equality of matched pairs of post-vaccination antibody titres between T1 and T2 was tested using Wilcoxon's matched-pairs signed-ranks test.
The proportional similarity index (PSI) [Reference Smid17] was used as a measure of the area of intersection of antibody titre distributions between T1 and T2. The PSI is calculated as PSI = 1–0·5 ∑ i |T1i − T2i |, where |T1i − T2i | is the absolute value of the difference in the relative frequencies of antibody titres with order of magnitude i out of all orders of magnitude determined at T1 and T2. PSI values range from 0 (no antibody titre in common) to 1 (identical antibody titre distributions). Confidence intervals (CIs) for the PSI were approximated by bootstrapping [Reference Smid17].
A special problem in analysing antibody titre data as response variable in regression models is that such response variable is incompletely observed, i.e. there are measurements (7% in this study) lying below the minimum detection limit of 1:100 (seronegative animals), and hence are left-censored. A Tobit regression model [Reference Tobin18] was therefore used for analysing antibody titres with a value <100 as being the left-censoring limit. The Tobit regression model allows for the use of all observations to estimate the regression line by assuming a latent variable that is linearly dependent on the predictors via their betas, and is generally to be preferred when dealing with censored data [Reference Tobin18]. Antibody titre values were log-transformed prior to Tobit regression analysis to meet the assumptions of homoscedasticity and normality of disturbances. Covariates included were the sampling time (dichotomous variable, T1 = 0 and T2 = 1), farm (dichotomous variable, O1 = 0 and O2 = 1), and cow's age at first vaccination expressed in months (continuous variable, treated as linear splines with knots at calves/heifers and heifers/adult cows). Effects of these covariates on log-transformed antibody titres are interpreted as the change in the expected geometric mean of the response variable of those above the left-censoring limit (uncensored antibody titres), weighted by the probability of being above that limit, while accounting for lack of independence of observations made on the same animals at T1 and T2 (repeated measurements) using a cluster-correlated robust variance estimator [Reference Williams19]. First-order interactions were also tested after establishing the main effect model. Statistical analysis was performed using Stata v. 11 (StataCorp., USA), and statistical significance was set at P < 0·05.
RESULTS
None of the animals had aborted or showed other symptoms of disease during the 27 weeks following antibiotic treatment and vaccination.
Serological response
Preliminary whole-herd serological screening revealed that 39/94 cows in O1 and 141/340 cows in O2 were naturally seropositive for serovar Hardjo, resulting in a seroprevalence of 41·5% (95% CI 31·4–52·1) in O1 and 41·5% (95% CI 36·1–46·9) in O2. Of the 55 and 199 seronegative cows in O1 and O2, respectively, 35 in O1 and 90 in O2 were re-tested serologically at T1, 3 weeks after vaccination. At T1, all of the 35 seronegative cows in O1 had seroconverted (100% seropositivity, 95% CI 90–100), with antibody titres ranging from 400 to 3200 and a median of 800 (geometric mean 1143, 95% CI 918–1422) (Fig. 2). In O2, 86/90 seronegative cows had seroconverted at T1 (96% seropositivity, 95% CI 89–99), with antibody titres ranging from 100 to 12 800 and a median of 800 (geometric mean 1003, 95% CI 779–1290) (Fig. 2). At T1, seroconversion rates did not differ significantly between O1 and O2 (P = 0·576), nor did their antibody titre distributions (P = 0·646).
