1. Introduction
Tobacco accounts for over 7 million deaths annually [Reference World Health Organization1]. Counseling and medication can increase more than double the success rate for quitting smoking [Reference World Health Organization1]. About 62% of those who attempt to quit smoking on their own with no pharmacotherapy had a relapse in the first two weeks, and only 5% succeed to quit smoking after a year [Reference Garvey, Bliss, Hitchcock, Heinold and Rosner2]. Smoking cessation is useful at any age for preventing the risk of co-morbidities progression and early mortality [3]. Current smoking cessation methods include counseling [Reference Goldstein, Niaura, Willey, Kazura, Rakowski and DePue4, Reference Zhu, Stretch, Balabanis, Rosbrook, Sadler and Pierce5], and nicotine replacement therapy (chewing gum, inhalers, and nicotine patches), with bupropion and Varenicline [Reference Heydari, Masjedi, Ahmady, Leischow, Lando and Shadmehr6]. Nevertheless, many smokers using these treatments also fail to quit smoking and maintain abstinence for at least six months [Reference Heydari, Masjedi, Ahmady, Leischow, Lando and Shadmehr6–Reference Silagy, Mant, Fowler and Lancaster8].
Over the past decade, transcranial Direct Current Stimulation (tDCS) has been employed as a safe [Reference Bikson, Grossman, Thomas, Zannou, Jiang and Adnan9], non-invasive brain stimulation method, for addiction treatment and research on this topic is expanding [Reference Dunlop, Hanlon and Downar10]. Research on the effect of tDCS on smokers was mainly focused on the reduction of cue-induced craving [Reference Fregni, Liguori, Fecteau, Nitsche, Pascual-Leone and Boggio11, Reference Mondino, Luck, Grot, Januel, Suaud-Chagny and Poulet12], ability to resist smoking [Reference Falcone, Bernardo, Ashare, Hamilton, Faseyitan and McKee13], reduction of negative affect but not cigarette craving in overnight abstinent smokers [Reference Xu, Fregni, Brody and Rahman14], and motivate smokers to quit smoking [Reference Brangioni, Pereira, Thibaut, Fregni, Brasil-Neto and Boechat-Barros15]. Furthermore, with repeated five sessions of stimulation per week, its cumulative effect decreased craving and even the number of cigarettes smoked [Reference Boggio, Liguori, Sultani, Rezende, Fecteau and Fregni16]. In other studies, anodal stimulation with tDCS has also decreased craving for alcohol and marijuana [Reference Boggio, Sultani, Fecteau, Merabet, Mecca and Pascual-Leone17, Reference Boggio, Zaghi, Villani, Fecteau, Pascual-Leone and Fregni18]. Treatment of patients with depression with tDCS revealed maximum therapeutic effect after 20 sessions of stimulation (5 sessions per week) in 4 weeks [Reference Martin, Alonzo, Mitchell, Sachdev, Gálvez and Loo19]. On the best of our knowledge, no study has so far examined the long-term clinical effects of tDCS on smoking cessation, nicotine dependency, and relapse rate on a 6-month follow-up. Bupropion was approved in the US in 1997 as a smoking cessation treatment [Reference Foulds, Burke, Steinberg, Williams and Ziedonis20]. Moreover, the Department of Health and Human Services (DHHS) guidelines for the treatment of nicotine dependence recommended bupropion in 2008 as the first line of treatment for smoking cessation [Reference Fiore, Jaén, Baker, Bailey, Benowitz and Curry21]. Therefore in this study the effectiveness of 20 sessions tDCS in two regimes (4 weeks & 12 weeks) has evaluated and compared with bupropion, on the treatment of tobacco dependence with a 6-month follow-up.
2. Methods
The present study is a randomized sham-controlled parallel-group clinical trial.
