INTRODUCTION
Hepatitis A virus (HAV) that is enterically transmitted, typically causes a mild, self-limited disease leading to life-long immunity. However, clinical symptoms are more frequent and more severe with increased age at infection; furthermore, in the case of underlying chronic hepatic burden due to hepatitis B or C viruses (HBV and HCV, respectively), superinfection with HAV may result in fulminant hepatitis [Reference Vento1–Reference Keeffe4]. Therefore, HAV infections are still of public health importance in the Western world. Travelling to highly endemic regions without having received the recommended immune prophylaxis constitutes a considerable risk factor of infection with HAV (reviewed in Franco et al. [Reference Franco5]), especially in backpackers and foreign-aid volunteers [Reference Steffen, Banos and deBernardis6].
Large-scale screening for antibodies to hepatitis A virus (anti-HAV) using various techniques in the late 1970s has provided significant insights into the epidemiology of the infection [Reference Frosner7–Reference Schenzle, Dietz and Frosner10]. More recent studies report a decline in the anti-HAV seroprevalence in most parts of the developed world that may be generally accounted for by improvements in socioeconomic and hygienic conditions and, in some instances, by the application of effective vaccination (reviewed in Jacobsen & Koopman [Reference Jacobsen and Koopman11]). This remarkable decline in HAV exposure rates particularly during childhood has resulted in a shift of the infection to adulthood, where symptoms are more severe.
Serological monitoring is essential to the design and evaluation of effective vaccination programmes; this is particularly true for HAV infections that are notoriously under-reported [Reference Jacobsen and Koopman11]. Seroprevalence studies of several vaccine-preventable diseases have been performed recently in countries participating in the European Sero-Epidemiology Network (ESEN) projects. The original ESEN project, which was established in 1996, aimed to coordinate and harmonize the serological surveillance of immunity to five vaccine-preventable diseases (measles, mumps, rubella, pertussis and diphtheria) in eight European countries [Reference Osborne, Weinberg and Miller12]. ESEN2 followed in 2001 and included three additional infections [varicella zoster virus (VZV) and hepatitis A and B] and further participant countries [Reference Nardone and Miller13]. In each case, national banks of several thousand age- and sex-stratified sera, termed ‘main serum banks’, were collected and tested using enzyme immunoassays (EIAs) for antibodies to the various antigens by a national laboratory.
Comparison of these seroprevalence data, generated at different national laboratories using diverse EIAs, depends upon the comparability of exchanged information [Reference Desquesnes14]. However, inter-laboratory variation, even when using the same EIA and international standards, is a well-recognized problem [Reference Hesketh15–Reference Kafatos21]; accordingly, differences mostly in sensitivity, but also in specificity, have been reported for both commercial and in-house anti-HAV assays [Reference Wiedmann22–Reference Sjogren31]. Standardization is a methodological approach that provides a means to overcome this limitation and to ensure the direct comparability of seroepidemiological results obtained during the project [Reference Kafatos, Andrews and Nardone32].
Herein, we describe the process of standardization for anti-HAV antibodies obtained in 15 countries across Europe, which was accomplished using a panel of sera that was prepared by a designated reference laboratory and tested by all participant national laboratories. Standardization equations were then generated by regressing each country's panel results against the results of the reference laboratory. Using these equations it is possible to transform each country's national results into common units and, hence, to derive directly comparable international HAV serological estimates.
METHODS
Standardization procedure
The methodology was based on that developed in the original ESEN project [Reference Osborne, Weinberg and Miller12]) and is described in detail by Kafatos et al. [Reference Kafatos, Andrews and Nardone32]. Briefly, for each antigen, a reference laboratory was selected, with the responsibility of constructing and distributing to the other participant countries a special panel of sera, ‘the standardization panel’, that included known negative, low-positive and positive specimens. The standardization panel was then tested by each national laboratory with their established assay and this same assay was used to test the national banks of serum specimens collected in each country, the main serum banks. The quantitative results of antibody testing for each antigen from each country were regressed or calibrated against those of the reference country by the Health Protection Agency, Centre for Infections, London, and standardization equations were derived, enabling the conversion of the results of the participating countries to the units of the reference laboratory.
