1. Introduction
Major depressive disorder (MDD) is a psychiatric disorder which is a state of extreme sadness characterized by a combination of symptoms including biochemical, environmental, genetic, and psychological factors that interfere with a person’s ability to work, sleep, study, eat, and enjoy once-pleasurable activities [Reference Penninx, Milaneschi, Lamers and Vogelzangs1]. There is now evidence that MDD is characterized by an activation of the immune-inflammatory response system (IRS), as indicated by increased levels of pro-inflammatory cytokines including interleukin (IL)-6 [Reference Maes, Scharpé, Meltzer, Bosmans, Suy and Calabrese2–Reference Mao, Zhang, Chen, Zhao, Zhou and Wang5]. Moreover, recently it was shown that this primary IRS also induces the compensatory immune-regulatory system (CIRS), which attenuates an overzealous inflammatory response, as indicated by increased levels of IL-10, a negative immune-regulatory cytokine [Reference Maes and Carvalho6]. Altered functioning of the IRS and CIRS is implicated in multiple medical morbidities of MDD including neuroinflammatory and autoimmune disorders [Reference Maes, Kubera, Obuchowiczwa, Goehler and Brzeszcz7–Reference Bica, Castello´, Toussaint and Monteso-Curto9].
Many patients with MDD report somatic pain symptoms, which are considered to be a multidimensional experience that entails sensory as well as emotional, cognitive and behavioral aspects [Reference Maes10–Reference Bai, Chiou, Su, Li and Chen12]. The most important biological pathways related to pain include pain matrix alterations, pro-inflammatory cytokines, including IL-6, and opioid levels [Reference Bai, Chiou, Su, Li and Chen12, Reference Torta and Ieraci13]. Some studies showed that MDD is not only associated with IRS/CIRS activation [Reference Jeon and Kim14], but also with changes in endogenous opioids [Reference Taylor and Manzella15] and opioid receptors [Reference Lutz and Kieffer16, Reference Slavich, Tartter, Brennan and Hammen17]. Endogenous opioid peptides including β-endorphin (β-EP) and endomorphin-2 (EM-2), are small molecules that are naturally produced in the central nervous system (CNS) and in various glands throughout the body, such as the pituitary and adrenal glands. These peptides produce the same effects as the well-known alkaloid opiates, which include morphine and heroine. β-EP and EM-2 peptides function both as hormones and as neuromodulators [Reference Janecka, Fichna and Janecki18]. There is strong evidence that opioid release is part of the organisms' defense mechanism against the harmful effects of stress [Reference Adam and Epel19], including increased HPA-axis activity, which is frequently observed in MDD [Reference Yang, Zhao, Wang, Liu, Zhang and Li20]. Moreover, opioid receptors are involved in various physiological and pathophysiological activities, including pain modulation, emotional responses, immune functions, feeding, cardiovascular and respiratory control as well as neurodegenerative processes [Reference Feng, He, Yang, Chao, Lazarus and Xia21]. Many of these functions are disturbed in MDD and therefore the study of opioid receptor levels in this disorder is important in addition to their ligands β-EP and EM-2. Endogenous opioids, including β-EP and EM-2, as well as their μ-opioid receptor (MOR) are found throughout the central and peripheral nervous systems and in other tissues as well. Moreover, immunocytes express opioid receptors, including MOR, which additionally may modulate immune functions [Reference Liang, Liu, Chen, Ji and Li22, Reference Nelson, Schneider and Lysle23]. A molecular basis of bidirectional interactions between the opioid system and the immune system had been elucidated previously [Reference Kraus, Börner, Giannini, Hickfang, Braun and Mayer24]. Furthermore, in animal models, pro-inflammatory cytokines affect opiate-dependent pathways by up-regulating the expression of MOR [Reference Byrne, Peng, Sarkar and Chang25]. Exogenous opioids show immunosuppressive effects [Reference Raghavan, Harvey and Humble26], while cytokines may elicit the release of endogenous opioids [Reference Parsadaniantz, Batsché, Gegout-Pottie, Terlain, Gillet and Netter27]. Thus, there are multiple reciprocal relationships between endogenous opioids and the immune system.
The present study aims to delineate the relation between IL-6, a pro-inflammatory cytokine, and IL-10, a CIRS cytokine, and two endogenous opioids (β-EP and EM-2) and MOR levels in MDD patients. The specific hypotheses are that MDD is accompanied by increased levels of IL-6, IL-10, β-EP and EM-2 and increased MOR levels.