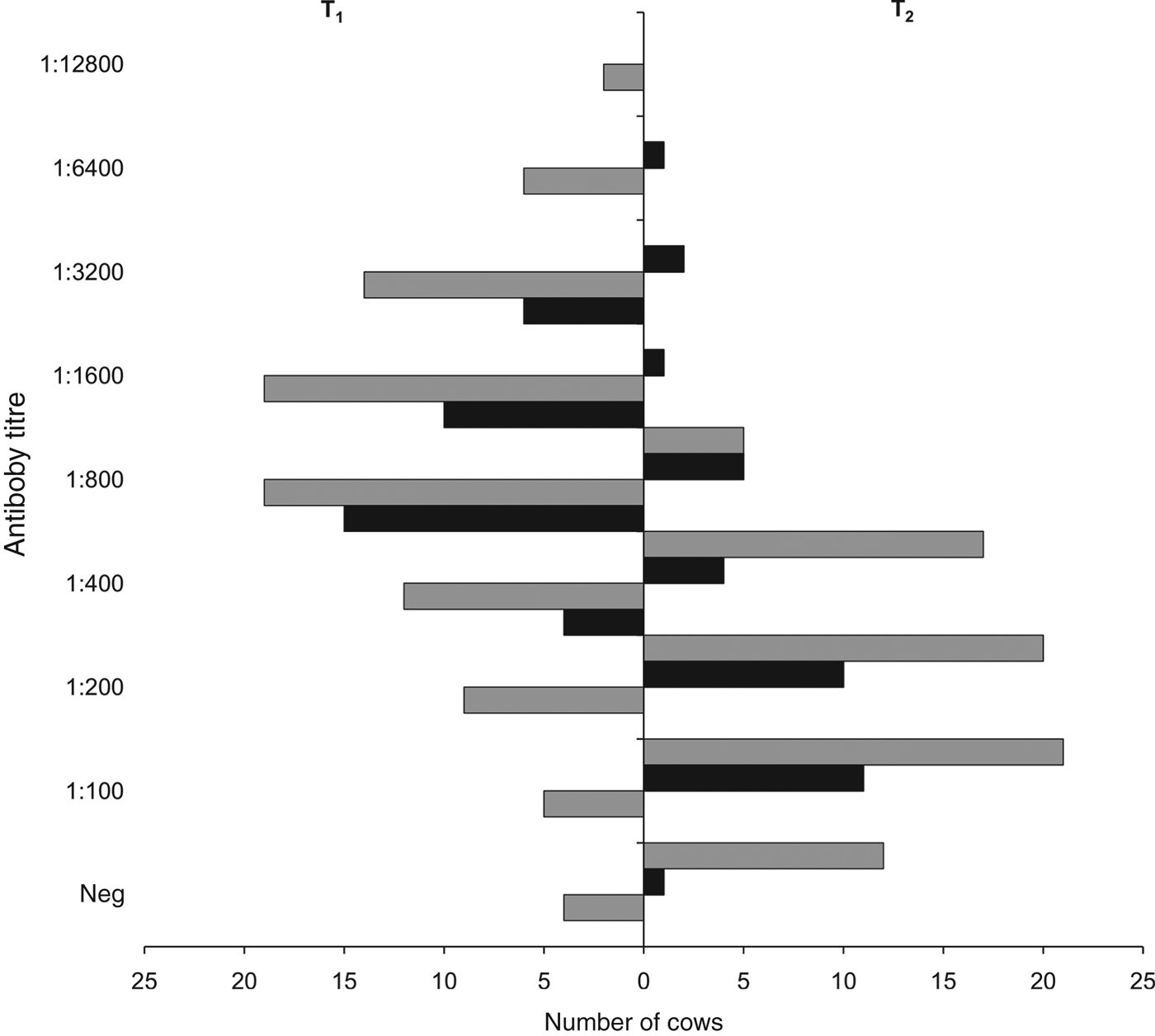
Fig. 2. Micro-agglutination test antibody titres for serovar Hardjo, 3 weeks (T1) and 24 weeks (T2) after vaccination, in the two affected dairy cattle farms in the provinces of Padua (O1, ■) and Vicenza (O2, ![]() ), northeastern Italy. Neg, Seronegative cows (antibody titre <100). Number of serum samples tested: in O1, 35 at T1 (100% seropositivity) and 35 (97% seropositivity) at T2; in O2, 90 (96% seropositivity) at T1 and 75 (84% seropositivity) at T2.
), northeastern Italy. Neg, Seronegative cows (antibody titre <100). Number of serum samples tested: in O1, 35 at T1 (100% seropositivity) and 35 (97% seropositivity) at T2; in O2, 90 (96% seropositivity) at T1 and 75 (84% seropositivity) at T2.