Within the framework of ESEN2, the Hellenic Centre for Infectious Diseases Control (HCIDC) represented by the National Retrovirus Reference Centre, Department of Hygiene and Epidemiology, University of Athens Medical School in Athens, Greece served as the reference laboratory for hepatitis A and was, therefore, responsible for the development of the standardization panel of samples. The panel was distributed to all participating national laboratories that were to test it for total anti-HAV antibodies with an assay of their choice, according to standard operating procedures, on two occasions: first, at the beginning of the project to evaluate the performance of the assays in comparison to the assay used by the reference laboratory, and, second, during the testing of the main serum bank in order to control for any assay drift. On the same two occasions, the standardization panel was tested twice at the reference laboratory to minimize between-test variability while calculating mean values of antibody titres.
An alternative method termed ‘back-standardization’ was undertaken by countries whose main serum banks had already been tested prior to the distribution of the standardization panel [Reference Kafatos, Andrews and Nardone32]. In addition to the testing of the panel with the country's established assay at the national laboratory, this procedure entailed the testing of a subset of about 150 titre-stratified (negative, low-positive, and positive) samples from the country's main serum bank at the reference laboratory with its chosen assay. Standardization was then performed in the same way as before, while taking into account the results of both the standardization panel and the titre-stratified specimens from the country's main serum bank. The regression line chosen for standardization in this case was the line of the best fit of the combined data, particularly in the area around the negative/positive cut-off point (equivocal range).
Standardization panel development and distribution
The standardization panel, which consisted of 150 plasma samples, reflected the immunity profile of a general population group (negative or positive status stemming either from disease or vaccination), since the samples were collected from blood donors or relatives of hospitalized patients in Athens, Greece [Reference Anastassopoulou33].
The standardization panel was designed to cover a broad range of quantitative results and was developed by combining samples with similar titres and/or by diluting high-titre samples. Thus, of 150 samples, 40 consistently exhibited anti-HAV titres <0·01 IU/ml, two exhibited titres ranging from >0·01 to 0·02 IU/ml, while 108 samples exhibited titres >0·02 IU/ml, as measured by the HAVAB 2.0 quantitative assay on the AxSYM system (Abbott Laboratories, Abbott Park, IL, USA) that was used by the reference laboratory. The corresponding samples were classified as ‘negative’, ‘low-positive’ (or ‘equivocal’), and ‘positive’.
The panel (400-μl aliquots of each specimen) was sent by courier post in a frozen state to all participating countries, where it was stored at −20°C until testing. Romania was the only country that did not receive it in a frozen state.
Utilized serological assays
Participating countries employed five commercially available enzyme-linked immunosorbent assay (ELISA) kits to test for anti-HAV, as shown in Table 1. The assays were performed and interpreted according to the manufacturers' instructions. Where possible, antibody titres were quantified and expressed in IU/ml based on the World Health Organisation (WHO) International Standard.
Table 1. Type of standardization undertaken by participant countries by utilized enzyme immunoassay for the determination of anti-HAV

Abbott (Abbott Laboratories, Abbott Park, IL, USA); Dade Behring (Marburg, Germany); DiaSorin (Turin, Italy).
Data analyses
Repeat testing of the standardization panel
Eight of the 16 participant countries tested the standardization panel twice: at the beginning of the project to identify any potential assay problems and during the testing of the main serum banks; the remaining countries (with the exception of the reference laboratory, which as previously mentioned undertook four rounds of testing), tested the panel only once (Table 2). The paired results produced by testing the panel twice with the same assay at each national laboratory were compared by plotting the logarithms (base 10) of the titres and drawing the slope through the origin. The agreement between the first and second round of testing of the standardization panel was good both at the national and the reference laboratories (data not shown), implying that the between-test variability was minimal. In all cases, the results of the second set of tests were used for the standardization of results since these measurements were closer in time to the testing of the national main serum banks.
