Introduction
Chickenpox (varicella) is a highly infectious disease caused by the varicella zoster virus (VZV), and VZV is transmitted by close contact, inhalation of aerosols from vesicular lesions and possibly through respiratory secretions [1]. Chickenpox is a common and mostly mild disease in children. However, it can cause serious disease in adults, pregnant women, immunosuppressed individuals and neonates. In the UK, it is estimated that 90% of adults who are UK-born are immune to chickenpox [Reference O'Moore and Howes2]. A person reporting a history of chickenpox is therefore usually sufficient evidence that a UK-born adult is immune, with a history of chickenpox having a positive predictive value of 90% [Reference Gétaz3]. In non-UK born populations, a history of chickenpox is less strongly predictive of immunity; therefore blood testing is required to confirm immunity [Reference O'Moore and Howes2, Reference Valdarchi4].
Chickenpox presents particular challenges in detention and other custodial settings such as prisons [Reference O'Moore and Howes2] and immigration detention centres [Reference Vairo5, Reference Ockerman6]. It is important to prevent the disease in the adult population because of the potential for more severe disease. Previous studies have indicated 6-fold higher susceptibility to chickenpox in predominantly migrant populations born or raised in tropical or subtropical climates compared to Western European adults [Reference Gétaz3]. Infection control has distinct challenges [Reference O'Moore and Howes2, Reference Vairo5, Reference Dapaah and Perkins7]. The physical environment varies between detention settings but often involves closeness of the population (such as room-sharing) with a high degree of population mixing, especially in immigration detention centres where there is usually internal free movement for detainees [8, 9]. Detention centres often have high levels of occupancy and high population turnover. There is variation between detention settings in staff levels, staff training in infection prevention and control and access to healthcare services. It is also crucial in detention settings to avoid disruption to core business, which can make infection control measures such as isolating cases and, quarantining and cohorting vulnerable contacts more challenging.
Here we describe the management of a chickenpox outbreak in a large immigration removal centre (IRC) in England from December 2017 to February 2018, including investigations carried out and control measures implemented.
Methods and models
Setting
The IRC where the outbreak took place is arranged across two separate but adjacent sites (A and B). Centre A is used mainly for the detention of adult males, but also has short-term holding capacity for 27 females; however, there is no direct contact between female and male detainees. Centre B is used solely for the detention of adult males. The capacity of Centre A is 312, and the capacity of Centre B is approximately 734 detained persons, including 22 Enhanced Care Unit beds. Operational responsibility (Care and Custody) for the IRC lies with the UK Home Office. Healthcare is provided by the local National Health Service (NHS) provider.
Case definition
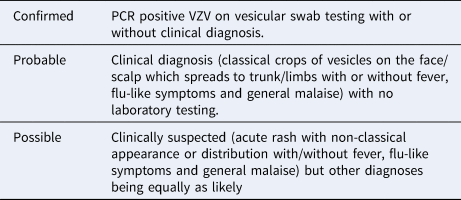
Case definitions are given in Table 1. Definitions for exposure, contact and immunity are given in Supplement 1.
Table 1. Case definitions used for epidemiological investigations in the IRC chickenpox outbreak
Outbreak investigation
In the UK, an incident in a prison, IRC or other prescribed place of detention will be declared an outbreak when two or more connected cases of varicella have been identified [Reference O'Moore and Howes2]. The outbreak is officially declared over when two incubation periods (42 days) for varicella have passed after the onset of the last case.
The outbreak management and investigation was guided by national guidelines published by Public Health England (PHE) specifically for detention settings [Reference O'Moore and Howes2]. An outbreak control team (OCT) was convened on notification of the second case. Immediate control measures implemented included isolation of the new case, identification of vulnerable close staff and detainee contacts and restrictions on movement into and out of the detention centres.
Microbiological investigation
Vesicular fluid swabs were taken on two cases to test VZV DNA by the polymerase chain reaction (PCR). Serology testing for VZV immunity (VZV IgG antibodies) was performed on significant contacts and the wider detainee population. All tests were undertaken at the Public Health Laboratories.