At T2, 6 months after vaccination, all the 35 cows that had seroconverted in O1 were re-tested and 34 of them were still seropositive (97% seropositivity, 95% CI 85–100), with antibody titres ranging from 100 to 6400 and a median of 200 (geometric mean 295, 95% CI 197–440) (Fig. 2). In O2, 75/90 cows tested at T1 were re-tested at T2 (the other 15 cows were sent to the abattoir) and 63 of them were still seropositive (84% seropositivity, 95% CI 74–91), with antibody titres ranging from 100 to 800 and a median of 200 (geometric mean 214, 95% CI 181–253) (Fig. 2). At T2, difference in seropositivity rates between O1 and O2 was borderline insignificant (P = 0·060), while their antibody titre distributions did not differ significantly (P = 0·399).
As there were no significant differences in seropositivity rates and antibody titre distributions between O1 and O2, their data were pooled and compared between T1 and T2. This revealed that, overall, the seropositivity rate at T2 (88%, 95% CI 82–94) was significantly lower (P = 0·011) than that at T1 (97%, 95% CI 93–100). Moreover, the antibody titres at T2 (median 200, range 100–6400; geometric mean 239, 95% CI 201–285) were significantly lower (P < 0·001) than those at T1 (median 800, range 100–128 00 geometric mean 1041, 95% CI 862–1257). PSI for antibody titre distributions between T1 and T2 was 0·37 (95% CI 0·12–0·59), i.e. they overlapped with one another by 37%, indicating that they were more different than they were similar (Fig. 3).
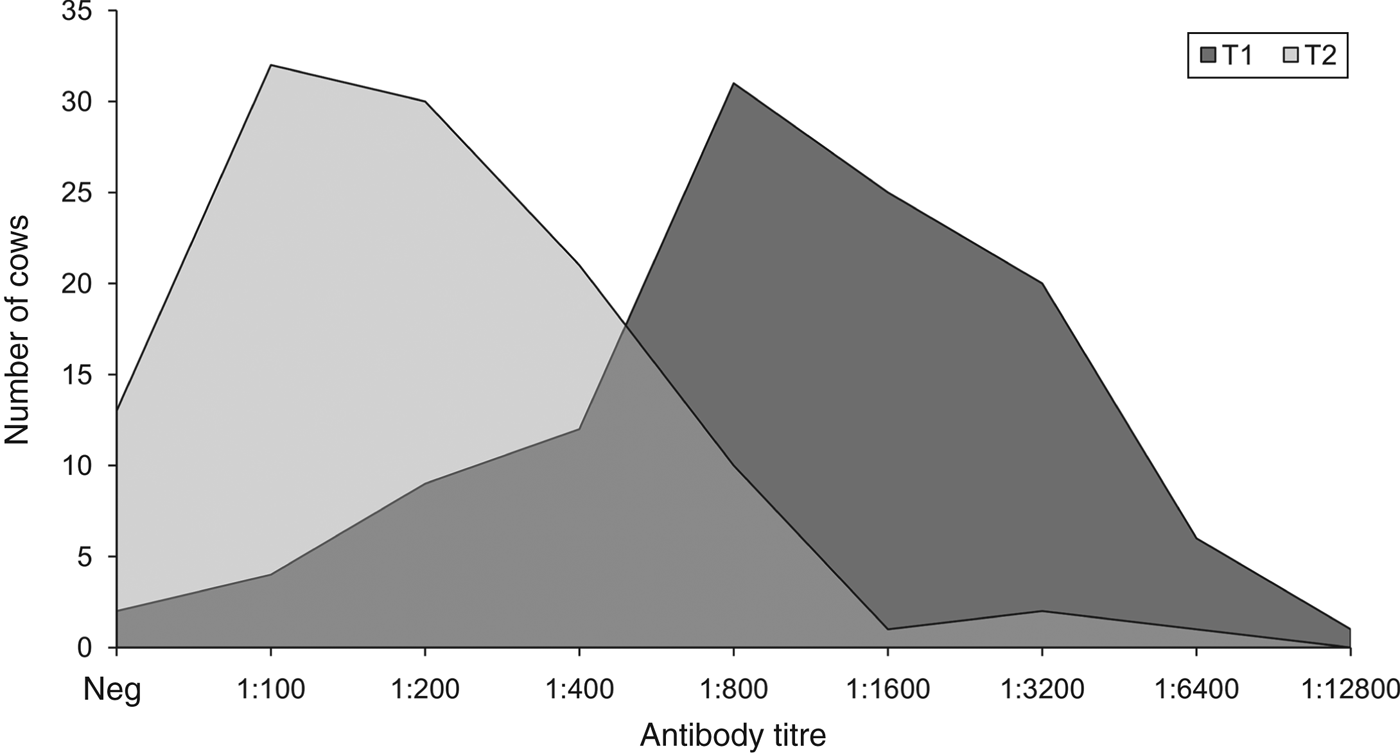
Fig. 3. Distributions of antibody titres for serovar Hardjo 3 weeks (T1) and 24 weeks (T2) after vaccination. Neg, Seronegative cows (antibody titre <100). Number of tested serum samples: 125 at T1 (97% seropositivity) and 110 at T2 (88% seropositivity).
