INTRODUCTION
Hand-foot-mouth disease (HFMD) is caused by a group of enteroviruses, with the most common ones being enterovirus 71 (EV71) and coxsackie A16 (Cox A16) [1]. EV71 is more strongly associated with severe cases compared to Cox A16 [1]. The latent period of HFMD is 2–7 days, and patients normally recover in 7–10 days [Reference Hii, Rocklov and Ng2]. Most affected individuals can recover without complications. However, many severely ill patients develop fulminant cardiorespiratory failures, which are often fatal [1]. The majority of affected cases are aged <60 months, but adults can also be infected. Precautionary measures, such as avoiding close contact with infected individuals and maintaining good hygiene, are effective means of preventing the spread of the disease [Reference Hii, Rocklov and Ng2].
HFMD epidemics have escalated in the Asia-Pacific region since the 1990s, with EV71-associated HFMD occurring in regular cycles [Reference Solomon3]. A total of 29 patients died between April and June in Malaysia in 1997, with the disease primarily caused by EV71 [Reference Chan4]. In 1998, a large EV71 epidemic occurred in Taiwan, where 405 severe complications were recorded, of whom 78 died [Reference Chen5]. The largest Asia-Pacific pandemic was reported in China in 2008, posing a challenge to public health. The disease first appeared in Fuyang, Anhui Province, then rapidly developed into a nationwide epidemic, with around 490 000 cases and 126 deaths reported [Reference Solomon3]. HFMD was categorized as a class C infectious disease by the Ministry of Health of China on 2 May 2008. Since then, medical institutions have been required to report HFMD cases within 24 h.
In 2009, the incidence rate of HFMD in mainland China was about 8·66/10 000 (1 155 525 cases), and its fatality rate was 0·03% (353 deaths) [1]. Better understanding of the transmission dynamics and epidemiological characteristics of HFMD may provide useful guidance in implementing preventive measures to control its outbreaks and minimize morbidity and mortality. The present study describes HFMD distribution with respect to age (year and month), birth month, time, area, etc., over the period 2008–2013. We aimed to assess the spatio-temporal distribution of HFMD.
MATERIALS AND METHODS
Study area
Fuyang is located northwest of Anhui Province, Eastern China (114° 52′ E to 116° 49′ E, 32° 25′ N to 34° 004′ N). It is situated near the southern fringe of the warm temperate zone; its annual average temperature is ~15·0 °C, and the annual average precipitation is 907 mm. Fuyang has the largest population among the cities in Anhui Province according to the sixth National Census in 2010.
Fuyang has 172 subdistrict offices, villages, and towns managed by three municipal districts (Yingzhou, Yingquan, Yingdong), four counties (Yingshang, Taihe, Linquan, Funan), and one county-level city (Jieshou). In this study, the three municipal districts were regarded as urban, and the other parts of Fuyang were regarded as rural according to administrative jurisdiction and economic development level. In China, subdistrict offices, villages, and towns are of the same administrative rank and are referred to in the present paper as ‘town level’.
Data collection
HFMD surveillance data from 2008 to 2013 were provided by Fuyang Centre for Diseases Control and Prevention (CDC). Ethical approval and patients' consent were obtained via the CDC's standard protocol for data collection. The data included patients' gender, birth date, onset date, diagnosis date, severity level, current address, etc. The geographical coordinates of the study area were obtained from Google Earth, and the administrative map of Fuyang at the town level was supplied by Fuyang Civil Affairs Bureau. Patient onset date was selected as the HFMD occurrence date; addresses and coordinate information were linked with the Fuyang administrative map. Demographic data at the county level were acquired from the 2014 Anhui Statistical Yearbook; however, accurate population data at the town level were unavailable. As a proxy, we divided a county's population by the number of towns and this value was taken as an estimate of the mean population of each town within the county, then HFMD incidence rate at town level was calculated.
