INTRODUCTION
Hepatitis E virus (HEV) can cause inflammation of the liver after an incubation period of 2–7 weeks. It usually causes mild disease, but mortality of up to 20% has been reported in pregnant women [Reference Greening and Goyal1]. The virus is transmitted following the faecal–oral transmission route, which for HEV mainly involves waterborne and foodborne transmission as secondary transmission from person-to-person is relatively uncommon [Reference Lewis, Wichmann and Duizer2]. Waterborne transmission is the most often reported transmission route in developing countries in Asia, Africa and Central America, where HEV can be considered hyper-endemic [Reference Labrique3]. HEV strains infecting mammals are currently classified into four genotypes (gt), i.e. gt 1–4, but two additional new genotypes have been proposed [Reference Takahashi4]. Genotypes 1 and 2 are commonly seen as causes of hepatitis in travellers to developing countries, whereas genotypes 3 and 4 were later found as causes of autochthonous infection in industrialized countries [Reference Kwo5, Reference Schlauder6]. Of the latter two genotypes, gt 3 is especially found to be widespread in pigs worldwide. The underreporting of human HEV infections, especially those caused by gt 3, is likely. These are newly recognized pathogens outside the tropical regions, and the treating physicians may not be aware of these pathogens becoming endemic in industrialized countries. Retrospective studies found HEV infection as a possible cause of 6–8% of all non-hepatitis A, B, or C cases [Reference Herremans7–Reference Waar9], and hepatitis E gt 3 is seen in patients without travel history living in regions with endemic HEV in pigs [Reference Borgen10–Reference Boxall12].
Although the proportion of infections attributed to food is currently unknown, foodborne infections due to the use of contaminated water used during production [Reference Zuckerman13–Reference Said15], as well as zoonotic foodborne transmission are possible [Reference Lewis, Wichmann and Duizer2, Reference Renou14]. Clusters of HEV cases in Japan were linked epidemiologically and genetically to the consumption of undercooked pig livers and deer meat [Reference Tei16, Reference Yazaki17]. HEV can be found in meat on the market in The Netherlands and USA, suggesting the potential for zoonotic foodborne transmission [Reference Bouwknegt18, Reference Feagins19]. In a case-control study in Germany, the consumption of offal and wild-boar meat was strongly associated with risk of HEV infection [Reference Wichmann20]. Shellfish may also be a source of HEV infection, although evidence of this is not conclusive [Reference Said15].
Exposure to HEV in The Netherlands does occur, as is illustrated with non-travel-related HEV infections [Reference Borgen10], and the presence of HEV in swine, commercially available livers, and surface waters [Reference Rutjes21]. However, the exact routes of transmission remain unclear and are merely based on case reports. Zoonotic (foodborne) transmission and blood transfusion were reported as possible routes in The Netherlands [Reference Borgen10]. Zoonotic transmission is possible with the omnipresence of HEV in pig farms in The Netherlands [Reference Rutjes22]. Although a case study indicated risk of raw pig liver consumption in Japan [Reference Yazaki17], this is unlikely to explain all cases in The Netherlands. The possibility of infection via blood transfusion and blood products has also been described [Reference Boxall12, Reference Colson23]. Blood products may be treated to inactivate infectious agents without impairing the physiological properties of blood compounds [Reference Pozzetto and Garraud24], but the exclusion of at-risk donors remains an important prevention measure for controlling emerging infections in blood products. Thus, the discussion about the need for screening of blood donors would profit from a clear risk profile of HEV infection [Reference Lewis, Wichmann and Duizer2].
The aims of this study were to estimate the seroprevalence of anti-HEV IgG antibodies in the Dutch population, and to obtain a risk profile of acquiring seropositivity in The Netherlands. A population-based seroprevalence study in the general population of The Netherlands in 2006–2007 resulted in a serum bank [Reference van der Klis25], which offered the opportunity to investigate these questions.