Seropositivity rates and antibody titres in calves, heifers and adult cows are reported in Table 2. Overall (O1 and O2 combined), significant differences in antibody titres in age groups were found at both T1 (P = 0·008) and T2 (P = 0·018).
Table 2. Seropositivity rate and MAT antibody titres to serovar Hardjo in calves, heifers and adult cows 3 weeks (T1) and 24 weeks (T2) after vaccination in the two affected dairy farms in Padua (O1) and Vicenza (O2) provinces, northeastern Italy
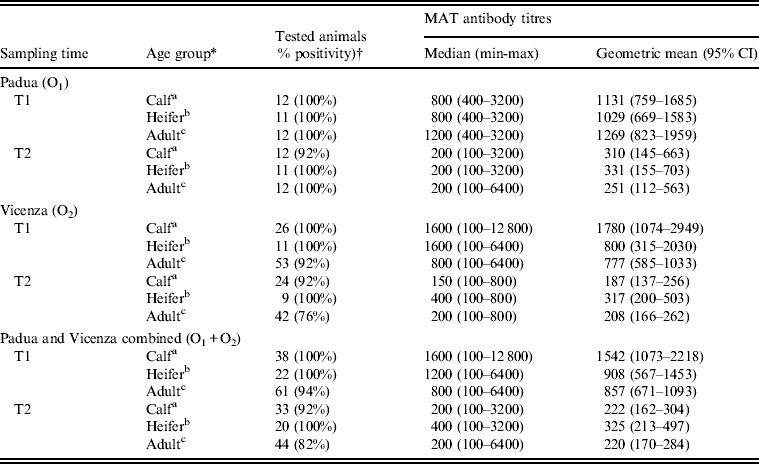
MAT, Micro-agglutination test; CI, confidence interval.
* Age group: a <12 months; b 13–35 months; c >36 months.
† Sera with antibody titres ⩾1:100 were considered positive.
The Tobit regression model included sampling time, age and farm as main effects. No significant interactions between these variables were found. It was estimated that the expected geometric mean of uncensored antibody titres decreased significantly from T1 to T2 by 84·7% (95% CI 76·2–90·1, P < 0·001). For each month increase in calves' age at first vaccination, the expected geometric mean of calves' uncensored antibody titres was estimated to increase by 26·3% (95% CI 6·1–50·2, P = 0·009), while for each month increase in adult cows' age at first vaccination, the expected geometric mean of adult cows' uncensored antibody titres was estimated to decrease by 2·5% (95% CI 1·0–5·1%, P = 0·039). No significant associations (P > 0·05) with farm of origin or with heifers' age were found.
Urinary shedding and renal colonization
Leptospires were demonstrated by PCR in urine samples, taken before antibiotic treatment and vaccination, of three animals (one from O1 and two from O2), while another two urine samples from O2 were only borderline positive by PCR (Table 1). These positive and borderline-positive samples were taken from cows with naturally acquired antibody titres between 800 and 6400. In the urine of one animal from O2, the attempt to culture leptospires gave positive results, and the cultured strain was identified as L. borgpetersenii serovar Hardjo strain Hardjobovis by the National Reference Centre for Animal Leptospirosis using serological methods (serogrouping by MAT with standard group-specific antisera) and PCR-based restriction fragment length polymorphism (RFLP) typing.
