Vancomycin-resistant enterococci (VRE) are established as major nosocomial pathogens worldwide [Reference Cetinkaya, Falk and Mayhall1] and the increasing incidence of infection has made the control of VRE a public health concern. Asymptomatic VRE colonization of the gastrointestinal tract typically precedes infection [Reference Zirakzadeh and Patel2] and colonization may persist for long periods and serve as silent reservoirs for the colonization of other patients [Reference Montecalvo3]. In addition VRE-colonized patients may become efficient vectors of environmental contamination, and this may constitute a risk for contaminating healthcare workers who can then spread VRE to non-contaminated surfaces or uncolonized patients [Reference Zirakzadeh and Patel2]. Dissemination of vancomycin resistance can occur through both clonal expansion of VRE and horizontal transfer of van genes to other bacteria. Generally, multiple clones and horizontal transmission of vancomycin resistance are frequently encountered as VRE become endemic over time [Reference Malathum and Murray4].
The Hospital Infection Control Practices Advisory Committee (HIPAC) of the Centers for Disease Control and Prevention (CDC) has issued guidelines to prevent the spread of VRE and recommended contact isolation for patients with VRE [5]. The guidelines suggest that patients colonized with VRE may be removed from isolation when three consecutive surveillance cultures, performed at least a week apart, are negative for the organisms. However, a notable proportion of patients who have been decolonized are often recolonized by VRE [Reference Donskey6] and this may be due to either a relapse of infection with the original strain or through acquisition of a new strain. To better understand the epidemiology of reacquisition of rectal colonization by VRE, we compared the molecular characteristics of primary colonization and recolonization isolates from hospitalized patients.
The study was carried out at a 1000-bed university hospital with an average of 43 000 patient discharges per year. From January 2000 to December 2007, patients who were positive for VRE on clinical culture were screened for intestinal carriage of VRE. Our Infection Control Committee policy regarding VRE specifies that patients with three consecutive negative stool cultures obtained at least 1 week apart are considered to be decolonized. We performed an 8-year retrospective study of all patients who were recolonized by VRE after they were documented as being clear of VRE. Any patients who received additional antibiotic therapy were excluded. A total of 15 patients were identified. The medical records of these patients were reviewed with regard to age, gender, medical service, source of specimen, invasive procedures, antibiotic use, duration of hospitalization, duration of intensive-care unit (ICU) stay and clinical outcome. Whenever VRE recurred in a patient this was recorded as a separate ‘event’; 24 events were identified from the 15 patients yielding 39 initial colonization and reacquisition stool isolates. Organisms were identified by conventional biochemical reactions, with the Vitek identification system (bioMérieux, USA), and with the API Strep system (bioMérieux).
All isolates were typed by pulsed-field gel electrophoresis (PFGE) on a CHEF-DR III apparatus (Bio-Rad Laboratories, USA) as described previously [Reference Murray7]. After digestion with SmaI, genomic DNA was separated by electrophoresis with ramped pulse times from 5 s to 30 s at 6 V/cm for 19 h. The banding patterns were interpreted according to Tenover et al. [Reference Tenover8]. Isolates with 2- to 3-band differences in DNA profiles were considered to be ‘closely related’, those with 4- to 6-band differences are ‘possibly related’ and a >6-band difference as ‘unrelated’.
Isolates were also characterized by their Tn1546 elements. DNA was extracted with the Qiagen DNeasy kit (Qiagen GmbH, Germany) according to the manufacturer's instructions. For structural analysis of Tn1546, the overlapping PCR amplification of internal regions of the element was performed as described previously [Reference Huh9]. The purified PCR products were directly sequenced on an ABI Prism 3100 DNA Sequencer (Applied Biosystems, USA) and analysed using DNASIS for Windows v. 2.6 (Hitachi Software Engineering, USA). Enterococcus faecium BM4147 was used as the control strain for Tn1546 elements [Reference Arthur10].
Statistical analysis was performed using SPSS version 15.0 biostatistics software (SPSS Inc., USA). Reacquisition types of VRE according to change of admission status after VRE clearance were analysed using Pearson χ2 tests. A P value of <0·05 was considered significant.
In our hospital, from 2000 to 2007 the prevalence of VRE in enterococcal isolates increased significantly from 13·5% to 21·4%. Fifteen patients with recurrent colonization were enrolled in the study. Enrolled patients had a mean age of 54 years (range 6–83 years), had received multiple courses of antimicrobial treatment, and had underlying diseases including diabetes mellitus, hypertension, chronic obstructive pulmonary disease, and congestive heart failure. Ten of the patients had tracheostomy. Three of the patients had died. Thirty-nine enterococcal isolates were evaluated. All isolates were confirmed to be E. faecium and carried the vanA gene.
Table 1 shows that six of the 15 patients with recurrent colonization continued to be hospitalized on the same floor during their stay and five were discharged home and subsequently readmitted; four were moved to another floor. Twelve of the 24 recorded events of recurrence of VRE occurred among the patients who remained on the same floor and in eight of these events patients were recolonized with a strain that was indistinguishable by PFGE or genetically related to the original colonizing strain. On four occasions recurrence of colonization occurred with a strain that had an identical Tn1546 gene cluster to the previous colonizing strain. Patients who were moved to another floor had de novo VRE colonization except for one event. In the first event of patient no. 5, he was recolonized with a strain that was indistinguishable from the original colonizing strain. He had been ‘cleared’ of VRE and moved to an ICU but subsequently became culture positive after 4 days. All five patients who were discharged and readmitted had de novo VRE colonization.