Table 2. Numbers of times the panel was tested, standardization equations, R2 values, and pre- and post-standardization equivocal ranges (in local units) for each participant country
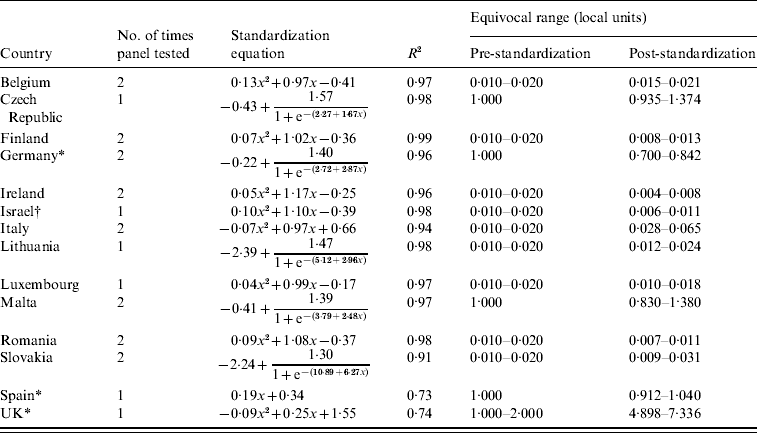
* Back-standardization.
† Quality assurance only (no main serum bank tested).
Regression analysis – quantitative comparison of standardized results
The results of the hepatitis A standardization panel testing from each country were calibrated against the results of the reference laboratory using a linear, quadratic, or sigmoid model. Reported results with concentrations outside the detection limits were assigned imputed titres; in particular, concentrations above the upper detection limit and below the lower detection limit were doubled and halved, respectively [Reference Lubin34]. All values were log10-transformed prior to analysis, with these results being plotted against the reference centre's results. Conversion factors were derived by regression, assuming normal errors on the logarithmic scale. The square of the multiple correlation coefficient (R 2) was calculated to quantify the proportion of variation between the testing and reference laboratory accounted for by the regression. An R 2 of at least 0·80 was considered to indicate that a high percentage of variation was explained by the model. The more parsimonious linear regression was used unless a significantly better fit (assessed using an F test) was obtained by the quadratic regression. Sigmoid regression was applied in situations where it provided a better fit of the data at the critical area around the negative/positive cut-off point (the equivocal range) [Reference Kafatos, Andrews and Nardone32].
Qualitative comparison of results
To assess the extent of qualitative agreement, the standardized panel results from each country were classified as negative, equivocal, or positive, by applying the cut-off values of the assay used by the reference laboratory (negative, <0·01 IU/ml; equivocal, i.e. basic protection, >0·01–0·02 IU/ml; positive, >0·02 IU/ml). These results were then tabulated against the reference country's qualitative results. Non-standardized results were similarly tabulated against the reference laboratory and the tables were compared to investigate the effect of standardization.
RESULTS
Pairwise quantitative comparisons and regression plots
The results of the testing of the standardization panel obtained by each country as well as the regression lines that were used in the standardizations are shown in Figure 1. Figure 2 shows the corresponding results generated from the three countries that undertook back-standardization by testing a subset of samples from their main serum banks. Four samples of the panel were found to be consistent outliers among laboratories and were, thereby, excluded from all analyses. The provision of quantitative results by all laboratories, wherever possible, limited the problem of arbitrary results (i.e. ‘censored data’). No evidence of significant influence of these censored data on the standardization equations was identified in any of the examined countries (data not shown).