None of the animals tested after antibiotic treatment and vaccination had cultivable and/or PCR-detectable leptospires in the urine or kidney samples. Difference in the overall proportion of PCR-positive urine samples before and after treatment was significant (Fisher's exact test, P = 0·001). Pathological analysis of kidney tissues showed no evidence of lesion formation or detectable leptospires.
DISCUSSION
In this study, two outbreaks of leptospirosis in dairy cattle were managed using an integrated approach based on enhanced biosecurity measures, whole-herd antibiotic treatment and vaccination. Evidence of herd immunization and control of infection was provided using a combination of molecular, cultural and pathological analyses in addition to serological tests.
One of the hazards we dealt with was the possible re-introduction of leptospires into the herds from outside sources (e.g. other livestock, pasture, wildlife, etc.). Indeed, it has been suggested that for effective control of bovine leptospirosis, contacts with other animals, including wildlife, have to be prevented [Reference Kuiken20]. To this purpose, the application of good biosecurity measures has valuable long-term benefits, even for improving the general herd health, compared to relatively short-term elimination of infection through, for instance, progressive replacement of the herd with healthy animals. Leptospira-targeted biosecurity measures for all potential exposures are often impractical in most dairy cattle operations and, when applied alone, may even fail in preventing further infections [Reference Little21]. Yet, antibiotic treatment and vaccination offer a means of control as a complement to biosecurity measures, with a whole-herd approach often being recommended [Reference Little21]. In this study, to ensure that all infections, detectable or not, were somehow controlled, all animals were treated and vaccinated, and putatively non-infected (seronegative) animals were housed away from infected animals. Antibiotic treatment was aimed at preventing further abortions and eliminating the carrier status, thereby stopping leptospiruria and limiting leptospiral load in the herd. Another goal of antibiotic treatment was to control infection until immunity was induced by vaccination or by the infection itself.
The type and quality of vaccine-induced immunity against leptospirosis primarily reflect differences in leptospiral serovars and isolates used for vaccine preparation. In this study, only a minor proportion of vaccinated animals (4/125) did not seroconvert. These were adult cows aged 54, 69, 78 and 117 months at first vaccination. Generally, the most common reasons as to why vaccinated animals may fail in mounting an immune response include improper vaccine handling and administration, stressors (e.g. adverse weather, acclimatization, weaning, deficient management, calving, nutritional disorders, etc.), age, immunodeficiencies and poor general health.
We found that most vaccinated animals developed relatively high antibody titres (⩾1:800) 3 weeks after vaccination and that these titres decreased significantly 24 weeks after vaccination. While the extent of such decrease in terms of seroprevalence was reasonably acceptable (−9%), this was much more substantial in terms of magnitude of antibody titres (−85%), which also skewed their distribution towards the minimum detection limit (Figs 2, 3). This indicates, again and convincingly, that the post-vaccination immune response against serovar Hardjo in cattle, as determined by MAT in field conditions, is only transient and a significant decline is to be expected 6 months after vaccination, at least in cattle herds that have never experienced vaccination against leptospirosis before. This in turn suggests that a semi-annual booster is generally to be preferred. It is also worth mentioning that, although immunization with killed whole-cell vaccines is not usually associated with induction of a long-lasting (cell-mediated) immune response in vivo, the vaccine used in this study has been shown to induce a potent Th1 immunity comprising response by CD4+ and γδ T cell proliferation and production of gamma-interferon (IFN-γ) in culture with antigens [Reference Naiman22]. Moreover, natural killer (NK) cells from vaccinated or infected cattle demonstrated an IFN-γ recall response when exposed to antigen ex vivo [Reference Zuerner23], suggesting that programmed NK cells may respond faster to infection by serovar Hardjo.