2. Method
2.1. Participants
This case control study involved 60 depressed drug free male patients aged 14–70 year and 30 age matched healthy males as a control group. The samples were collected at “The Psychiatry Unit”, Al-Hakeem General Hospital and a private psychiatric clinic run by an assistant professor in psychiatry, Najaf Governorate-Iraq during the period January to July 2017. C-reactive protein (CRP) was evaluated in all samples and we excluded subjects with CRP values >6 mg/L to eliminate any effects of overt inflammation on the results [Reference Al-Hakeim, Al-Rammahi and Al-Dujaili3]. Patients were diagnosed by psychiatrists according to DSM-IV criteria (5th revision of the Diagnostic and Statistical Manual of Mental Disorders). Severity of clinical symptoms was assessed using the 24-item Hamilton Depression Rating Scale (HDRS) one or two days before blood was drawn. Only MDD patients with a total HDRS score >21 were included. Informed consent was obtained from all participants after approval from the ethics committee (IRB) of the College of Science, University of Kufa, Iraq.
Patients were evaluated by full medical history. We excluded subjects with systemic disease that may affect immune parameters, including diabetes, liver disease, and renal disease. We also excluded MDD patients who were medicated, and subjects with other-axis I diagnosis including substance abuse. Individuals with highly increased hsCRP values (CRP >6 mg/L) were also excluded to participate.
2.2. Measurements
Five milliliters of venous blood samples were drawn, utilizing disposable needle and plastic syringes, from patients and controls. The samples were transferred into a clean plain tube. Haemolyzed samples were discarded. The blood was left at room temperature for 15 min for clotting, centrifuged 3000 rpm for 10 min, and then serum was separated and transported into two Eppindroff tubes to be stored at −80 °C until analyzed. Serum CRP was measures using a kit supplied by Spinreact®, Spain. The test is based on the principle of the latex agglutination. Commercial ELISA sandwich kits were used to measure serum EM2 and MOR (MyBioSource®, Inc. CA, USA) and β-EP, IL-6, and IL-10 (CUSABIO® Co., China). The procedures were followed exactly without modifications according to manufacturer’s instructions.
2.3. Statistical analysis
Normality of distribution of the variables was examined using the Kolmogorov-Smirnov test. The results were expressed as (mean ± standard deviation). Pooled t-test were used for the comparison between patients and controls. Pearson's correlation coefficients (r) were calculated to estimate the correlation between parameters. The differences between groups are considered to be statistically different when p < 0.05 (two tailed). The p-values were adjusted using the Benjamini–Hochberg method for multiple-hypothesis testing and false discovery rate (FDR). Statistical differences in categorical variables were evaluated by using χ2-test. All statistical analysis were performed using SPSS Statistics Version 25 (2017) by IBM-USA. Figures were made using the Excel program of Microsoft Office 2013.
3. Results
3.1. Demographic and clinical characteristics
The demographic and clinical characteristics of the patients with MDD and control groups are presented as observational data in Table 1. Patients with MDD and control groups showed no significant differences in age.
These data showed that most MDD subjects were married. About less than half of the patients were smokers. Most of them are urban and educated patients. All patients were newly diagnosed cases (drug free) in order to eliminate the effect of drugs on the measured parameter levels. The results in Table 1 showed a significantly lower BMI (p < 0.05) in MDD patients as compared with controls.
3.2. Comparison between patients with MDD and control groups
Serum β-EP, MOR, IL-6 and IL-10 concentrations in the MDD were significantly higher than in the control group (p < 0.05, Table 2), while EM-2 was not significantly different between both groups.
3.3. Comparison between smoking and nonsmoking MDD patients
Table 3 presents the comparison between smokers and nonsmokers MDD groups in addition to control group. There were no significant differences between smoking and nonsmoking MDD patients in IL-6 and EM-2. β-EP was significantly higher in both smoking and nonsmoking MDD patients than in controls (p < 0.05). IL-10 and MOR were significantly higher in both MDD subgroups than in controls, whilst there were no significant differences between both MDD subgroups (p < 0.05).
Table 1 Demographic and clinical characteristics of patients and controls.
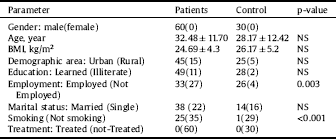
Table 2 Comparison between MDD patients and control group.

Table 3 Comparison between groups (control, nonsmoker MDD, and smoker MDD) in cytokine levels after adjusting for Age and BMI.

A: Significant difference (p<0.05) between Control and nonsmokers MDD groups.
B: Significant difference (p < 0.05) between Control and smokers MDD groups.
C: Significant difference (p < 0.05) between Smokers and nonsmokers MDD groups.