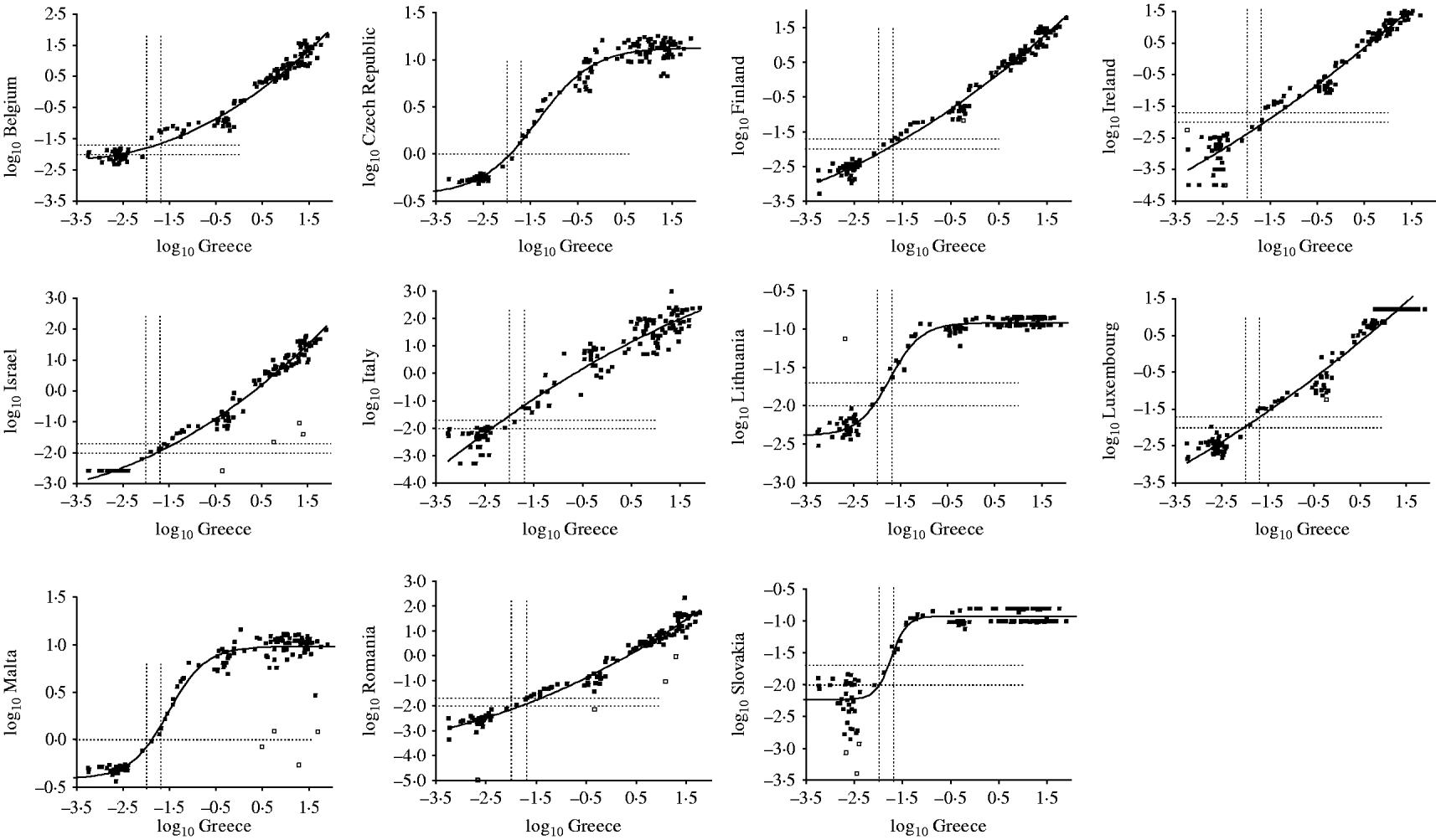
Fig. 1. Anti-HAV assay results of the standardization panel obtained by each country (y-axis) that undertook ordinary standardization plotted against the corresponding results of the reference laboratory (Greece, x-axis) on the logarithmic (base 10) scale. Open squares denote outlier samples, while dotted lines show the equivocal ranges; solid lines represent the regression models.
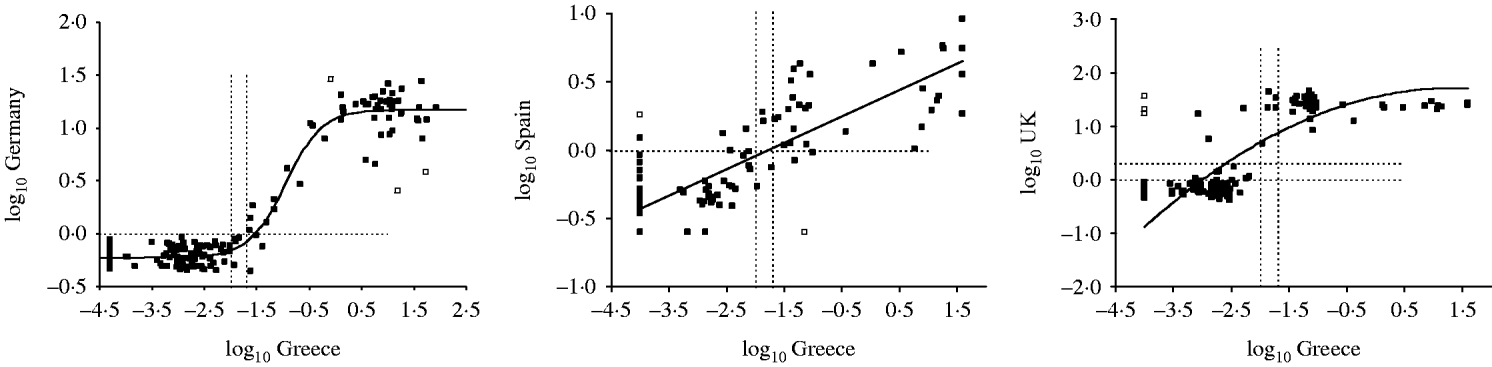
Fig. 2. Anti-HAV assay results of the standardization panel obtained by each country (y-axis) that undertook back-standardization plotted against the corresponding results of the reference laboratory (Greece, x-axis) on the logarithmic (base 10) scale. Open squares denote outlier samples, while dotted lines show the equivocal ranges; solid lines represent the regression models.