Table 1. Characteristics of 39 vancomycin-resistant Enterococcus faecium isolates from 15 patients
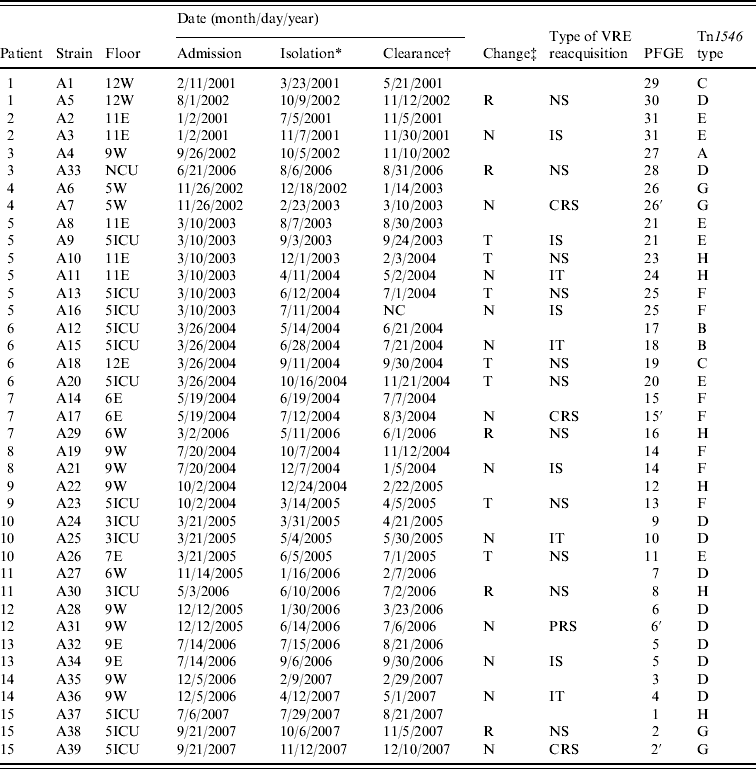
VRE, Vancomycin-resistant enterococci; PFGE, pulsed-field gel electrophoresis; W, general ward in the west wing; E, general ward in the east wing; NCU, neurosurgical intensive-care unit; 5ICU, medical intensive-care unit; 3ICU, surgical intensive-care unit; NC, not cleared; R, Discharge and readmission after VRE clearance; N, No change of admission; T, transfer patient to other ward after VRE clearance; NS, new strain; IS, identical strain; CRS, closely related strain; IT, identical Tn1546 type; PRS, possibly related strain.
* Date of VRE isolation.
† Date of VRE clearance.
‡ Change of admission status due to readmission or transfer of patient to other ward.
Statistical analysis of the 24 patient events categorized into three groups: (i) no change in admission status (n=12); (ii) transfer to another ward (n=7); and (iii) discharge and readmission (n=5) showed significant differences among the groups in the type of relatedness of VRE acquired after decolonization (P<0·05).
Reacquisition of VRE strains by patients after apparent successful decontamination of the bowel – three consecutive negative stool cultures – is widely recognized to occur [Reference Donskey6]. In this study, we found that the reacquisition type of VRE was influenced by changes of admission status of patients. Patients who remained on the same floor invariably had a recurrence of colonization with a strain that was indistinguishable or closely related to the original colonizing strain. It could be due to either an increment of the density of colonization or re-inoculation of the individual with their predominant resident strain excreted into their immediate environment. Four events showed a recurrence of colonization with a strain that was unrelated from the original colonizing strain by PFGE but had an identical Tn1546 gene cluster. This might be indicative of horizontal spread of Tn1546 into genetically diverse E. faecium strains in the gastrointestinal tract of same patient as it has been demonstrated that even transient colonizers may provide a significant donor potential for transfer of resistance genes to the permanent commensal flora [Reference Dahl11].
Six of seven events involving four patients who were moved to another floor had de novo VRE colonization with a strain that was genetically unrelated. It is possible that these patients harboured different E. faecium types in low numbers relative to the original strain isolated. The populations of E. faecium may be perturbed by antimicrobial treatment for other concomitant infections as was the case with patient no. 5 who had received ticarcillin-clavulanate for a Pseudomonas aeruginosa infection and vancomycin to treat a methicillin-resistant Staphylococcus aureus infection, leading to the selection and proliferation of the more resistant VRE types over time. Alternatively, low numbers of VRE below the threshold of detection by culture persisted in the intestinal tract of the patient, with subsequent overgrowth during antibiotic therapy. This represents a decrease in the density of VRE rather than true eradication, and surveillance cultures should be obtained from these patients when antibiotics are administered. In the cases of de novo VRE colonization previous colonizing strains were not responsible for recurrent colonization and their movement to another floor could have exposed them to VRE that were contaminating environmental surfaces and equipment on the new floor as well as healthcare workers who are recognized to be efficient vectors of transmission [Reference Salgado12]. Of note is the finding that all five patients who were discharged from the hospital had different strains on readmission to hospital; this suggests that recolonization occurred during the period of admission as asymptomatic carriage in healthy people in Korea has not yet been reported. Nevertheless, the frequency of VRE in food-producing animals and healthy individuals in other countries may suggest that acquisition of new types is just as likely to occur in the community as in hospital [Reference Guimaraes13, Reference de Niederhausern14]. Indeed, an in-depth study of the incidence of VRE in the general public in Korea as well as in food animals is called for to establish the origin of these strains in the medical setting and provide evidence of the effectiveness of infection control measures such as isolation in single-bed rooms or isolating a floor for VRE patients, improved nursing staff-to-patient ratios, and strict observance of contact precautions to combat the spread of these organisms and decrease colonization and infection rates of VRE.
DECLARATION OF INTEREST
None.