3.4. Correlation among parameters in MDD group
Correlation analyses among the parameters showed a significant correlation between IL-10 and MOR in the MDD group as seen in Fig. 1. This association remained significant after p-correction for FDR (p = 0.012). There were no significant correlations between IL-6 and any of the opioid system measurements and between IL-10 and either β-EP and EM-2.
4. Discussion
The first major finding of this study is that MDD is characterized by significant increases in serum β-EP and MOR levels. This increase may indicate stimulation of the synthesis of opioids and its receptor as a result of immune-inflammatory activation in MDD. The involvement of the opioid system in depression has been previously studied by several groups [Reference Slavich, Tartter, Brennan and Hammen17, Reference Fichna, Janecka, Piestrzeniewicz, Costentin and do Rego28–Reference Merenlender-Wagner, Dikshtein and Yadid30]. The results of β-EP assays in different papers is controversial. Hegadoren et al. [Reference Hegadoren, O’Donnell, Lanius, Coupland and Lacaze-Masmonteil31] summarized findings on serum baseline levels of nineteen published studies. Of these studies, seven reported increases in β-EP levels in MDD compared to controls; five reported decreased β-EP levels; and seven reported no changes. Nevertheless, measurements of post-dexamethasone β-EP levels in MDD indicate that there are aberrations in the EP system in MDD. Thus, post-dexamethasone β-EP levels are significantly higher in MDD with melancholic and psychotic features as compared with controls, although there were no differences in baseline β-EP levels [Reference Fichna, Janecka, Piestrzeniewicz, Costentin and do Rego28]. The variability in the β-EP results across studies may be explained by differences in subgrouping, comorbidity issues, sex difference, drug state of the patients, sample size, etc. (31). All in all, our results that β-EP levels are in increased in MDD are in agreement with a number of other studies revealing that plasma β-EP levels and MOR levels are elevated in MDD [Reference Goodwin, Austin, Curran, Ross, Murray and Prentice32, Reference Gabilondo, Meana and Garcia-Sevilla33].
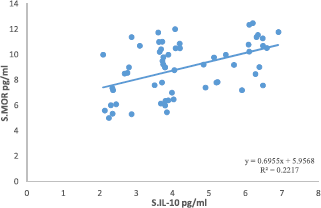
Fig. 1. Correlation between IL-10 and MOR in MDD group.
There is evidence that endogenous opioids may modulate human mood and that the endogenous μ-opioid tone is dysregulated in depression [Reference Kennedy, Koeppe, Young and Zubieta34]. Accumulating evidence from animal research shows that MOR exerts a significant control over mood-related processes [Reference Lutz and Kieffer35]. β-EP binds to opioid receptors thereby activating the endogenous analgesia system, which is located in the CNS. Moreover, this activation inhibits pain conduction and agitation of nociceptors to exert an analgesic effect [Reference Luan, Wang, Yu and Chai36]. These phenomena may be a reaction of the body against depression symptoms to overcome the psychological and somatic pain caused by MDD [Reference Nezi, Mastorakos, Mouslech, De Groot, Chrousos, Feingold, Grossman and Hershman37].
In the present study we found no significant differences in EM-2 levels between MDD patients and controls. To the best of our best knowledge, serum EM-2 levels in MDD were not previously studied. The possible role of endogenous opioid peptides in depression is supported by neurochemical and neurobehavioral findings [Reference Fichna, Janecka, Piestrzeniewicz, Costentin and do Rego38]. It has been demonstrated that EMs and MORs are present in brain regions containing monoamine neurotransmitters (serotonin, dopamine, and noradrenaline), which play a role in the physiopathology of depressive disorders.
In fact, EMs have been shown to modulate dopaminergic [Reference Huang, Chen and Tao39], noradrenergic [Reference Al-Khrasani, Elor, Abbas and Ronai40] and serotoninergic [Reference Hung, Wu, Mizoguchi, Leitermann and Tseng41] neurotransmission. In animal studies, EMs and MOR may be involved in the pathophysiopathology of depressive disorders, whilst the endomorphinergic system could serve as a novel target for the development of antidepressant drugs [Reference Fichna, Janecka, Piestrzeniewicz, Costentin and do Rego42]. The analgesia produced by EMs is short and its effect may not be significant because EMs are easily degraded by various proteases (poor metabolic stability) [Reference Perlikowska, Fichna, do-Rego, Gach and Janecka43]. This effect may also limit possible increases in EMs in MDD patients. However, in a comprehensive survey [Reference Berrocoso, Sánchez-Blázquez, Garzón and Mico44], no consistent changes in endogenous opioids levels were observed in MDD patients.