The participants were selected from among those responding to a public invitation for cigarette cessation. They were all right-handed, aged 15–65 years, addicted to cigarette nicotine based on DSM-5 criteria, without underlying physical or psychological disease based on interviews with a psychiatrist and WHO Disability Assessment Schedule 2.0 [Reference Gold22], and interested in quitting smoking. Exclusion criteria were the consumption of drugs containing sodium, potassium, and calcium; epilepsy; malnutrition; history of neurosurgery; consumption of any other addictive substance except nicotine, and the use of other nicotine-containing products such as pipe, hookah, or cigars. All of the participants refrain from smoking 2 h before baseline measurement and tDCS sessions during the study. This study has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The participants were informed by a clinical psychologist of all therapeutic steps, duration of the study, randomized nature of interventions, and possible complications of interventions, and entered the study upon signing informed consent forms approved by the Ethics Committee (Ethics Code: IR.SHMU.REC.1395.75) of Shahroud University of Medical Sciences.
2.1. Interventions
Interventions were provided in five groups: A) pharmacotherapy, using 300 mg bupropion (GlaxoSmithKline 2006) for 8 weeks; B) tDCS (Soterix 1 × 1 tDCS) for 20 sessions over 4 weeks, (5 sessions per week); C) The sham tDCS for 20 sessions over 4 weeks, (5 sessions per week); D) active tDCS for 20 sessions over 12 weeks, (5 sessions per week for two weeks, followed by one session per week), and E) The sham tDCS for 20 sessions over 12 weeks, (5 sessions per week for 2 weeks, followed by one session per week). The electrode placement montage was anode F3 and cathode F4 in tDCS groups based on standard 20/20 EEG system. Stimulation intensity was 2 mA for 20 min in tDCS groups and only 30 s in sham groups. This short period is the time when the device becomes ready to start the 2 mA stimulation. Carbon electrodes, placed inside sponges smeared with saline solution, were utilized. Anode and cathode size was 35 and 100 cm2, respectively.
2.2. Outcomes
Primary outcome of this study was abstinence at 6-months confirmed by salivary cotinine <4 ng/ml. Nicotine dependence severity confirmed by Fagerstrom test for nicotine dependence (FTND) and self-reported cigarette per day (CPD) were secondary and tertiary outcomes respectively. All outcomes measured at time points of baseline (time 1), post-intervention (time 2), and 6-month follow-up (time 3). The length of follow-up was the same for all groups (6 months after the beginning of interventions). Point abstinence prevalence was calculated by the percentage of individuals with salivary cotinine of lower or equal to 4 ng/ml at different time points.
2.3. Sample size
Given that other studies [Reference Aubin, Lebargy, Berlin, Bidaut-Mazel, Chemali-Hudry and Lagrue23–Reference Piper, Smith, Schlam, Fiore, Jorenby and Fraser25], reported a 31% abstinence following treatment with bupropion at six-month follow up, α = 0.05, β = 0.2 (95% confidence interval and 80% power), and 5% quit rate with tDCS; the sample size was calculated 35 per groups. The Sample size was smaller for salivary cotinine and Fagerstrom score which were evaluated based on mean scores of these outcomes. Therefore, the sample size was considered as 35 per group.
2.4. Randomization
Random allocation was designed by a nurse using a similar balls method. In this method, 175 similar balls were prepared, and the names of the five groups (A–D) were placed inside balls. Then, each participant selected one ball, thus selecting their group randomly.
2.5. Blinding
Interventions were conducted by one researcher who was not blinded, while salivary cotinine measurement was taken by a colleague working at the laboratory who was blinded to participant’s groups. In addition, questionnaires were completed by the participants and handed to the psychologist of the group who was also blind to group classification. All data were given to the analyzer, who was blinded to the type of interventions. The participants were unaware of the type of intervention in all active and sham tDCS groups.