Descriptive epidemiology analysis
The incidence rate of HFMD was calculated annually and by area. The analyses of demographic characteristics were conducted by year. The number of reported cases was summarized monthly and by age to describe the temporal and age distribution of HFMD. The number of months between birth month and onset month was calculated as the patient's age in months. Patients aged <60 months were classified into 144 groups by their birth month and onset month (12 birth-month groups, 12 onset-month groups). Then onset-month distribution was described for each birth-month group. The majority of cases were children aged <60 months who accounted for 96·1% (102 405/106 565) of the total number of cases over the entire study period; hence, age 60 months was selected as the upper age threshold in analyses regarding patients' age in months, birth month, onset month and isolated virus serotype.
To assess the impact of birth month and age on infection risk, patients born in 2008 were selected as the analysis sample and the number of births in 2008 was summarized monthly. We assumed that there was no HFMD-related population migration over the study period. The surveillance data recorded the comprehensive information about all reported HFMD cases from 2008 to 2013 in Fuyang. Therefore, for individuals who were born in 2008, the standard information was recorded in our surveillance data if they were infected and hospitalized with HFMD in their first 60 months of life. The absolute risk of infection was calculated for each birth-month group in 2008 (e.g. the number of HFMD patients born in January divided by the number of births in January). We then quantified the relative risks (RRs) of HFMD infection for each birth-month group and peak age groups with 95% confidence intervals (CIs). January was selected as the reference month for each age and birth-month group because of the lowest incidence.
Spatio-temporal cluster analysis
The space–time scan statistic was introduced by Kulldorff et al. in 1998; Kulldorff's SaTScan (http://www.satscan.org/) is one of the most popular software packages for the space–time scan statistic method [Reference Chen6]. The space–time statistic is defined by a cylindrical window, with the base referring to the circular geographical region and the height representing time. The window undergoes dynamic changes in its base and height to detect potential spatio-temporal clusters [Reference Kulldorff7]. SaTScan scans for clusters of geographical size between zero and a pre-set maximum value. The maximum cluster size can be specified as a percent of population at risk in the study, or in terms of geographical size using the circle radius. The cluster radius is the circular size of a detected cluster, a geographical region is included in the cluster if its centroid is included in the scanning circle [Reference Kulldorff8].
The space–time scan statistic can be used for prospective and retrospective analyses based on several types of probabilistic models, such as discrete/continuous Poisson model, discrete Bernoulli model, and space–time permutation model. At present, the discrete Poisson and space–time permutation models are extensively adopted in cluster detection and early warning of different kinds of diseases, such as cervical cancer, brain cancer, and HFMD [Reference Chen6, Reference Kulldorff7, Reference Liu9]. The main difference between these two models lies in their initial data requirements. The former requires the demographic data of study areas, whereas the latter does not; therefore, the latter is especially practical when population information is inaccessible. Hence, the space–time permutation model was utilized in this study to detect spatio-temporal areas in Fuyang with abnormally high HFMD incidence.
SaTScan detects possible clusters by calculating the log likelihood ratio (LLR) for each cluster as follows:
where C is the total number of cases, c is the observed number of cases within the scan window, and n is the expected number of cases within the window. The relative risk (RR; equivalent to c/n) and the P value of the LLR are calculated for each cluster. The scan window with maximum significant LLR is considered the most likely cluster; other scan windows with significant LLR are identified as secondary clusters and ranked according to their LLR test statistic [Reference Kulldorff7, Reference Kulldorff8, Reference Kulldorff10]. In this study, clusters with P values <0·001 were reported.
SaTScan has two limitations. First, the software lacks cartographic support. Thus, scan results cannot be visualized by this software. Second, scan results are very sensitive to parameter settings [Reference Chen6]. To overcome these limitations, ArcGIS 10·2 (ESRI, USA) was utilized to visualize scan results, and a method for optimal selection of parameters was employed in this study as follows.