METHODS
Study population and questionnaire
The study design and details on the data collection of the cross-sectional population-based seroprevalence study have been published elsewhere [Reference van der Klis25]. In short, 40 municipalities were sampled within five geographical Dutch regions. An age-stratified sample was randomly taken from each municipality. Overall, 24 147 persons were invited: 19 781 in the national sample including oversampling of 2574 non-Western immigrants from 12 municipalities; and 4366 in municipalities with low immunization coverage of diseases included in the Dutch national immunization programmeFootnote †. Subjects were asked to provide a blood sample and to complete a questionnaire. From adults a maximum of 22 ml blood was taken and depending on their age and the degree of discomfort, less blood (0·1–8 ml) was taken from children. The questionnaire addressed demographic characteristics, vaccination history, perceived health and diseases, activities possibly related to infectious diseases (travel, profession, food habits, gardening) and information related to sexually transmittable diseases for 15- to 79-year-olds. Samples and data of the seroprevalence study were collected in the period from February 2006 to June 2007.
Informed consent was obtained for all participants included in this study. The study proposal was approved by the Medical Ethics Testing Committee of the Foundation of Therapeutic Evaluation of Medicines (METC-STEG) in Almere (clinical trial number: ISRCTN 20164309).
Serology
The sera were stored at −80°C. Anti-HEV IgG antibodies were detected in serum by a commercial enzyme immunoassay (EIA) (HEV ELISA, MP Diagnostics, France) according to the manufacturer's instructions. Based on assay validation by Herremans et al., a combined testing regimen was applied to increase specificity: all positive samples were confirmed by Western blot analysis (RecomBlot HEV IgG; Mikrogen, Germany) according to the manufacturer's instructions as described previously [Reference Herremans26]. This study was performed prior to the launching of a newer version of the assays, in which gt 3 antigens were included. As this change was made by the manufacturer without notice, only minimal comparative validation was done. This suggested that the sensitivity of the current assay may be slightly higher.
Seroprevalence analysis
The participation rate of subjects providing combined sera and questionnaire data was 33%. Sociodemographic data from non-responders and comparison of the response group to the Dutch population have been described previously [Reference van der Klis25, Reference Verhoef27], with non-responders being similar with regard to region, educational level and health status. The seroprevalence was calculated for the national sample representative of the Dutch population. Migrants were also included in the seroprevalence estimation. Therefore, the seroprevalence estimates were weighted within each municipality for age, gender, ethnicity and degree of urbanization, up to their proportion in the total population of The Netherlands as of 1 January 2007 [28]. Seroprevalence was adjusted for the two-stage cluster sampling by taking into account the strata (five regions) and clusters (40 municipalities) [Reference van der Klis25]. Prevalence rates per year of age and 95% confidence intervals (CI) were estimated using smoothing splines with logit link and binomial distribution [Reference Hastie and Tibshirani29]. Seroprevalence was calculated for groups based on country of birth: (1) The Netherlands, (2) Surinam and Netherlands Antilles and Aruba, (3) Morocco and Turkey, (4) other non-Western countries, and (5) other Western countries. In addition, seroprevalence was calculated for people with and without ‘risk abroad’, with risk abroad defined as being born abroad, ever travelled to Africa, Asia, South America, or Central America, or having received a blood transfusion abroad.
Determinants of anti-HEV antibody positivity
Univariate logistic regression analysis was performed to determine which of 86 investigated variables could be identified as determinants of anti-HEV antibody positivity, i.e. seropositivity for HEV, after adjustment for age, gender and ethnicity if cell counts were ⩾5. If cell counts were <5, variables were adjusted for age and ethnicity, or age only. These analyses were based on the total study population (i.e. both the national sample and the low-vaccination-coverage sample), as well as the national sample only. In sub-analysis, we investigated whether comparison of EIA-positives (irrespective of blot result) compared to EIA-negatives would influence the determinant profile for the total group. In sub-analysis, to identify potential determinants of seropositivity after endemic HEV exposure, similar analysis was performed while separating all persons with risk abroad from those without risk abroad, as defined above. Variables were included in a multivariate model if their P value was <0·20 in adjusted univariate analysis. The variables remained in the multivariate model if the P values were <0·10 while the backward selection procedure was used, or if they were found to be confounding factors for other variables in the model. To ensure a valid model, further reduction was performed until the number of parameters was ⩽10% of the number of seropositive subjects. Missing values were classified as ‘unknowns’, in order to be able to include the maximum number of participants in the multivariate logistic regression. Analysed variables were included as numerical instead of categorical where possible (i.e. age in years, number of persons contacted). Variables were considered significant if P<0·05 and borderline significant if 0·05⩽P<0·10. Data were analysed using SAS v. 9.2 for Windows (SAS Institute Inc., USA).