Immunity testing was offered to the wider detainee population in Centre B from 19 January 2018 and not from the start of the outbreak because it was only later in the outbreak that the IRC's core business was being severely impacted by the control measures already in place. As we were nearing the end of the incubation period of the two cases for Centre A at this stage, and to make the best use of limited resources, we only performed wider immunity testing in detainees in Centre B. The aim of wider immunity testing was to identify immune detainees across Centre B which could be safely moved to other IRCs to free up capacity to quarantine non-immune possible contacts, or to place non-immune detainees with immune detainees where single rooms were insufficient. This process also allowed safe management of new incoming detainees whose immune status was not known.
Epidemiological investigation
A descriptive epidemiological investigation was conducted to support the management of the outbreak by PHE Field Epidemiology Service.
Study design and population: A descriptive analysis of chickenpox cases, immunity status of significant contacts and the wider detained population and demographic characteristics of non-immune detainees was performed. The study population was adult males in detention at the IRC, with wider immunity testing only performed in detainees in Centre B. Centre B is made up of seven residential wings of predominantly double rooms and a few single rooms. Detainees are allowed to move freely within the Centre and dining, outdoor time and activities are shared between detainees across the Centre. Therefore, there is the potential for a high degree of mixing and risk of exposure between detainees depending on individual social mixing behaviour. Length of stay varies widely and can be 3 days to 5 months. There are on average 13 daily new admissions.
Data collection and management: Data collected for cases included: demographic details, including age and country of origin, rash onset date, infectious period, area of residence with dates, laboratory results and known epidemiological links with other cases. Data collected for contacts and the wider detainee population included: age and country of origin, if known to have had significant exposure to a case of chickenpox, place of exposure if linked to a known case, area of residence and VZV immunity results. Patient identifiable information was stored and managed in compliance with local Caldicott guidelines and the Data Protection Act (1998).
Modelling
Mathematical modelling was performed on 26 January 2018, 1 month into the outbreak, to assist the OCT decision making process, by enabling an estimation and comparison of the impact of different proposed control strategy scenarios. This was requested when it became clear that continuing widespread immunity testing of detainees was challenging to sustain because it required significant resources. Therefore, this approach was having a severe impact on operational capacity and ability to maintain core business activities at the IRC. Specifically, modelling results were used to assess the impact of scaling back mass VZV immunity testing of the wider detainee population, compared to continuing with full testing of all detainees in Centre B. The analyses used data and results available up to 24 January 2018.
To test how different control measures would affect the course of the outbreak, a susceptible-exposed-infectious-recovered transmission model of VZV infection was proposed (see Supplement 2 for details). All standard infection control measures which had already been implemented in the IRC were fixed in the model. Three scenarios of VZV immunity screening were tested by comparing their difference in impact on the outbreak:
Condition 1 (no testing): No testing and no quarantining and cohorting for non-immune detainees;
Condition 2 (full testing): From 19 January 2018 testing all detainees in Centre B and quarantining and cohorting those found to be non-immune for 21 days;
Condition 3 (partial testing): Due to resource constraints, continuing widespread immunity testing of detainees was challenging to sustain. Therefore, the OCT wanted to consider the impact of interrupting mass immunity testing of detainees in Centre B. This condition considers this scenario by assuming testing of 300 detainees in Centre B (out of 706) and quarantining and cohorting all non-immune detainees (7% of 300) up to 29 January 2018.
Results
The outbreak investigation
Cases
There were two confirmed and two probable cases of chickenpox amongst detainees. All were male, aged between 22 and 30 years old, and from Somalia, Nepal, Ghana and Bangladesh, respectively. None of the cases experienced complications or required hospitalisation. There were no cases amongst IRC staff.