No consensus exists regarding the selection of parameters in SaTScan; most previous studies set the maximum spatial cluster size (scan radius) to 50% and/or 20% of the total population at risk; the maximum temporal cluster size was set to 50% of the study period [Reference Liu9, Reference Liu11–Reference Qi13]. However, scan results tend to include large cluster areas and regions where no significant elevated risk exists by using 50% as the threshold value [Reference Haining14]. Scan results are closely related to parameter settings. The hotspots and scope of infected areas may keep changing over the entire study period. Thus, the optimal scan radii should probably be changed every year instead of being kept constant. An exploratory analysis was performed by using the retrospective space scan statistic based on the discrete Poisson model to determine favourable parameters [Reference YIN15]. Specifically, the retrospective space scan statistic based on the discrete Poisson model was employed to analyse 2008 county-level HFMD data in Fuyang. The maximum circular size of the scanning window was set to the default value, which is 50% of the total population at risk. The results of this method showed that the maximum cluster size was 29·82 km; that is, the largest cluster would be detected if the scan radius was set to 29·82 km. Therefore, when an optimal setting for the retrospective space–time scan permutation statistic was determined, 30, 25, and 20 km were selected as the upper bounds of the scan radius. The corresponding maximum temporal cluster size was set to 7 days (the incubation period of HFMD is 2–7 days, and according to the Guidelines for Prevention and Control of Hand, Foot and Mouth Disease formulated by the Ministry of Health of China in 2009, the cluster of HFMD cases is defined within 7 days).
Tango & Takahashi [Reference Tango and Takahashi16] recommend that the number of regions included in the largest cluster should be deemed unfavourable when it exceeds 10–15% of the total number of regions. The total number of geographical regions in the present study is 172. Therefore, the number of regions included in the largest cluster should be <25. The results obtained with different scan radii show that the scan radius of 20 km satisfied the required criteria (the number of regions included in the largest cluster was 15). Similarly, this approach was employed to obtain favourable parameter choices for the remaining 5 years of the study. The optimal scan radii were set as 30 km in 2009, 15 km in 2010, 20 km in 2011, and 15 km in 2012 and 2013. The maximum temporal cluster size was set to 7 days for all study years.
RESULTS
Incidence of HFMD
As shown in Figure 1, the incidence rate of HFMD demonstrated a rising trend over the 6-year study period (the χ 2 for trend statistic is 12 460 with P < 0·001), increasing from 9·46/10 000 in 2008 to 24·34/10 000 in 2013. More specifically, in 2009 the morbidity of HFMD was only marginally smaller than that in 2008. After that, the rate increased markedly to 19·67 in 2010, which was twofold higher than that in the preceding year. After declining to 13·97 in 2011, the next year recorded a sharp rise again, reaching the highest point at 26·93 and then finally the incidence rate dropped slightly in 2013 to 24·34. Overall, during the study period, HFMD generally grew and reached a peak every other year.

Fig. 1. Incidence rate of hand-foot-mouth disease (HFMD) in Fuyang from 2008 to 2013 (per 10 000).
Temporal distribution
As shown in Figure 2, the monthly distribution of HFMD exhibited strong seasonality, with seasonal peaks occurring between April and June in almost every year since 2008.

Fig. 2. Temporal distribution of hand-foot-mouth disease (HFMD) in Fuyang from 2008 to 2013.
Demographic characteristics
Table 1 summarizes the demographic characteristics of the HFMD cases. A total of 106 565 HFMD cases were reported in Fuyang from 2008 to 2013, including 67 927 (63·7%) male patients and 38 638 (36·2%) female patients. The male-to-female ratio ranged from 1·6:1 to 1·9:1 (the average ratio was 1·8:1, cf. Table 1). According to the Anhui Statistical Yearbook, the proportion of male population was slightly higher than that of female over the study period (52·0% and 48·0%, respectively). Children aged <60 months predominated in reported cases. In terms of patient status, Table 1 shows that most of the cases were preschool children who lived at home or attended kindergarten (95·2% and 3·5%, respectively). The proportion of students and others are relatively small (students are defined as those who receive formal education in school or other places. The rest of patients are categorized as other cases). In total, the number of HFMD cases in rural areas was higher than that of urban areas.