RESULTS
Overall, of 24 147 invited persons a total of 7904 (33%) provided a serum sample, of which 7072 (89%) were of sufficient volume for anti-HEV antibody testing. Of these, a total of 5642 were provided by persons from the nationwide sample, whereas the additional 1430 sera were provided by the sample group from the lower vaccination coverage areas. The median age was 34·2 years (range 0 to 79 years). A total of 186 sera tested positive using EIA, of which 134 were confirmed by immunoblot.
Seroprevalence
The overall weighted seroprevalence of HEV antibodies in the Dutch population was estimated to be 1·9% (95% CI 1·5–2·3). The seroprevalence was somewhat higher for men (2·2%, 95% CI 1·6–2·8) than for women (1·7%, 95% CI 1·1–2·2), although not significantly so. Table 1 shows the seroprevalence of the different groups of origin, showing that people originating from The Netherlands, Turkey, Morocco and other non-Western countries have the highest seroprevalence, but no significant differences are seen. Table 1 also shows the seroprevalence for people with and without risk abroad, showing a marginally significant difference between these groups. The seroprevalence of anti-HEV IgG antibodies increases with age (Fig. 1).

Fig. 1. Weighted age prevalence estimates (mid black line) of hepatitis E antibodies and 95% confidence intervals (outer black lines) presented in age per year in a nationwide sample of the Dutch population in 2006–2007 (n=6386). Black square symbols (▪) represent the weighted seroprevalence estimates of 5-year age groups.
Table 1. Weighted seroprevalence of HEV antibodies in the Dutch population in the national sample, 2006–2007, by gender and ethnic origin (n=5642)
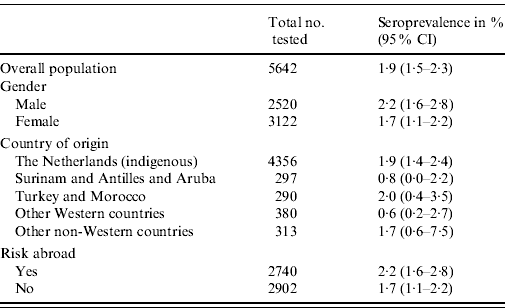
CI, Confidence interval.
Determinants of anti-HEV antibody positivity
In logistic regression analysis 21/86 variables were found to be associated (P<0·20) with prevalence of anti-HEV IgG antibodies in univariate analysis, of which five variables were significant (P<0·05) after correction for age, gender and origin (Table 2). Positive association with anti-HEV IgG antibodies was seen for people where the youngest household member was aged <5 years (i.e. pre-school) or aged between 19 and <65 (adult) years, who had a partner from abroad, who were pregnant (based on only two cases), and who adhered to a religion that considers pigs unclean. The higher the number of persons that a subject had spoken to the previous day was found to be significantly negatively associated after correction for age, gender and origin. In multivariate analysis older age, being male, recent (i.e. during past month) diarrhoeal complaints, adhering to a religion that considers pigs unclean, working with patients, and working with animals were independently positively associated with the presence of HEV antibodies (P<0·10). Exclusion of the low-vaccination area did not result in a different determinant profile, so in order to increase power we considered the inclusion justified. Using the different case definition based on EIA-positives did not result in a more evident determinant profile; e.g. gender was no longer associated. In accord with other studies, we considered the use of blot confirmation necessary. Risk abroad – i.e. history of travelling to Africa, Asia, Southern America or Central America, being born abroad or having received a blood transfusion abroad – could explain 56% (75/134 subjects) of all HEV seroprevalence in the Dutch population.