2.6. Instruments
1: The Expiration Carbon Monoxide (CO) was measured by Smokerlyzer (Bedfont Scientific Ltd, UK); each participant with CO more than 9 ppm was classified as a smoker [Reference Jarvis, Tunstall-Pedoe, Feyerabend, Vesey and Saloojee26]. 2: Fagerstrom Test for Nicotine Dependence (FTND) is a reliable and valid scale for determining the severity of nicotine dependence [Reference Heatherton, Kozlowski, Frecker and Fagerström27, Reference Sarbandi, Niknami, Hidarnia, Hajizadeh, Azaripour and Eslampanah28], and results vary from 0 to 10. 3: Salivary cotinine measurement with ELISA method is a very sensitive quantitative test for estimating the status of cigarette smoking in the past week [Reference Wall, Johnson, Jacob and Benowitz29, Reference Cooke, Bullen, Whittaker, McRobbie, Chen and Walker30]. Salivary cotinine level in non-smokers is less than or equal to 4 ng/ml.
This clinical trial was registered in the Iranian Registry of Clinical Trials (registered code: IRCT2016072629093N1).
2.7. Statistical methods
After examining the normality of variables using Shapiro–Wilk’s test and skewness and kurtosis indices, data were analyzed using repeated-measures analysis of variances and the generalized estimation equation (GEE) model. Maximum alpha error for hypothesis testing was determined as 0.05 (p < 0.05).
3. Results
In this study, 210 individuals volunteered following a general invitation. 10 persons did not meet the inclusion criteria; 25 did not accept terms of research, including pharmacotherapy, tDCS, and 6-month follow-up, and 5 did not visit during the intervention and follow-up due to work-related problems. Finally, 33 were allocated to Group C, 32 to Group D, and 35 to other groups, with 170 participants completing all steps of the study. Fig. 1 illustrates the number of participants in the five groups over different steps, from inclusion to analysis. Recruitment occurred from August 2016 to November 2016, and interventions and follow-up lasted until August 2017.

Fig 1. Flow diagram of study.
Mean ± SD of participants’ age was 42.88 ± 10.96 years, ranging from 21 to 64 years. All participants were male, with 121 (71.2%) being married, 75 (44.1%) with high school-level education, and 114 (67.0%) being employed. Baseline demographic characteristics of the participants in different groups are reported in Table 1.
The comparison of participants succeeding in completely quitting smoking (Salivary Cotinine < = 4) at each time point (point abstinence rate) revealed that 43 people (25.3%) succeeded in the first month and 20, (11.8%) in the six months after intervention. Examination of this status in different intervention groups at the 6-month follow-up indicated that 7 participants (20%) in Groups A, 2 participants (5.7%) in group B, one participant (2.8%) in Groups C and E, and 9 participants (25.7%) in Groups D successfully quit smoking. Therefore, treatment success was markedly higher in Groups A and D compared to other groups (p < 0.001). Results of the GEE model in Table 2 revealed that failure in complete smoking cessation was higher in Groups C, E, and B, compared to A (p < 0.001) and there was no statistically significant difference between groups A and D (p = 0.266).
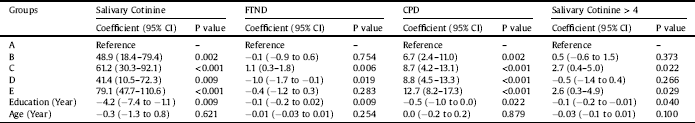
Results of repeated-measures ANOVA for salivary cotinine revealed that this value showed a significant difference at three- time points (baseline, post-intervention, and follow-up) (F = 60.3, df = 2, P < 0.001). Moreover, the value of salivary cotinine differed across groups (F = 6.5, df = 4, P < 0.001). Beside, an interaction effect exists for the values of cotinine in groups by time points (F = 9.1, df = 8, P < 0.001). As the time and group interaction effect was significant, further tests (Tables 3 and 4) revealed that the cotinine level differed across Groups A, B, and D in different time points (P < 0.001). On the other hand, it was not significant in Groups C (p = 0.859) and E (p = 0.876). Examination of the effect of different interventions at three- time points showed no significant difference in baseline measurements (time 1) of cotinine level across groups (F = 1.8, df = 4, P = 0.130), which was expected considering randomization. On the second and third time points, however, cotinine level differed across groups (P < 0.001). Table 3 presents the effect of interventions at three- time points. Examination of the trend of changes in cotinine over different periods showed that, in Groups A, B, and D, a significant linear and quadratic trend exists for cotinine levels (p < 0.001). Nevertheless, these trends were not significant for the sham groups (C, E). A significant increase in salivary cotinine existed in group A, while in groups B and D there were an steady state between times 2 and 3 (Table 3 and Fig. 2).