Table 1. Demographic characteristics of reported HFMD cases in Fuyang from 2008 to 2013

Birth month and onset month distribution
The relationship between patient's birth month and month of illness onset is shown in Figure 3. Each cell in Figure 3a represents the number of patients who were born in m and developed HFMD in n, i.e. the size of previously mentioned 144 groups (n, m = January, February, …, December). As shown in Figure 3a , a large number of patients were born between April and June. Each row in Figure 3b shows the onset-month distribution for each birth-month group (the sum of the percentages in each row is 100%). For instance, the first cell in Figure 3b represents patients born in January who developed HFMD in January and accounts for 53·9% of the total number of January-born patients (2683/4979; 4979 is the sum of numbers in the first row in Fig. 3a ). It was found from Figure 3b that more than half of the patients were identified as having HFMD in the month they were born (the diagonal from upper left to lower right in Fig. 3b , the proportion ranges from 52·5% to 89·0%). In addition, an increased chance of infection occurred during the peak months of HFMD, i.e. April–June (percentages in the fourth to sixth column in Fig. 3b ) as well as in the month before the birth month. For example, in the February birth group (the second row in Fig. 3b ), 62·2% of patients developed HFMD in February, 5·9% in April, 7·2% in May, 4·3% in June, and 4·0% in January.

Fig. 3. Birth month and onset month distribution of hand-foot-mouth disease in Fuyang from 2008 to 2013.
Age distribution
The age distribution of HFMD patients was investigated and the results are presented in Figure 4. The age (months) distribution plot reveals a particular trend with strong periodicity every 12 months in individuals with HFMD in Fuyang. Three large peaks occurred when the patients were aged 12, 24 and 36 months. Medium peaks appeared at the 48th and 60th month after birth, with several small peaks occurring before age 11 months and at 11th the 23rd and 35th month of life.

Fig. 4. Age distribution of hand-foot-mouth disease (HFMD) in Fuyang from 2008 to 2013.
Impact of birth month and age
Figures 3 and 4 indicate that birth month and age might be important risk factors of HFMD. The absolute risk and relative risk of HFMD infection for each birth-month group are presented in Table 2. Birth month is associated with increased HFMD risk in the first 60 months of life, with April, May and June being the peak birth-month risk groups. In particular, 34·5% of children born in May 2008 were diagnosed with HFMD in their first 60 months of life, compared to 3·5% of children born in January 2008 (RR 9·79, 95% CI 8·88–10·79).
Table 2. Absolute and relative risk of HFMD infection by birth month in the first 60 months of life with January as the reference group for each birth-month group
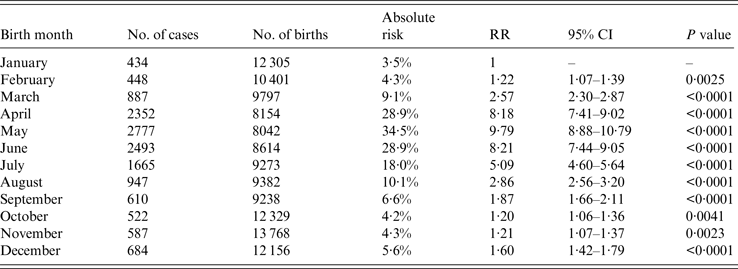
RR, Relative risk; CI, confidence interval.
As shown in Figure 5, in peak age groups (children aged 12, 24, 36, 48, 60 months), 12-, 24-, and 36-month-olds born between April and June had a statistically significant elevated risk of HFMD infection compared to children of the same ages born in January. In fact, for those aged 12, 24, 36, and 48 months, most birth-month groups had significantly higher risk than in January. The largest increment in risk of HFMD infection compared to the reference January birth group was age 24 months, born in May (RR 34·85, 95% CI 26·49–43·25), while age 60 months, born in August (RR 0·21, 95% CI 0·08–0·55) exhibited the lowest risk.

Fig. 5. Relative risk of hand-foot-mouth disease infection by age and birth month with January as the reference month for each age and birth-month group.
Virus serotype distribution
In practice, given the limited laboratory capacity, most HFMD cases are retrieved from clinical reports, with a small sample of reported cases having virus isolated. Clinical samples were collected by medical facilities to confirm the virus serotype based on established procedures [Reference Xing17]. Out of the reported cases, 1032 (1%) were laboratory confirmed, 529 (0·5%) were severe, and 34 (0·03%) were fatal (22 in 2008, one in 2009, five in 2010, none in 2011, one in 2012, and five in 2013). Of laboratory-confirmed cases, EV71, Cox A16 and other enteroviruses accounted for 60·1%, 7·1% and 32·8%, respectively.