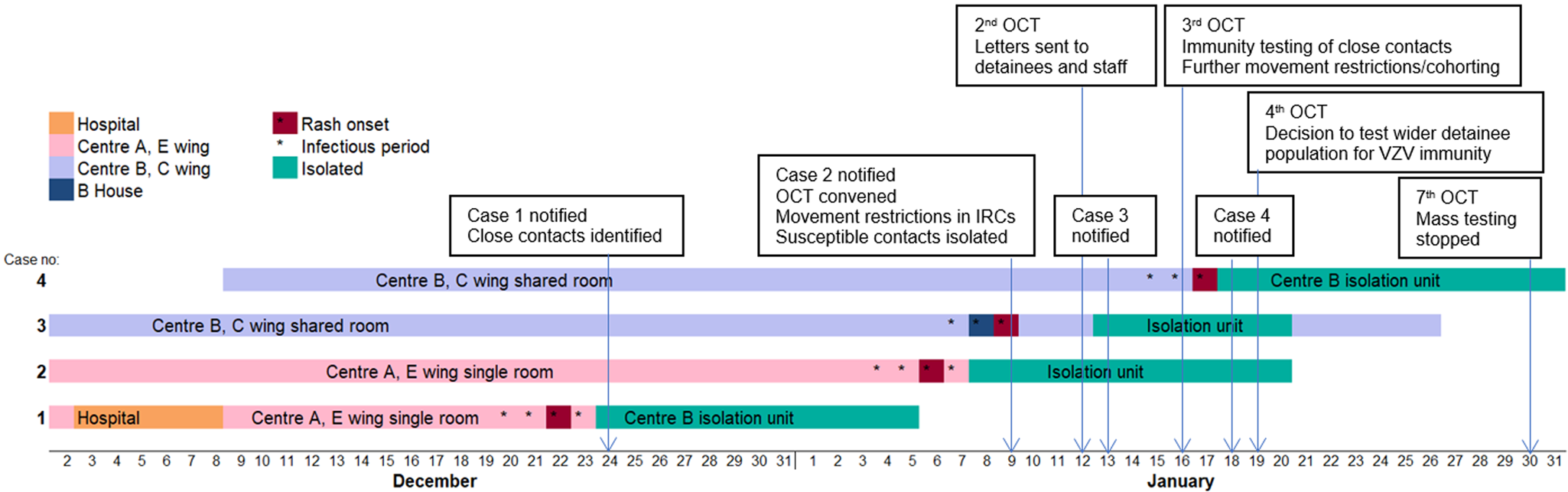
Reported date of rash onset in Case 1 was 22 December 2017 (see Fig. 1). This individual had been detained in the IRC since August 2017, but had been an inpatient at the local hospital during the incubation period for chickenpox (21 days). Therefore, the hospital admission was considered the likely source of his exposure to chickenpox. During the index case's infectious period, he was a resident in Centre A on E wing in a single room. Case 2 was also resident on E wing and during the investigation of Case 1 was identified as having had direct contact with the index case. Case 3 was a resident in Centre B on C wing. No definitive epidemiological links were established between this individual and the first two cases. It is not likely that Case 3 was infected from Case 2 since the onsets between these two cases were only 3 days apart. It is more likely that Case 3 was potentially exposed to Case 1 even though our investigations did not identify any reported mixing of cases between Centres A and B. The fourth case was also a resident in Centre B on C wing, and had direct contact with Case 3. As both centres were running at almost full occupancy, the attack rates on Centres A and B were 0.64% and 0.27% respectively, with an overall attack rate of 0.38% across the IRC.