The derived standardization equations and corresponding R 2 values for each country are given in Table 2. Linear, quadratic and sigmoid equations were used. The least amount of correlation accounted for by the regression was observed in the United Kingdom and Spain, where both undertook back-standardization (with R 2 values of 0·74 and 0·73, respectively). For the remaining participant countries, R 2 values were all >0·80, with values ranging from 0·91 for Slovakia to 0·99 for Finland.
Table 2 also shows the equivocal range in local units before and after standardization. For most countries, the line of best fit passed through or very close to the point of equivalence, hence minimizing the impact of the process of standardization on the final sero-profiles obtained from each country (Figs 1, 2). However, the local assay overestimated antibody titres in two instances (Italy, United Kingdom), while the converse effect was observed in three instances (Ireland, Israel, Germany) (Table 2, Figs 1, 2).
Qualitative comparisons
To investigate further the qualitative improvement of standardization, all measurements were compared before and after the application of the reference laboratory's cut-off (i.e. pre- and post-standardization, respectively) (Table 3). Most laboratories, especially those that utilized the same or a similar assay as the reference laboratory (namely, the Czech Republic, Israel, Malta, Romania; Table 1), were almost in complete agreement with Greece, the reference laboratory, on the non-standardized results of negative sera. The greatest discrepancies in the identification of negative sera were noted in the three countries that used the DiaSorin ETI-AB-HAVK-3 assay (DiaSorin, Turin, Italy): Belgium, Slovakia, and Italy (with 17, 10, and five samples, classified as equivocal, correspondingly). Standardization alleviated these differences to a great extent, without affecting the general good agreement in the classification of negative sera in the other countries. Only one discrepant result, classified as negative in the reference laboratory and as positive in Lithuania, was noted; the standardization procedure did not adjust for this local observation.
Table 3. Qualitative comparison of national ELISA results pre- and post-standardization vs. the respective results obtained by the reference laboratory (non-standardized results are given in parentheses)
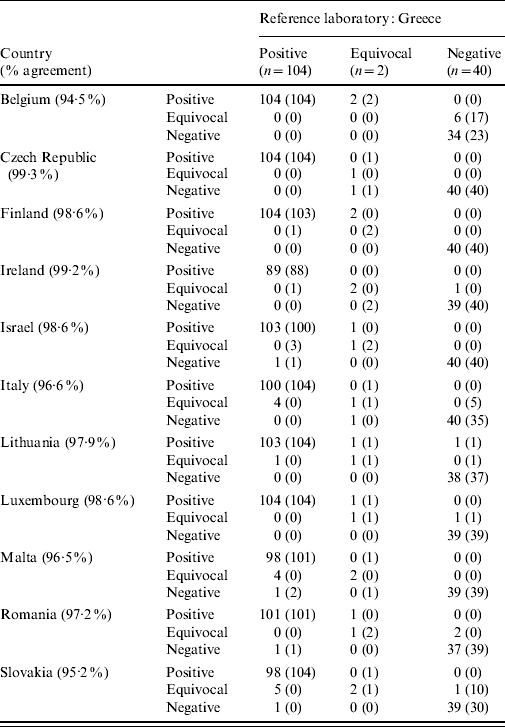
Four of the 150 samples of the standardization panel were excluded from all analyses as outliers. Moreover, four countries did not test all these 146 samples of the panel; in particular: Ireland did not test 15 positive samples, Lithuania did not test one negative, Malta did not test one positive and one negative, and Romania did not test two positive and one negative samples.