EV71-associated cases predominated the laboratory-confirmed virus serotype for almost every year since 2008. The proportion of patients infected by other enteroviruses demonstrated an upward trend and exceeded EV71 infections in 2012. In contrast, the number of Cox A16 cases were minimal and relatively small. Cox A16 and other enteroviruses were almost equally distributed in 2009 (Fig. 6a ).

Fig. 6. Virus serotype distribution of hand-foot-mouth disease in Fuyang from 2008 to 2013. (a) Virus serotype distribution by year. (b) Virus serotype distribution by patients' age (month). (c) Virus serotype distribution by patients' birth month. (d) Virus serotype distribution by month of illness onset.
EV71 infections were more severe than Cox A16 and other enterovirus infections across different age groups, especially in those aged 12, 24, 36, 48 and 60 months. The percentage of other enterovirus infections accounted for 43·1% in other age groups (Fig. 6b ).
Figure 6(c,d) illustrates that the isolated virus serotype had similar distribution in birth-month and onset-month groups. EV71 predominance also occurred in patients who were born or infected with HFMD between April and June. The majority of September-born/infected patients was due to other enteroviruses.
Spatial distribution
HFMD affected all 172 regions in Fuyang, but the influence varied by region and time. Figure 7 shows the morbidity map of the HFMD epidemic in Fuyang over the 6-year study period. As shown in Figure 7, the incidence rate of HFMD increased significantly, and the infection continued to spread wider except in 2011. Most epidemic hotspots (incidence rate >50·01/10 000) were located in urban area, especially in Yingzhou and Yingdong districts. In total, the incidence rate in urban area was higher than that in rural areas over the study period (31·42 and 13·78/10 000, respectively).

Fig. 7. Morbidity map of hand-foot-mouth disease (HFMD) in Fuyang at the town level from 2008 to 2013.
Spatio-temporal cluster analysis
SaTScan 9·4 was utilized to identify the risk regions and time periods of HFMD in Fuyang from 2008 to 2013, and ArcGIS 10·2 was applied to create HFMD cluster maps (Table 3, Fig. 8). One most likely cluster was detected each year, which was least likely to have occurred by accident [Reference Kulldorff8]. The most likely cluster included 1365 cases over the study period, and was located in different places in Fuyang. In 2009 and 2011, the centre of the most likely cluster was located in almost the same place (Yingshang), but the cluster time was different (between May and June in 2009, October in 2011). The cluster centre resided in Linquan and the surrounding area in 2010, 2012 and 2013, with a similar cluster time in 2012 and 2013 (March–April). The scanning result in 2008 was different from other years, Yingzhou was detected to be the most likely cluster at the end of April. The cluster radius stabilized between 10 and 14 km except for 2009 and 2011 (18·07 and 19·41 km, respectively). The time-frame of the most likely cluster emerged in different months over the entire study period. The largest LLR and RR appeared in 2013 and 2011, respectively. Overall, the scale and RR of the clusters were large in 2009 and 2011, and cluster intensity was strong in 2008 and 2013. Further investigations revealed that the dominant virus serotype in the most likely cluster was EV71 from 2008 to 2013 (100%, 81·3%, 83·9%, 83·1%, 48·5%, 82·0%, respectively. Due to the limited number of laboratory-confirmed cases, the proportion was calculated for detected cluster areas within a whole year instead of the cluster time). Furthermore, the proportion of children attending kindergarten within the most likely cluster was 28·2% in 2008, but was small for the remaining 5 years (<4·0%).

Fig. 8. Cluster map of hand-foot-mouth disease in Fuyang at the town level from 2008 to 2013.
Table 3. Most likely clusters of HFMD cases detected using space–time permutation model, 2008–2013
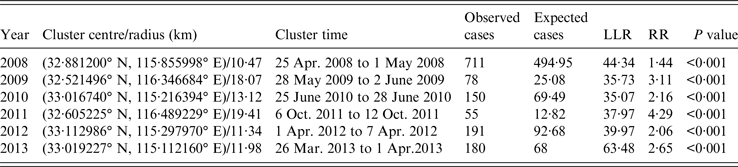
LLR, Log likelihood ratio; RR, relative risk.