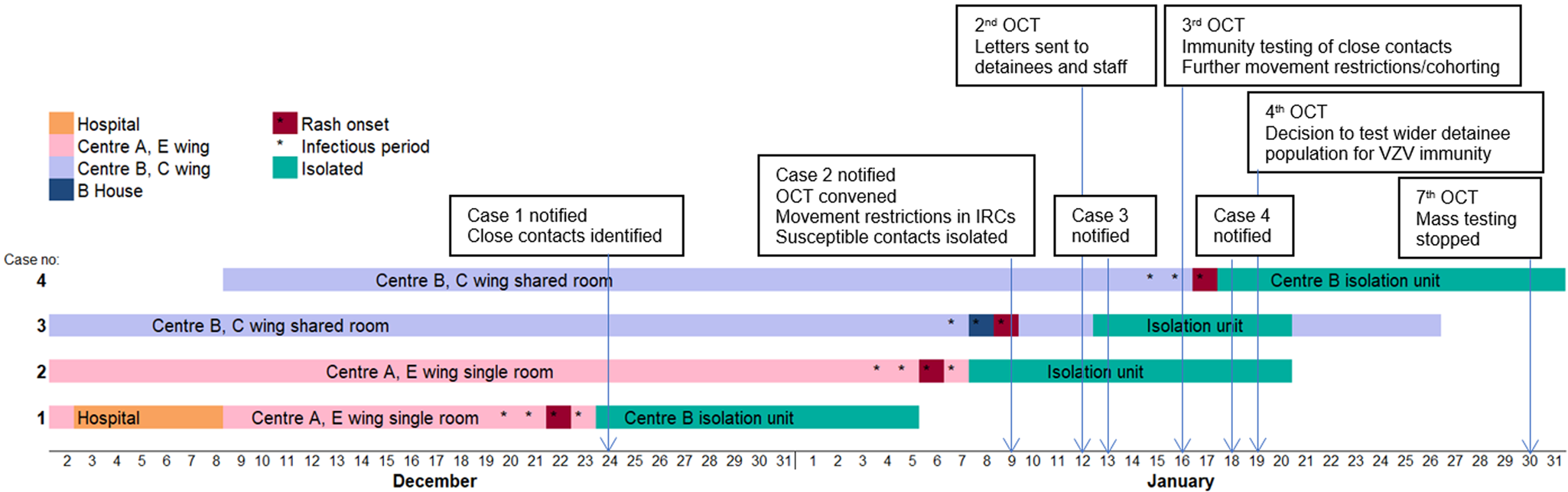
Fig. 1. Timeline of cases of chickenpox among detainees and early public health actions, December 2017 – January 2018. (OCT: Outbreak Control Team. 2nd, 3rd, 7th refers to the 2nd, 3rd, 7th OCT meeting – For example, the decision to stop mass immunity testing was taken at the 7th Outbreak Control Team meeting)
Samples were collected from two cases of chickenpox (Case 2 and Case 3). VZV DNA was detected by the PCR from vesicular fluid swabs. The other two cases were diagnosed clinically, and swabs were not obtained for virological confirmation.
VZV immune status of detainees within the IRC
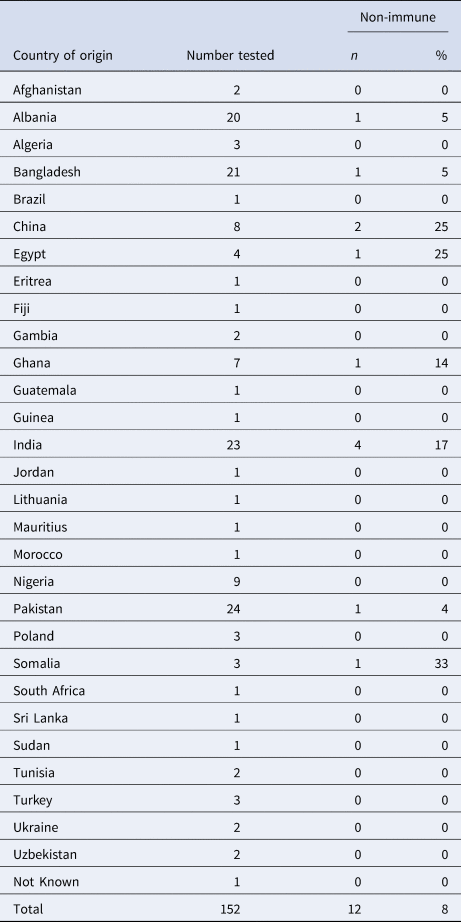
Overall, results were available for 301 detainees within the IRC; this included 27 detainees across the two IRC sites with significant contact to one of the four cases, and a further 274 detainees (probable or possible contacts) from the wider detainee population in Centre B without known significant exposure to a case of chickenpox. A phased implementation approach to VZV immunity testing was used across Centre B giving higher priority to detainees residing or frequenting areas where cases had been accommodated during their infectious period and based on likely social mixing patterns between areas. Thus, in C Wing where the two cases resided in Centre B, detainees were tested first followed by detainees accommodated in adjacent Wings. Screening coverage across Centre B was 53% (274/518) before the decision was taken to interrupt mass immunity testing of detainees. Twenty-four detainees were non-immune (8%), one of those was a significant contact; and 265 detainees (88%) were immune. The immune status was unknown for 12 detainees (4%) because three results went missing, seven declined testing, and two results required clinical confirmation of whether the detainees were immunocompromised for the correct interpretation, but this confirmation was never reported back to the OCT.