Table 2. Odds ratios (OR) and 95% confidence intervals (CI) for significant (P<0·05) and borderline significant (0·05≤P<0·10) associations between different variables and the prevalence of HEV antibodies in the Dutch population in 2006–2007, as found in univariate logistic regression adjusted for age, gender and origin, and in a multivariate logistic regression modelFootnote * Footnote † (n=7072)
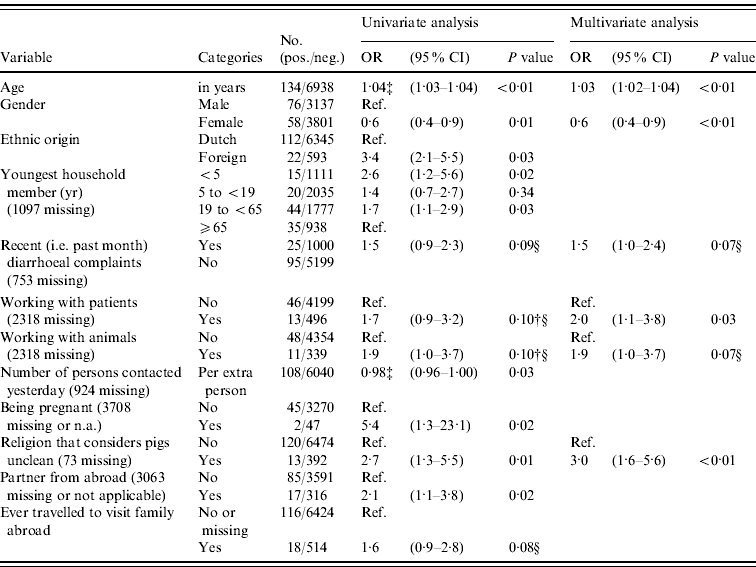
* The following factors were associated in univariate analysis (P<0·20) after correction for age, gender and origin and entered in a multivariate stepwise selection model: blood in stool; recent diarrhoeal complaints; fever or health complaints resulting in sick leave in the previous month; ever having had a blood transfusion; presence of hepatitis A antibodies; first sexual contact at age <18 years; steady relationship; partner from abroad; being pregnant; number of persons in household; youngest household member (categorical); ever travelled to visit family abroad, travelling duration >3 weeks; religion that considers pigs unclean; number of persons spoken to yesterday (categorical or numerical per person); hours per week of gardening without gloves in the past 12 months; working with patients; working with animals; military job; eating raw or half-cooked meat during the past 12 months; monthly consumption of raw vegetables (yes/no). The following factors were not associated (P>0·20) in univariate analyses after correction and not entered in a multivariate stepwise selection model: presence of children in household attending day-care, risk per extra day attending day-care; food allergy; vomiting, nausea or visiting a physician in the previous month; number of sexual partners; age of first sexual experience (in years); gardening without gloves (categorical); living in a highly urbanized area; low income or education level; self-reported bad health conditions; holidays or working abroad; having kept a pet or farm animal during the past 5 years (dog, cat, bird, fish, rabbit, goat, pig, cow, sheep, poultry); medical job necessitating vaccination; critical opinion towards vaccination; food allergy; asthma or chronic obstructive pulmonary disease; eczema; hay fever; having been a blood donor; ever having donated blood; number of sexual partners; male-to-male sex; tattoo; playing in sand box; working with clients or children; being vegetarian; monthly consumption of raw vegetables (yes/no). In this multivariate model, also age, gender and ethnic origin were included as associated factors.
† Associations that had P value >0·10 but that were included in the final multivariate model are also presented.
‡ Results for the variables age in years or in number of persons are given to two decimal places, with odds ratios provided for each extra year, person or day.
§ Borderline significant values may be significant by chance, and these should be interpreted with caution.
Determinants of anti-HEV antibody seropositivity after endemic exposure
When confining the logistic regression analysis for persons that did not have risk abroad, 4022/7072 (57%) were included of which 59 (1·5%) were positive for HEV antibody testing. Of these suspected locally exposed seropositive subjects, 14 (24%) reported ever having donated blood, and of those three donated blood in 2006. Compared to the 578/3963 (15%) HEV-negative persons without travel abroad that had donated blood, donating blood was not associated with HEV exposure (P=0·48). Recent diarrhoeal complaints, youngest household member being aged <5 years or between 19 and <65 years, being pregnant, working in the garden without gloves, and having a tattoo were found to be positively associated with HEV antibodies after correction for age (Table 3). In multivariate analysis, only ‘youngest household member being aged <5 years or between 19 and <65 years’ and older age remained as independent associations.
Table 3. Odds ratios (OR) and 95% confidence intervals (CI) for associations (P<0·10) between different variables and the prevalence of HEV antibodies in a subset of the Dutch population that had no risk abroad (n=4022), as found in univariate logistic regression adjusted for age and in a multivariate logistic modelFootnote * Footnote †

n.a., Not applicable.
* Subset 1: exclusion of all persons that had travelled outside Europe or who had received a blood transfusion abroad. Risk factors for non-travel-related HEV exposure were analysed (n=4022, of which 59 were positive for HEV antibody testing).