A particularly interesting result of this study was that the cow's age at first vaccination had a significant effect on the mounted immune response. A recent Canadian study examining antibody titres for seven Leptospira serovars, including serovar Hardjo, in beef herds during the grazing season showed that age was not associated with the odds of an increase in antibody titres for any of the examined serovars [Reference Van De Weyer24]. However, older cattle had a significantly greater chance of being Leptospira seropositive compared to the young, a finding consistent with repeated exposure to leptospires over time [Reference Van De Weyer24]. We found that the magnitude of post-vaccination antibody titres was positively associated with calves' age and negatively associated with adult cows' age. This is consistent with decreasing immunoimmaturity in calves and increasing immunosenescence in adult cows over time, resulting to an increased (in calves) or a reduced (in adult cows) response to vaccination associated with ageing. This agrees with previous studies reporting that 3-month-old calves have a significantly poorer serological response to vaccination compared to 6-month-old calves [Reference Schollum and Marshall25], suggesting that vaccination in calves aged <6 months should be boosted at ⩾6 months. It is widely recognized that immunosenescence affects the host's competence to respond to both infection and vaccination. This seems to occur in both short- and long-living animal species relative to their life expectancy, and it is reasonably well documented in dogs [Reference HogenEsch and Thompson26] and horses [Reference Horohov27], whereas relatively little is reported on cattle, e.g. [Reference Ohtsuka28]. Further investigations are therefore needed to clarify the effect of ageing on the immune response of cattle to leptospiral vaccines.
One of the theoretical disadvantages of vaccination in absence of an efficacious antibiotic treatment is the possible development of carriers, which are sufficiently immune to resist the disease but not to resist renal colonization, leading to transient leptospiruria. Although the kidneys of only 12 cows could be examined, it is difficult to ignore that there were no cultivable, (histo)pathologically detectable and/or PCR-detectable leptospires therein, not to mention the 79 urine samples, taken after treatment and vaccination, that tested negative to both cultural and molecular analyses (Table 1). This supports, to some extent, the value of a whole-herd treatment and vaccination plan in supplementing biosecurity measures against bovine leptospirosis.
Serological testing is widely used for diagnosing bovine leptospirosis, with MAT being the method of choice [Reference Bolin1, Reference Bolin10]. Yet, MAT is best suited as a herd screening test as it has some limitations in the detection of carriers on an individual basis and endemic infections in herds. For instance, a recent Brazilian study [Reference Otaka29] showed that 50% of PCR-positive urine samples came from MAT-negative cows, suggesting that there is no single test that can be used alone to prove leptospiral infection, as failure to detect leptospires in the urine of seropositive cows is also quite common because of intermittent leptospiruria. In this study, MAT was the best affordable tool for screening the two herds and then measuring the post-vaccination immune response in those that were seronegative. Although serological findings were supplemented with molecular, cultural and pathological analyses, the apparent limitation of this approach is that some infected animals with a MAT-undetectable immune response might have been assigned to the putatively non-infected group. Therefore, results reported here are particularly representative of those animals with a MAT-undetectable response, rather than a strictly naive, pre-vaccination immune response as for serovar Hardjo.
In conclusion, there is evidence that outbreaks of leptospirosis in dairy cattle herds can be managed effectively using an integrated approach based on enhanced biosecurity measures, whole-herd antibiotic treatment and vaccination. However, it is recommended to supplement serological tests with other techniques to demonstrate leptospires in tissues or bodily fluids. As bovine leptospirosis is a herd, rather than an individual animal problem, vaccination is an affordable insurance, provided that proper management procedures and chemoprophylaxis are implemented. Results of this study can be generalized to other situations globally, providing a practical example of how bovine leptospirosis outbreaks can be managed in an integrated fashion and particularly how active immunization operates in vaccine-naive (affected) populations under field conditions. While further information is needed to make sound vaccine recommendations for bovine leptospirosis, this study clearly shows that factors such as animal's age and related boosting schemes cannot be summarily ignored when vaccination has to play a major role.
ACKNOWLEDGEMENTS
The authors are grateful to Dr Tagliabue for technical support.
DECLARATION OF INTEREST
None.