DISCUSSION
International comparisons of serosurvey results that provide the most accurate reflection of the immune status of the population often constitute unattainable targets due to the lack of direct comparability of obtained serological data; standardization is a methodological approach that overcomes this limitation by adjusting for laboratory and assay differences [Reference Kafatos, Andrews and Nardone32]. This methodology has been applied successfully for various vaccine-preventable infections during the ESEN projects, i.e.: (i) measles, mumps, rubella [Reference Andrews16, Reference Tischer20]; (ii) pertussis [Reference Giammanco17]; (iii) diphtheria [Reference von Hunolstein19]); (iv) VZV [Reference de Ory18]; and (v) HBV [Reference Kafatos21].
The present collaborative work describes the development of a standardization procedure that allows for direct comparisons of HAV seroprevalence data generated at 15 European laboratories. This aim was achieved through the establishment of a standard panel of 150 sera by a designated reference laboratory and its subsequent testing by all participating countries by assay methods of their choice. The obtained results were regressed against those of the reference laboratory and standardization equations were derived to provide the means to convert local (national) results to common (reference laboratory) unit measurements.
The standardization procedure was generally successful, as reflected by the very high R 2 values ranging from 0·91 (for Slovakia) to 0·99 (for Finland), which were obtained in all but two cases, namely Spain and the United Kingdom (with R 2 values of 0·73 and 0·74, respectively, Table 2). Despite the fact that the chosen regression models appeared to describe the Spanish, but not the British, data reasonably well around the critical positive/negative cut-off area (Fig. 2), the variability that remained unexplained was unacceptably high for both these countries that undertook back-standardization. Nevertheless, this reason, by itself, does not explain satisfactorily these results, since undertaking this type of analysis evidently did not prevent Germany from obtaining a very high R 2 value (0·96, Table 2). Thus, the most likely explanation for these discrepant results seems to lie in the utilized assays.
As shown in Table 1, Spain used IMx HAVAB (Abbott), a test performed on an automated immunoassay system that was first introduced in 1988 and has since been substituted by the following analysers: AxSYM in 1994, Prism in 1995, and Architect in 1999 (all Abbott). Although both the Spanish and the reference laboratory used microparticle enzyme immunoassays (MEIA) that are based on the same fundamental principles, the results they produce may still differ. This is not surprising given that the IMx HAVAB assay was designed for the qualitative determination of anti-HAV (positive or negative status), in contrast to the assay used by the reference laboratory that was designed for quantitative determinations of anti-HAV titres. It should be noted, however, that when the standardization panel was tested at the Spanish reference laboratory, an agreement of 95·3% was obtained (data not shown).
The United Kingdom used ETI-AB-HAVK-3, another competitive binding ELISA assay for total antibody to HAV (DiaSorin). Interestingly, the qualitative comparisons of the results obtained showed that the greatest discrepancies in the negative sera prior to the application of the reference laboratory's cut-off (or, in other words, prior to standardization) were noted in the cases of Belgium, Slovakia, and Italy that all used this same assay by DiaSorin (with 17, 10, and five samples, correspondingly, classified as equivocal, Table 3). However, these differences were alleviated to a great extent by standardization.
This study demonstrated the practical usefulness of standardization for serological results that cannot be directly compared. To our knowledge, this is the first time that such an international seroepidemiological project has taken place for HAV. The results are expected to contribute to the evaluation and potential improvement of the currently employed immunization strategies for hepatitis in Europe.
ACKNOWLEDGEMENTS
This work was funded by a grant from DG X11 of the European Union under contract QLK2-CT-2000-00542 (concerted action). Further sources of funding include CRP-Santé (Luxembourg) and ISCIII MPY1075/01 (Spain). Z. Moschidis and V. Gonianaki (Greece) are gratefully acknowledged for their excellent technical assistance.
DECLARATION OF INTEREST
None