Several secondary clusters were identified each year in Fuyang, which are less likely to be true clusters [Reference Kulldorff8]. As shown in Figure 8, the number of secondary clusters varied by year, covering different areas with different cluster times. Relative risk for these secondary clusters ranged from 1·44 to 23·08. The largest and least number of secondary clusters occurred in 2012 and 2010. Nearly half of secondary clusters occurred during April–June (47·4%).
DISCUSSION
HFMD is one of the most frequently occurring infectious diseases in China, and half a million to one million patients suffer from this epidemic each year [Reference Wang18]. HFMD characteristics vary with region, time, and spatial scale level. Previous studies have investigated the demographic, spatial, and temporal distributions of HFMD at large or medium spatial-scale levels [Reference Liu9, Reference Liu11–Reference Qi13]. In China, the county- or city-level CDC is responsible for the implementation of preventive and control measures. Information on HFMD characteristics at the country, province, city, or countylevel is insufficient. In particular, the features of HFMD in Fuyang at the town level remains vague. Since the large HFMD epidemic in China ‘originated’ from Fuyang city of Anhui Province in 2008, it is worthwhile describing the epidemiology of HFMD in this city. In this study, the distribution of HFMD in Fuyang was examined with respect to time, age in months, birth month, onset month, virus serotype, area, etc. Town level was employed as the basic research unit for spatial and spatio-temporal analyses.
Descriptive analysis revealed a rising incidence rate of HFMD over the study period. April, May and June were the high-epidemic months of HFMD in Fuyang. Possible explanations for such seasonal pattern may be associated with meteorological parameters. HFMD is caused by various human enteroviruses whose survival and propagation are facilitated by a thermal and/or humid environment [Reference Hii, Rocklov and Ng2]. Previous studies revealed a significant association between HFMD and specific weather conditions [Reference Hii, Rocklov and Ng2, Reference Xing17–Reference Ma20]. The number of HFMD cases was related to climate factors, such as average temperature and relative humidity, in Singapore, mainland China, Japan, and Hong Kong [Reference Hii, Rocklov and Ng2, Reference Xing17, Reference Onozuka and Hashizume19, Reference Ma20]. Fuyang has a warm, temperate, semi-humid monsoon climate. According to statistical data in recent decades provided by Fuyang Meteorological Bureau, the average temperature increases from 8·8 °C in March to 27·7 °C in July and then begins to decline. Therefore, it is likely that weather condition is the most likely reason for the seasonal pattern of HFMD in Fuyang.
The gender ratio in patients with HFMD in Fuyang was 1·8:1. A similar male predominance has been observed in other studies of HFMD from Asia, the gender ratios were approximately 1·7:1 in Shandong, 1·8:1 in Guangdong, 1·5:1 in Taiwan and 1·3:1 in Singapore [Reference Liu11, Reference Deng12, Reference Chen21, Reference Ang22]. Preschool children living at home predominated the reported cases, which is consistent with the findings in Sichuan, Guangdong Province, and Beijing [Reference Liu9, Reference Deng12, Reference Wang23]. One possible reason is that children usually start kindergarten at the age of 36 months in China. Most of the children aged <36 months are living at home. In the present study, 93·5% of preschool children living at home were aged <36 months. A particular pattern of birth-month and onset-month distribution among HFMD patients aged <60 months was observed in the present study. Patients born between April and June were relatively numerous compared to other birth-month groups. Patients had a high risk of HFMD infection in: (1) the birth month; (2) peak months of HFMD; (3) the month before birth month. Cao et al. reported a similar pattern of risk period of HFMD, ‘In addition to summer season, HFMD poses a high risk in birth month for a child and the risk of infection decreases as the gap from the birth month increases'. This study was based on 18 445 reported HFMD cases in Beijing in 2008 [Reference Cao24]. More research is needed to verify the generality of these findings.
A periodical age (month) distribution of HFMD was detected in the present study. Across the first 60 months of life, the onset of HFMD in Fuyang was age-dependent and exhibited a periodic pattern (a 12-month periodicity), with children aged 12, 24 and 36 months being the most susceptible. A duration of about 1-year periodicity of age distribution of HFMD cases was also observed in Beijing [Reference Cao24]. However, the age-specific vulnerable groups (12, 24, 36 months) have not been explicitly identified. Whether the age (month) distribution pattern of HFMD is universal remains uncertain.