† Results for variables per year of age or per number of persons are given to two decimal places.
‡ Borderline significant P values (between 0·05 and 0·10) may be significant by chance, and these should be interpreted with caution.
DISCUSSION
On the basis of our cross-sectional serological study the overall seroprevalence of anti-HEV IgG antibodies in the Dutch population was estimated to be 1·9% in 2006/2007. Older age, being male, recent diarrhoeal complaints, adhering to a religion that considers pigs unclean, working with patients, and working with animals were independently associated with risk of a past or recent exposure to HEV. Of those with no risk abroad – i.e. probably locally exposed – older age and youngest household member being aged <5 years or between 19 and <65 years were found to be independent determinants of seropositivity. However, the small numbers generated in this study prevented a robust risk-factor analysis, especially for the endemic risks, and further confirmatory work is necessary. Nevertheless, although donating blood was not found to be associated with HEV exposure, 14/59 (24%) of endemic seropositive individuals reported ever having donated blood, of which four had donated recently. This demonstrates that HEV-exposed persons are not excluded from donating blood, which may indicate a potential public health risk in The Netherlands.
Although the seroprevalence in men was somewhat higher than that found in women, this difference was not significant. However, hepatitis E patients in The Netherlands were more likely to be male on the basis of descriptive case studies [Reference Borgen10]. This was also observed by other studiess [Reference Lewis, Wichmann and Duizer2, Reference Ijaz30], suggesting that men, compared to women, are at higher risk of developing disease following exposure. The seroprevalence of anti-HEV in The Netherlands is comparable to the 3·2% found in healthy blood donors in France [Reference Boutrouille11], and low compared to 9·3% in people age- and geographically matched to pig farmers in Sweden (age 40–60 years) [Reference Olsen31], 7·3% in Catalonia [Reference Buti32], or 13% in England [Reference Ijaz30]. However, these studies are difficult to compare due to different populations studied, and different diagnostic tests used. For example, the study in England expected that anti-HEV IgG titres would be low and deliberately chose a more sensitive ELISA assay [Reference Renou14]. Moreover, positive ELISA results were not confirmed with immunoblot, as was done in our study. We used this confirmative immunoblot based on findings by Herremans et al. [Reference Herremans26]. We assume that ELISA-positive results that can not be confirmed by immunoblot are false positives.
Detection of anti-HEV IgG was done using a commercially available ELISA that contains peptides derived from ORFs 2 and 3 of HEV viruses belonging to genotypes 1 and 2, whereas locally endemic viruses belong to HEV gt 3 [Reference Borgen10]. This raises the question of whether the actual seroprevalence of HEV might be higher in our population. Studies comparing peptides or complete ORF2 capsids as antigens in ELISA have found discrepancies in favour of the use of complete ORF2 from homologous strains for clinical diagnosis. When comparing this ELISA for detection of IgG in patients with HEV gt 3 infection against those with gt 1 infection, sensitivity was lower in gt 3-infected persons [Reference Herremans26]. A comparative analysis of peptide-based assays for detection of antibodies to genotypes 1 and 4 viruses showed that antibodies to heterologous strains were detected with lower sensitivity [Reference Ma33]. Therefore, use of a genotype-specific immunoassay could have increased the seroprevalence in our study, but not uniformly for all exposures. In our study, approximately 56% of subjects with seropositivity were potentially exposed to HEV abroad, which was more likely to be gt 1 or gt 2, for which the ELISA was optimal. Potential under-ascertainment could apply selectively for endemic exposure. Based on the kinetics of antibody response and the lower sensitivity and threshold for detection of heterologous antibodies, positive findings in this group may be biased towards recent exposure, as IgG levels peak in the weeks following acute infection [Reference Bendall34]. This could explain the association with recent diarrhoeal complaints, although this is not a frequently reported symptom of HEV infection.