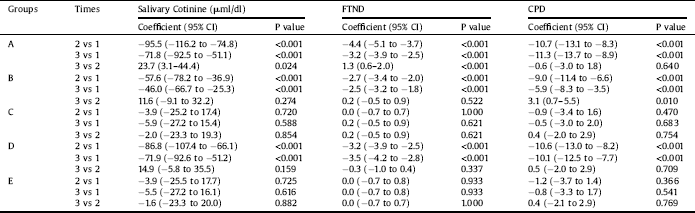
Further inter groups analysis revealed that compared to group A, cotinine levels were higher in other groups at times 2 and 3 (Table 4). Similarly, results of the GEE model, adjusted with other independent variables, including education level and age revealed that the levels of cotinine were higher in different intervention groups compared to Group A (Table 2). This model also showed that an increase in education level leads to a decrease in cotinine level (p = 0.009).
Results of repeated-measures ANOVA for FTND revealed that this value showed a significant difference across the three- time periods (F = 95.7, df = 2, P < 0.001). Furthermore, the value of FNDT differed across groups (F = 5.9, df = 4, P < 0.001). Also, there was an interaction effect for this value for groups by time (F = 18.9, df = 8, P < 0.001). As the time and group interaction effect, was significant, further tests revealed that the FTND score differed across Groups B, A, and D in different time points (P < 0.001). On the other hand, it was not significant in Groups C (p = 0.850) and E (p = 0.995) (Fig. 3). Examination of the effect of different treatments at three- time points showed that, at all-time points, FTND scores differed across groups (p < 0.001).
Table 1 Demographic characteristics of the participants (n = 170).
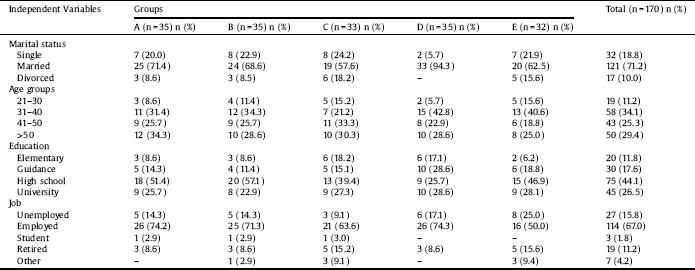
Table 2 The effects of different interventions on various outcomes in Generalized Estimating Equation models.
A, Bupropion Intervention; B, 20 sessions Active tDCS in 1 month; C, 20 sessions Sham tDCS in 1 month; D, 20 sessions Active tDCS in 3 month; E, 20 sessions Sham tDCS in 3 month; FTND, Fagerstrom Test of Nicotine Dependence; CPD, Cigarette Per Day; CI, Confidence Intervals.
Examination of the trend of change in FTND over different time periods indicated that, in Groups A, B, and D, a significant linear and quadratic trend existed (p < 0.001). None of these trends was significant in sham groups. Further analysis revealed that compared to group A, FTND were different in other groups at different time points, except for group B (Table 4). Similarly, results of the GEE model, adjusted with other independent variables (Table 2), showed that dependence severity did not differ in Groups B, and E compared to Group A but differed in Groups C and D compared to Group A. This model also showed that an increase in education level lead to reduction in nicotine dependence (p = 0.009), but age had no such effect (p = 0.254).