The median age of detainees screened from the wider population was 33 (range 18 to 67 years). For the detainees where country of origin was reported, they came from 30 different countries, most commonly the Indian subcontinent (Table 2). The highest number of detainees who were non-immune were from India, four individuals, which was 17% of all those screened from India. The highest proportion of non-immune was among those screened from Somalia (33%), but this only represented one individual out of three in the total screened.
Table 2. Country of origin of detainees in Centre B tested for VZV immunity without known significant exposure to cases, January 2018
Characteristics of non-immune detainees: Of the 24 individuals found to be non-immune, age was known for 23 of these: the median age was 36 years (range 23 to 47 years). For the 13 individuals where country of origin was known, five were from India, two from China and one from each of Albania, Bangladesh, Egypt, Ghana, Pakistan and Somalia.
No information on country of origin was collected at the time for Centre A. However, operationally the IRC allocates detainees to Centre A or B based on available bed space and not on individual detainee characteristics. Therefore, we have no reason to suspect that there were any systematic differences between detainees in the two Centres.
Modelling
Table 3 (and Supplement 2 for further details) shows the results and projections under the three scenarios. Under Condition 1 (no testing), there was a 95% chance that chickenpox transmission in both IRC sites would have died out by 24 January 2018. In Centre A, there was a small chance of an additional three cases of chickenpox within 4 weeks of the analysis (2.5% probability). In Centre B, there was a 97.5% chance that chickenpox transmission would die out by 6 February 2018. There was a small chance of an additional 18 cases of chickenpox in Centre B in the 4 weeks after the analysis (2.5% probability).
Table 3. Summary of results of mathematical analysesa
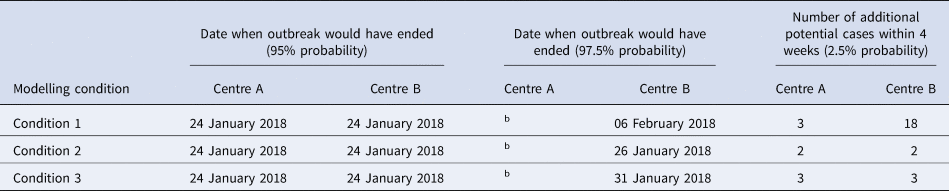
Date when outbreak would have ended was defined as onset of last case.
a Note:
• Three conditions are:
Condition 1: No testing and no quarantine for non-immune detainees.
Condition 2: From 19 January 2018 testing all detainees on Centre B and cohorting all those found to be non-immune for 21 days.
Condition 3: Testing 300 detainees in Centre B (out of 706) and cohorting all non-immune detainees (7% of 300) up to 29 January 2018.
• Model assumptions for all conditions tested are that current control measures are in place and continuing: infection control, management of cases with identification of contacts and movement restrictions in the IRCs.
• Modelling analysis performed 26 January 2018 using data available up to 24 January 2018.
Modelling results and projections applied into the future 4 week period after analysis.
b Date when outbreak would have ended at centre A (97.5%) could be a long time in the future see Supplement 2 for details.