We could use the above-mentioned findings regarding birth-month and onset-month distribution to explain the observed age distribution in Figure 4. The risk of developing HFMD in birth month is very high, which will inevitably lead to high frequencies in children aged 12, 24, 36, 48, and 60 months. For example, if a patient is born in January, 12, 24 or 36 months later, it is still January (i.e. the birth month of the patient). Conversely, from Figure 4, it could be found that a child would be more susceptible to HFMD in the first, second and third birth month (i.e. 12, 24 and 36 months). Recent study has established that high maternal antibody levels in serum and in breast milk confer protection against enterovirus infection, and enterovirus antibody levels in breast milk wane over time [Reference Sadeharju25]. In the present study, the HFMD frequencies increase with age in patients under aged <11 months (cf. Fig 4), which could be explained by the same principle.
Across the first 60 months of life, children born between April and June had an eight- to nine-fold higher risk of HFMD infection than children born in January; the risk, relative to an individual's birth month and age, was highest for 24-month-olds born in May (RR 34·85, 95% CI 26·49– 43·25). In the literature, the age- and birth-month disease associations have been found in respiratory syncytial virus infections and other diseases [Reference Lloyd26, Reference Boland27]. However, to the best of our knowledge, research reporting the impact of birth month and age on the risk of HFMD infection is very limited for pertinent cross-referencing.
Unlike in Guangdong Province, Singapore, and Beijing where the predominating enterovirus changes with time [Reference Deng12, Reference Ang22, Reference Wang23], EV71 has been the dominant virus serotype (compared to Cox A16) in Fuyang since 2008. An entirely opposite pattern has been observed in Hong Kong, with Cox A16 always making up the majority of the reported cases [Reference Ma28]. The main virus serotype in those aged 12, 24, 36, 48, or 60 months as well as in patients who were born or infected between April and June was EV71. In the case of other enteroviruses, it was the predominant strain in 2012 and was prevalent in September-born/infected patients.
In the present study, the HFMD incidence rate in urban area was higher than that in rural areas in Fuyang, which is consistent with findings of previous studies [Reference Qi13, Reference Cao24]. However, more HFMD cases were reported in rural areas. A possible reason for this finding is the high population density in urban areas. Over the study period in Fuyang, the average density of population was 357 inhabitants/km2 in urban areas and 204 inhabitants/km2 in rural areas. Moreover, urban and rural areas have different diagnostic capacity, timeliness and accuracy of reporting, which make the urban surveillance system more effective and might lead to the higher incidence rate in urban areas [Reference Qi13].
After the descriptive analysis, SaTScan was utilized to perform a spatio-temporal cluster analysis of HFMD in Fuyang. ArcGIS 10·2 was used to visualize the results. Town-level scan results indicated that the time-frame, scale, RR, and intensity of the most likely cluster varied over the study period. The most likely cluster was located in Yingzhou in 2008, Yingshang in 2009 and 2011, Linquan and its surrounding area (Jieshou) in 2010, 2012 and 2013. Generally, the most likely cluster was formed during the peak months of HFMD (April–June) except for 2011, so the time period of detected clusters is reasonable. EV71 dominated the virus serotype in most clusters, which is consistent with the findings in Shandong Province [Reference Liu11]. The change of proportion of children attending kindergarten in a most likely cluster may be associated with relevant effective public health action. The largest number of secondary clusters occurred in 2012, which may be related to the highest HFMD incidence rate in this year (cf. Figs 1 and 8). Preventive interventions towards detected spatio-temporal hotspots might help to reduce HFMD incidence. Moreover, knowledge on the characteristics of these clusters could be useful to identify high-risk clusters in the future. However, it should be noted that the spatial scan statistics are based on the assumption of circular or cylinder scanning windows to detect potential clusters, which may not represent the actual shape of the clusters [Reference Wang23]. We plan to explore finer methods to conduct spatio-temporal analysis.