Results of repeated-measures ANOVA for CPD showed a significant difference across the three- time periods (F = 81.3, df = 2, P < 0.001). Moreover, the value of CPD differed across groups (F = 8.1, df = 4, P < 0.001). Also, there was an interaction effect for this value for groups by time (F = 11.3, df = 8, P < 0.001). As the time and group interaction effect, was significant, further tests revealed that CPD differed across Groups A, B, and D in different time points (P < 0.001). On the other hand, it was not significant for Groups C (p = 0.770) and E (p = 0.654) (Fig. 4). Examination of the effect of different interventions at three- time points showed that, at all-time points, CPD differed across groups (p < 0.001). Examination of the trend of changes in CPD over different periods showed that, in Groups A, B, and D, a significant linear and quadratic trend existed for CPD (p < 0.001). Further analysis revealed that compared to group A, CPD was higher in other groups at different time points (Table 4). Similarly, results of the GEE model, adjusted with other independent variables, revealed that the level of CPD differed in different intervention groups compared to Group A (Table 2). This model also indicated that an increase in education level significantly decreased the CPD (p = 0.025).
In this study, there were no complications reported from bupropion (except for 2 cases of insomnia and 3 cases of dry mouth) or tDCS (4 cases of the mild headache) to motivate participants to withdraw from interventions. All the noted minor side effects were resolved without pharmacotherapy.
4. Discussion
This study investigates two different tDCS protocols with their sham controls and compares these to standard treatment for tobacco dependence. The main finding of the study was that the longer duration tDCS protocol (20 sessions over 12 weeks: 2 weeks of daily stimulation followed by weekly booster sessions for 10 weeks) resulted in the highest abstinence rate at 6 months (25.7%) and was significantly more effective than the shorter stimulation protocol (20 sessions over 4 weeks) (7%) and both sham controls. So it can be claimed that the effects of tDCS on smoking cessation are more stable in long-term interventions. The 6-month abstinence rate for standard treatment with bupropion was 20% which did not show any significant difference with Group D (25.7%) (p = 0.266). However, this difference is clinically significant and the lack of statistical significance may be attributed to the small sample size in groups.
Table 3 The effects of interventions on outcomes in different times in repeated measure analysis of variance models.
A, Bupropion Intervention; B, 20 sessions Active tDCS in 1 month; C, 20 sessions Sham tDCS in 1 month; D, 20 sessions Active tDCS in 3 month; E, 20 sessions Sham tDCS in 3 month; 1, baseline; 2, after intervention; 3, fallow up; FTND, Fagerstrom Test of Nicotine Dependence; CPD, Cigarette Per Day; CI, Confidence Intervals.
Table 4 The comparison of different interventions with group A in different times in repeated measure analysis of variance models.
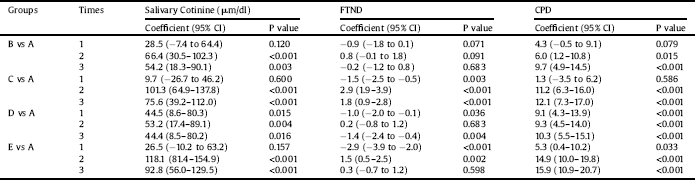
A, Bupropion Intervention; B, 20 sessions Active tDCS in 1 month; C, 20 sessions Sham tDCS in 1 month; D, 20 sessions Active tDCS in 3 month; E, 20 sessions Sham tDCS in 3 month; 1, baseline; 2, after intervention; 3, fallow up; FTND, Fagerstrom Test of Nicotine Dependence; CPD, Cigarette Per Day; CI, Confidence Intervals.

Fig 2. Salivary Cotinine changes according to different times and groups.