A general feature of serological surveys measuring IgG antibodies is that they may reflect current exposures and exposures in the past. The window of detection is, however, uncertain for hepatitis E, for which antibodies can still be present after 14 years [Reference Emerson, Purcell, Knipe and Howley35], but can also be gone after 9 months [Reference Goldsmith36]. This may explain the weak association with older age. Even more so, the kinetics may differ between genotypes. Thus, it is unclear when the exposure occurred, and for which time span the requested associations are of relevance. As a consequence, it is not possible to tell whether the potential associations addressed in the questionnaire took place before or after the actual exposure. We recommend a systematic analysis of the kinetics of antibody response of people infected with gt 3 and measured with the assays used in our study to enable the identification of a more definite risk profile of exposures, especially since genotypes are described as differing both epidemiologically and clinically. Gt 3 infections are thought to cause milder disease [Reference Mizuo37], and considered to be potentially zoonotic infections rather than waterborne [Reference Lewis, Wichmann and Duizer2]. Thus, the analysis of all genotypes as one group may have diluted the effect of endemic associations. We aimed to solve this problem by performing our sub-analysis while excluding all persons with potential risk abroad.
The investigation of a large number (n=86) of variables may have led to finding four or five associations by chance. This may be especially true for the borderline significant associations, and these, in particular, should be interpreted with caution. Still, the associations found are interesting. Working with animals may indicate the zoonotic potential of HEV gt 3 as described previously [Reference Lewis, Wichmann and Duizer2]. However, this association was found for the overall population, and did not remain significant during sub-analysis of endemic exposures. Interestingly, this association was not found in Germany either, instead foodborne zoonotic transmission was reported [Reference Wichmann20]. The extra risk of HEV seropositivity when working with patients may suggest person-to-person transmission in the overall population. Although this is generally reported to be rare [Reference Lewis, Wichmann and Duizer2], it may occur after contact with patients [Reference Nubling, Hofmann and Tiller38]. The positive association in the overall population adhering to a religion that considers pigs unclean is not surprising, because people from such a religion are more likely to have lived in, or visited North Africa or Arabic countries and thereby be associated with HEV seropositivity. This association was apparently stronger than ethnic origin, which was less clear in discriminating such countries and was not included in the final multivariate model for the overall population. These ethnic differences were filtered out when confining the analysis to endemic exposures.
With regard to the group with potential endemic exposure, gender was no longer found to be significantly associated, which may be due to lower numbers. The youngest household member being aged <5 years is an interesting independent association in this group of possible endemic infections, especially since it could not be explained by having a child attending day-care. It could be an indicator of other common behaviour such as more frequent visits to children's farms or a zoo. Children's farms are visited by 9–11 million people each year in The Netherlands [Reference Berends39], and 21% of the visitors are aged 0–5 years [Reference Evers, Horneman and Doorduyn40]. Unfortunately, we do not have more detailed information to confirm all of the associations identified as potential risk factors. For future studies we recommend including the possibility of contacting study subjects, so that additional detailed information can be obtained afterwards, when required.
We considered stratification of analysis for risk abroad in order to find potential risk factors for assumed travel-associated HEV exposure. However, apart from travelling, no significant associations were found in this group other than age being positively associated and ‘number of persons contacted yesterday’ being negatively associated with HEV exposure. The latter is difficult to explain but may be related to differences in cultural behaviours, since the group of people with risk abroad was heterogeneous and represented a combination of several ethnic backgrounds as well as travellers.
Overall, our data show that risk of HEV exposure in the Dutch population is present. Although the seroprevalence seems to be lower compared to surrounding European countries, endemic exposure appears to occur with associations suggesting zoonotic potential. Several other identified potential associations, like older age and gender, are consistent with findings in studies. Other additional associations for HEV IgG antibodies were found which may assist in further development of a risk profile for HEV infection. Given a weighted seroprevalence of 1·9% in the general population, of which 44% are people with no risk abroad and including blood donors, this indicates a potential public health risk, even though the viraemic phase is know to be short. In the absence of a clear risk profile, people at risk of HEV infection cannot be excluded from donating blood, certainly since the proportion of asymptomatic HEV infections is unclear. We advise increased surveillance and serological follow-up of HEV cases and their potentially asymptomatic HEV seropositive family members in The Netherlands, in order to obtain a better understanding of the kinetics of gt 3 infections and sources of autochthonous exposure to HEV.
ACKNOWLEDGEMENTS
We thank Liesbeth Mollema and Fiona van der Klis for technical assistence, and the public health services, the municipalities involved, and the Pienter2 Project Team for their contribution to the realization of the Pienter2 Project. We are grateful to all participants of the Pienter2 study for their contribution.
DECLARATION OF INTEREST
None.