Compared with Condition 1 (no testing), Condition 2 (full testing) could reduce the number of additional cases and stop the transmission earlier. There was a small chance that transmission may continue in Centre A, with a 2.5% probability of an additional two new sporadic cases in the following 4 weeks. These two cases are likely to be linked as their symptom onset dates are separated by about 10 days which is less than the incubation period (21 days) (Figure S3). In Centre B, there was a 97.5% chance that chickenpox transmission would have stopped by 26 January 2018. There was a small chance of two new chickenpox cases in Centre B in the following 4 weeks (2.5% probability). This demonstrates a large effect of full control measures in Centre B, with fewer potential late cases of chickenpox.
Condition 3 (partial testing) modelled the outbreak if mass VZV immunity testing of detainees in Centre B was interrupted on 29 January 2018. In this situation, 42% of detainees (300/706) had been tested by this date, allowing partial assessment of VZV immunity in the detainee population for supporting decisions around quarantining and cohorting of non-immune detainees. Under Condition 3, the potential number of additional chickenpox cases in Centre B could also be significantly reduced compared to Condition 1. However, under Condition 3, there was a small chance of three new chickenpox cases in Centre A in the following 4 weeks (2.5% probability). Otherwise, the effect of Condition 3 was very similar to Condition 2. Condition 3 was predicted to result in one more additional case of chickenpox in Centre B compared to Condition 2.
Outbreak control measures
A range of measures was taken to limit VZV transmission and prevent further cases of chickenpox.
Infection control
Standard infection control precautions were recommended in accordance with the PHE guidance on infection control in prisons and places of detention [Reference O'Moore and Howes2], and were implemented by the IRC staff [Reference Dapaah and Perkins7].
Isolation of cases
Healthcare staff were advised to have a low index of suspicion and to isolate promptly any detainees who presented with symptoms compatible with chickenpox [Reference O'Moore and Howes2]. However, there were some delays in reporting of symptoms by detainees and therefore not all cases were isolated immediately on rash onset.
Management of confirmed contacts amongst detainees
Significant contacts of cases were identified and risk assessed. Confirmed contacts who were non-immune were quarantined for 21 days from exposure (the length of the chickenpox incubation period), or until confirmation of their VZV immunity. From 16 January 2018, the OCT advised serology testing for VZV immunity for all confirmed contacts, to enable effective quarantining and cohorting arrangements (Fig. 1). Immunity testing was advised because a history of chickenpox is a less reliable indicator of VZV immunity in non-UK born populations [Reference O'Moore and Howes2]. In view of operational challenges in quarantining contacts, with insufficient single rooms, it was agreed that contacts could be cohorted according to immune status as a pragmatic approach to limiting VZV transmission.
Varicella vaccination in a prison or IRC outbreak is one of the numbers of control measures which may be implemented. Selected vaccination of non-immune individuals who had been in contact with cases was considered by the OCT. However, given the logistical difficulties in assessing immune status, including assessing for immunosuppression, and issues around completing the two-dose vaccine course prior to a detainee being transferred, released or deported it was decided not to offer vaccination in this situation.
Movement restrictions at IRC level
These were applied at the IRC level as per national guidance [Reference O'Moore and Howes2], suspending new admissions of vulnerable individuals and inter-site moves between Centre A and Centre B. A medical hold was put in place on transfers for non-immune detainees who had been identified as contacts of chickenpox cases and for those with immunity test results pending.
Immunity testing of wider detainee population
All detainees in Centre B were considered as potentially having been exposed because of the free movement of detainees within the Centre and also because of the poor history given by the cases in terms of specific close contacts. From 19 January 2018, immunity testing was offered to the wider detainee population in Centre B (Fig. 1), the site with the most recent cases. The aim was to identify immune detainees who could be moved to free up capacity to quarantine non-immune possible contacts, or to place non-immune detainees with immune detainees where isolation rooms for cases and quarantine rooms for contacts were insufficient. This process also allowed safe management of new incoming detainees whose immune status was not known. By 25 January it was clear that continuing widespread immunity testing was not sustainable within the operational capacity of the IRC's healthcare provider. On 29 January the OCT agreed that mass immunity testing could be stopped (Fig. 1) based on the conclusions from the modelling analyses.