Salivary cotinin and FTND values increased significantly during 6-month follow-up compared to post intervention measurements in bupropion users (group A), while these changes were not significant for both types of tDCS protocol (Table 3 and Fig. 2). These findings suggest that the bupropion group was relapsing to smoking whereas the tDCS groups seemed to maintain abstinence. This is important because relapse following pharmacotherapy for smoking cessation is very common.
To the best of our knowledge, this is the first study that investigates tDCS in tobacco dependence for 6-month abstinence. Therefore the comparison of results with other studies is a bit difficult. In a study in which 615 smokers treated with 300 mg bupropion and counseling, the 6-month abstinence rate was 26.9% which was higher than placebo group [Reference Hurt, Sachs, Glover, Offord, Johnston and Dale31]. Similarly, in a study on 600 adult smokers, treated with slow-release 300 mg bupropion and counseling, the quit rate at 26 weeks was 21.0% vs. 13.7% in placebo group [Reference Ahluwalia, Harris, Catley, Okuyemi and Mayo32]. However, What should be considered in comparison of these studies is that counseling was not provided in the current study, and the smoking abstinence measurement scale was a quantitative and more reliable method compared to most previous studies which using self-reported measurements [Reference Scheuermann, Richter, Rigotti, Cummins, Harrington and Sherman33]. Previous studies have shown that five F3 anodal stimulations reduce CPD by about 30% compared to the sham group [Reference Boggio, Liguori, Sultani, Rezende, Fecteau and Fregni16]. However, we did not find any study on the persistence effects of tDCS on dependence severity and CPD for six months.

Fig 3. Nicotine dependent severity according to different times and groups.

Fig 4. Cigarette per day smoking changes according to different times and groups.
Smoking cessation is related to a reduced dopaminergic activity which induces craving and relapse [Reference Diana34]. Although little is known about the mechanism of effect of tDCS, various theories have been proposed in this regard. Studies indicate the mediation of neuromodulators of dopamine (DA-D2) [Reference Nitsche, Lampe, Antal, Liebetanz, Lang and Tergau35], and N-Methyl-B-Aspartic Acid (NMDA) [Reference Nitsche, Fricke, Henschke, Schlitterlau, Liebetanz and Lang36], in post-effect changes (cortical excitability changes) and, consequently, long- lasting synaptic plasticity changes [Reference Bindman, Lippold and Redfearn37]. The severity of this plasticity effects is based on size and forms of evoked potentials stimulations [Reference Bindman, Lippold and Redfearn38]. Anodal stimulation increases and cathodal stimulation reduces the excitability of cortical neurons [Reference Nitsche, Nitsche, Klein, Tergau, Rothwell and Paulus39, Reference Jansen, Daams, Koeter, Veltman, van den Brink and Goudriaan40]. Therefore, the stimulation of dorsolateral prefrontal cortex (DLPFC) can induce the release of dopamine in mesolimbic pathways, which leads to a transient increase in dopaminergic activity and, thus, mimics the effects of substances in the mesolimbic pathways, thereby assisting the temporary reduction in craving [Reference Pedron, Monnin, Haffen, Sechter and Van Waes41]. However, it is believed that the long-term effects of tDCS on cortical excitability are due to the glutamatergic mechanism [Reference Liebetanz, Nitsche, Tergau and Paulus42]. This change in neural excitability occurs at the time of stimulation and continues in the delayed form afterward, depending on the duration and intensity of stimulations as well as the person’s baseline neural excitability [Reference Nitsche and Paulus43]. It seems that the most crucial factor in relapse and failure of smoking cessation is negative affects in nicotine abstinence state [Reference Shiffman, Gnys, Richards, Paty, Hickcox and Kassel44, Reference Shiffman and Waters45]. But it must be noted that the anti-depressant effects of bupropion comprise less than 20% of its effects as a smoking cessation drug [Reference Lerman, Roth, Kaufmann, Audrain, Hawk and Liu46]. Human and animal studies indicate several possible cognitive mechanisms which may play a role in reducing smoking and smoking cessation by the stimulation of the DLPFC region. These include reducing nicotine craving [Reference Fregni, Liguori, Fecteau, Nitsche, Pascual-Leone and Boggio11, Reference Boggio, Liguori, Sultani, Rezende, Fecteau and Fregni16, Reference Pedron, Monnin, Haffen, Sechter and Van Waes41, Reference Wing, Barr, Wass, Lipsman, Lozano and Daskalakis47], emotion regulation and stress control [Reference Peña-Gómez, Vidal-Piñeiro, Clemente, Pascual-Leone and Bartrés-Faz48, Reference Nitsche, Koschack, Pohlers, Hullemann, Paulus and Happe49], decreasing high-risk behaviours [Reference Fecteau, Knoch, Fregni, Sultani, Boggio and Pascual-Leone50], and enhancement of decision-making skill [Reference Fecteau, Agosta, Hone-Blanchet, Fregni, Boggio and Ciraulo51], due to the direct anodal stimulation of DLPFC regions and, therefore, subcortical synchronization activity [Reference Rodriguez, George, Lachaux, Martinerie, Renault and Varela52].