There are several limitations in this study. First, potential reporting bias might exist in the surveillance data. HFMD cases could be underreported because of the self-limiting nature of this disease, or patients who did not seek medical care. Second, further analyses stratified by virus serotype are constrained by the small proportion of laboratory-confirmed cases (only 1% of reported cases were laboratory confirmed in the surveillance data). Third, the population assumption made to calculate the disaggregated incidence rates and the hypothesis of no HFMD-related population migration. The demographic data at town level were unavailable, which may reduce the accuracy of spatial distribution analysis of HFMD at town level. This was not necessarily a problem for other analyses in the study. The relative risks for each age and birth-month group were estimated by monthly birth population in 2008 and surveillance data of HFMD. Missing migration information could affect estimated risks to some extent. Last, due to the limited access to surveillance data in wide areas, some conclusions arising from this study such as the upward trend incidence, the spatial distribution and spatio-temporal clusters of HFMD, might be region-specific and thus restrict the large-scale application.
CONCLUSIONS AND SUGGESTIONS
To summarize, the present study offers information that is essential in understanding the epidemiological characteristics and spatio-temporal patterns of HFMD in Fuyang over the period 2008–2013. This research provides important bases for the prediction of pandemic trends, such as high-risk season, population, and areas. Key findings in the study are as follows: (1) the peak months of HFMD are April–June; (2) the onset of HFMD is age-dependent and exhibits a 12-month periodicity, with children aged 12, 24 and 36 months being the most vulnerable groups; (3) birth month and age might be risk factors of HFMD infection, with children born between April and June being 8–9 times more at risk compared to the January birth-month group, and 24-month-olds born in May had the highest relative risk compared to the reference January birth-month group; (4) a large proportion of laboratory-confirmed cases were associated with EV71.
To our knowledge, only one study by Cao et al. [Reference Cao24] refers to the possible birth-month disease and age disease association in HFMD researches. In their study, although these authors noted a periodical age distribution in HFMD patients, the specific age groups were not determined precisely [Reference Cao24]. Therefore, further studies are warranted to confirm whether the detected pattern and associations are universal. More in-depth insights from medical and biological perspectives are needed to elucidate the inherent mechanism underlying the relationship between birth month/age and HFMD in future studies.
In view of current prevention and control measures and findings in the present study, we would suggest that: (1) enhancement of laboratory infrastructures to expand the capacity of serotype identification. More clinically reported HFMD cases are needed to be virus isolated for further data analysis; (2) preschool children living at home require more public attention and medical resources; (3) for children aged <60 months, special attentions should be paid to age-specific groups (12, 24, 36 months) and birth-month groups (children born between April and June). Clinicians and families could be made aware of the increased likelihood of HFMD in birth month, peak months of HFMD and perhaps the month before birth month; (4) in addition, as suggested by Sadeharju et al., exclusive breastfeeding is also important in reducing the risk of enterovirus infections in infants [Reference Sadeharju25].
Currently, multiple groups and medical scientists in Singapore, Taiwan and mainland China remain committed to developing safe and effective EV71 vaccines [Reference Hwa29–Reference Mao31]. Innovative progress and critical barriers to implementation co-exist in researches. The first EV71 vaccine was approved by the China Food and Drug Administration on 3 December 2015 [Reference Mao31]. However, EV71 vaccination cannot prevent infections caused by Cox A16 and other enteroviruses [Reference Liu32], which limits the wide application of EV71 HFMD vaccine. A surveillance system is also needed to assess the long-term impact of the introduction and deployment of the vaccine. Results in current study may provide new perspectives for developing multivalent vaccines against HFMD by taking into account the disease incidence pattern with respect to birth month and age. In addition, the foregoing findings will help regulatory authorities to formulate and implement efficient vaccination strategies. For example, targeted vaccination towards high-risk birth-month and age groups may contribute to herd immunity.
ACKNOWLEDGEMENTS
The authors are grateful to the Editor and four reviewers for providing very helpful suggestions and comments. We thank Fuyang Centre for Disease Control and Prevention for providing HFMD surveillance data from 2008 to 2013. Gratitude is also extended to Fuyang Civil Affairs Bureau for providing the administrative map. This research was supported by the National Natural Science Foundation of China (grant no. 71 571 176) and the Research Grants Council of Hong Kong (grant no. T32-102/14N). All authors contributed equally to this work.
DECLARATION OF INTEREST
None.