Occupational health measures for IRC staff
Risk assessment was advised for all IRC staff to identify staff contacts of chickenpox cases and to determine VZV immune status by history of chickenpox in UK-born staff, and/or blood tests for VZV immunity. Advice was given that staff duties should be modified as appropriate for non-immune or vulnerable staff contacts. Pre-employment checks of healthcare staff at the IRC included documentation of VZV immunity and the provision of vaccination for non-immune staff as per NHS national policy [1]. This allowed a timely risk assessment of all IRC healthcare staff.
At the time of the outbreak checking VZV immunity was not a requirement of pre-employment checks for IRC Care and Custody (non-healthcare) staff. Therefore, the VZV immune status of these staff was not known. The OCT recommended, as part of the control measures, that the IRC occupational health provider should undertake proactive checks of VZV immunity in all IRC Care and Custody staff and vaccinate non-immune staff. The total number of Care and Custody staff working at the IRC was 450. They were all asked about the history of chickenpox and/or evidence of VZV immunity. Of these 450 staff, 51 Care and Custody staff were identified with uncertain VZV immunity status and therefore advised blood testing for immunity to exclude or modify their duties if non-immune. There were delays and operational challenges implementing this recommendation. All 51 staff were eventually tested for VZV immunity, but only after the outbreak was declared over. As the outbreak was over, for confidentiality reasons, these results were never fed back to the OCT.
Communications
A reactive press statement was drafted by the UK Home Office with support from PHE. Warn and inform letters were sent to all detainees, staff and visitors.
Discussion
Key lessons learned
Managing an outbreak within an IRC provided us with an opportunity to collect data on VZV immunity from a large cohort of people in detention representing a wide range of countries of origin, of whom 8% were non-immune to VZV. These results demonstrate fewer non-immune individuals than expected in a predominantly non-UK-born population, compared to previous findings documented in the literature [Reference O'Moore and Howes2–Reference Valdarchi4]. However, it is worth noting that the seronegativity rate of 8% was obtained from only 301 detainees in one detention centre. We acknowledge that populations in other detention facilities may differ, and not every outbreak is the same. Therefore, more studies on VZV seroprevalence of non-UK born populations need to be done to support our findings.
The results of mathematical modelling were invaluable in guiding the OCT in making an informed decision regarding whether or not to stop widespread screening of detainees for VZV immunity in the context of unsustainable levels of testing. Mathematical modelling of infectious diseases provides a useful tool for management and control of infectious disease outbreaks [Reference Bernoulli10–Reference Anderson and May13]. For example, during the 2001 outbreak of foot-and-mouth disease in British cattle farms, the timely and reliable information from mathematical modelling helped the government effectively control and stop the outbreak [Reference Ferguson, Donnelly and Anderson14, Reference Woolhouse15]. Although mathematical modelling can help the management and control of infectious diseases, its use to date, particularly in acute situations such as outbreaks, has been limited. Part of the reason is thought to be the lack of understanding of the potential usefulness [Reference Little16]. We hope our experience provides further evidence highlighting the important contribution mathematical modelling can make to successful outbreak management.
Successful aspects
The sustained effort and commitment of all stakeholders were crucial to the successful containment of the outbreak in the context of a challenging situation.
Challenges and areas for improvement
Certain challenges were inherent to the detention environment. Under usual operating conditions, each centre has, on average, 13 daily new admissions, with an average length of stay of 64 days (range 3 days to 5 months) in Centre A and 60 days (range 3 days to 5 months) in Centre B. The daily detainee movements between the two centres are on average 1.4 persons. With such a high degree of population turnover and high levels of social mixing within the IRC, identification of significant contacts was difficult. Control measures such as isolation of cases, and quarantining and cohorting of contacts involving restriction of movement represented a challenge given the rights of people in detention to free movement combined with high levels of close contact in the detention context [Reference O'Moore and Howes2]. Movement restrictions required for outbreak control pose significant challenges to IRC operations as restrictions may be required for up to 3 weeks from the onset of rash in the last case in a chickenpox outbreak. This prolonged period is particularly problematic given the population flows and management required by IRCs where detainee transit times through the system are usually shorter than this period [17].