Repeated electrical stimulation in the reduction of nicotine dependence severity (FTND) was more stable than drug treatment (Tables 3 and 4). It appears that the effects of neural adaptation and synaptic plasticity in the meso-cortico-limbic reward pathway through multiple sessions of stimulation cause this effect [Reference Gersner, Kravetz, Feil, Pell and Zangen53]. The lower FTND in group D compared to group A (Coefficient = −1.0, P = 0.019) (Table 2), may be due to the tDCS effects on the ability to resist smoking [Reference Falcone, Bernardo, Ashare, Hamilton, Faseyitan and McKee13], emotion regulation [Reference Xu, Fregni, Brody and Rahman14] and decrease high-risk behaviours [Reference Fecteau, Knoch, Fregni, Sultani, Boggio and Pascual-Leone50].
Analysis of CPD data (Table 3) showed a decreased value in post-intervention compared to the baseline (time 2 vs. 1) and follow-up compared to the baseline (time 3 vs. 1) in Groups A, B, and D (p < 0.001). Nevertheless, comparison of CPD in post-intervention compared to follow-up (time 3 vs. 2) showed no significant changes for Groups A (p = 0.640) and D (p = 0.709) and an increased value for Group B (p < 0.010) (Table 3 and Fig. 4), which indicated on cumulative effects of tDCS on CPD. In previous studies, the median decrease in cigarettes at the end of 5 sessions of stimulation was 30% compared to 10% in the sham group and similar to our findings, magnitude of tDCS effects increased after each session of stimulation [Reference Falcone, Bernardo, Ashare, Hamilton, Faseyitan and McKee13].
Finally, it is important to note that there are many inter-correlation between outcomes of this study (i.e. Cotinin, CPD and FTND) so it is not surprising that they all show the same pattern of results.
We are aware that our research has some limitations: 1. In the past decade, the NRT treatment with Varenicline in addition to counseling has mostly been used for smoking cessation [Reference Heydari, Masjedi, Ahmady, Leischow, Lando and Shadmehr6]. However, we used bupropion in this study as a drug intervention. 2. No woman responded to the public invitation and all participants were men. This limits the generalizability of results to the female population. 3. The sample size for some comparisons was low. Indeed, further studies on different races and with larger sample sizes are required to generalize results to all communities.
Funding
This work was supported by Shahroud University of Medical Sciences, Iran as a PhD dissertation (Project No. 9521).
Decleration of interest
The authors of this manuscript did not have any conflict of interest.
Acknowledgments
This study is a part of Ph.D. dissertation on addiction studies in Shahroud University of Medical Sciences (Project number: 9521). The authors are grateful to the deputy for research in this university and the respected participants who collaborated in this study.











Comments
No Comments have been published for this article.