Operational challenges included difficulties with healthcare staffing levels and sickness, especially over the Christmas holidays. This led to problems meeting surge capacity requirements to deliver phlebotomy services for serology testing, in the context of significant additional workload for IRC staff. These workforce challenges have implications for how healthcare is commissioned in detention settings. Alternative ways to understand potential vulnerability to infection and avoid the need for surging capacity in outbreaks include at or near reception testing for VZV immunity. Reception screening for immunity to vaccine preventable diseases, and offering catch-up vaccination where necessary, would also facilitate future outbreak management as timely information regarding the immune status of detainees would be available, provided this is documented and readily accessible.
Finally, there were significant delays in assessing the immune status of Care and Custody staff with uncertain VZV immunity status which resulted in delays in offering vaccination to non-immune staff. Discussions are ongoing between PHE, NHS England (National) and the UK Home Office regarding occupational health policy for non-healthcare staff in custodial settings who are considered at risk of infectious disease transmission due to the nature of their duties and the environment in which they work having similarities to health and social care settings. The degree to which the occupational health recommendations applicable to healthcare workers to document staff immunity to VZV should apply to non-healthcare staff in custodial settings is also being considered.
Conclusion
Given the challenges of managing outbreaks in these complex settings [Reference O'Moore and Howes2, Reference Vairo5], a pragmatic approach was required. In managing this outbreak, we have demonstrated the importance of serology testing to indicate susceptibility to VZV infection, and of mathematical modelling, in guiding outbreak control decision-making. Consideration should be given to reviewing current UK guidelines for managing chickenpox outbreaks in detention settings which assume much lower levels of VZV immunity within non-UK born individuals [Reference O'Moore and Howes2]. Our findings suggest that VZV immunity among non-UK born individuals in detention could indeed be comparable to estimates in UK-born adults in the general population [Reference O'Moore and Howes2]. However, more studies on seroprevalence of non-UK born populations need to be done before assuming that all populations in detention in the UK have rates of VZV seroprevalence similar to the UK-born population. Also, a seronegativity rate of 8% in adults is still high given that adults are at risk of severe disease. Control measures for managing chickenpox outbreaks should still continue to include identification of contacts to chickenpox cases, identification of non-immune or susceptible persons and managing these contacts appropriately. In addition, lessons learnt from this outbreak will contribute to ongoing policy discussions regarding reception medical screening protocols for people in detention and occupational health standards for non-healthcare staff in custodial settings.
Additional members of the outbreak control team
Dr Claude Seng, Dr Margie Meltzer, Terry Gibbs, Charlotte Skillern, Duncan Partridge, Richard Brown, Amanda Gillett, Jane Leaman, Maciej Czachorowski, Chris Kelly, Elsie Acheampong, Zara Fido, Emma Donoghue, Lisa McCarthy, Brian Milliken, Ellie Sewell, Theresa Tinsely, Trish Walter, Andrew Willock, Lesley Halford, Dr Gee Yen Shin, Geraldine Leong, Martin White, Hayley Kennedy, Tycie West.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S095026882000014X
Acknowledgements
With many thanks to all OCT team members, all IRC staff, PHE Field Epidemiology Service SEaL for epidemiological support and Public Health Laboratory staff.
Authors' contributions
Xu-Sheng Zhang, Alexandra Smith and Christina Atchison were involved in drafting the manuscript. Charlotte Anderson conducted the epidemiological analysis. Xu-Sheng Zhang conducted the modelling analysis. Bharat Patel and Gillian Higgins were responsible for serology and PCR testing of samples. Laura Pomeroy, Éamonn O'Moore and Yimmy Chow were involved in critical revision of the manuscript for important intellectual content. All authors were involved in the final approval of the manuscript and the decision to submit the manuscript for publication.
Conflict of interest
None