Our results that IL-6 is increased in MDD is in agreement with several studies, reviews and meta-analyses [Reference Maes, Scharpé, Meltzer, Bosmans, Suy and Calabrese2, Reference Al-Hakeim, Al-Rammahi and Al-Dujaili3, Reference Maes, Anderson, Kubera and Berk45–Reference Köhler, Evangelou, Stubbs, Solmi, Veronese and Belbasis47]. The IL-6 results indicate that MDD is accompanied by increased proinflammatory IL-6 trans-signaling [Reference Maes, Anderson, Kubera and Berk45]. Moreover, our results that IL-10 is increased in depression is in line with other studies and meta-analyses [Reference Al-Hakeim, Al-Rammahi and Al-Dujaili3, Reference Köhler, Evangelou, Stubbs, Solmi, Veronese and Belbasis47, Reference Köhler, Freitas, Maes, de Andrade, Liu and Fernandes48]. These results further support the view that MDD is accompanied by a simultaneous activation of the IRS (increased IL-6 signaling) and CIRS (increased IL-10 production) amongst other immune pathways [Reference Maes and Carvalho6]. In fact, increased IL-6 signaling is a key characteristic of the activated IRS in MDD, while IL-10 is one of the hallmarks of the CIRS [Reference Maes and Carvalho6]. Other findings [Reference Raison and Miller49] suggest that IL-10 may have a regulatory effect on the HPA axis. Furthermore, it is required for regulating immune functions by promoting the widespread suppression of immune responses through its pleiotropic effects [Reference Maes and Carvalho6]. Stress can induce nerve cells in the hypothalamus to produce and release CRH, which stimulates the release of β-EP. CRH is transported to the anterior pituitary gland where it stimulates production of a proopiomelanocortin (POMC), which is the precursor for a number of stress-related hormones, including adrenocorticotropic hormone (ACTH) and β-EP. ACTH stimulates cells of the adrenal glands to produce and release the stress hormone cortisol [Reference Stephens50]. Cortisol has many effects when it binds to glucocorticoid receptors, including effects on cardiovascular function, immunologic status, arousal, and learning and memory. All of these systems are affected when the HPA-axis is activated in response to stress [Reference Adinoff, Best, Ye, Williams and Iranmenesh51]. Thus, the anti-inflammatory effects of cortisol are brought about by reducing proinflammatory cytokines, histamine secretion, stabilizing the membranes of cell components and lysosomes [Reference Hardya, Razac and Cooper52], and stimulating immune complements [Reference Al-Hakeim53].
The finding of the present study show that the differences in IL-10, MOR and β-EP between MDD patients and controls were not affected by smoking. Table 3 shows a significant difference in serum β-EP between smoking and nonsmoking MDD patients. In one study, β-EP level was found to be higher in light smokers (less than fifteen cigarettes daily) than in controls [Reference del Arbol, Muñoz, Ojeda, Cascales, Irles and Miranda54]. Increased nicotine induces transient increases in circulating β-EP [Reference Gilbert, Meliska and Plath55], suggesting that there may be individual differences in the response of β-EP to nicotine, its derivatives, or other components of cigarette tobacco [Reference del Arbol, Muñoz, Ojeda, Cascales, Irles and Miranda54]. There are few studies reporting on the impact of smoking on inflammatory markers in MDD. Nunes et al. [Reference Nunes, Vargas, Brum, Prado, Vargas and de Castro56] showed that smoking and MDD act synergistically to increase inflammatory markers. Another paper found an association between IL-6 and smoking status where male current smokers had significantly higher levels of serum IL-6 compared to male former smokers [Reference Aldaham, Foote, Chow and Hakim57]. Our negative MOR results with regard to smoking status are in accordance with Kuwabara et al. [Reference Kuwabara, Heishman, Brasic, Contoreggi, Cascella and Mackowick58] who found no significant difference in MOR availability between controls and smokers.
The second major finding of this study is that IL-10 levels are strongly correlated with MOR levels (Fig. 1), while there was no significant correlation between endogenous opioids and IL-6 and IL-10. To the best of our knowledge, this is a first study that MOR levels are associated with IL-10, a key component of the CIRS. Cytokines regulate growth and proliferation of glial cells, modulate the activity of endogenous opioid peptides, release endogenous opioids, activate the HPA-axis [Reference Garcia, Cardoso and Dos-Santos59] via increased production of corticotrophin releasing hormone (CRH) and elicit changes in neurotransmitter activity [Reference Parsadaniantz, Batsché, Gegout-Pottie, Terlain, Gillet and Netter27]. Endogenous opioids, in turn, exert a negative tonic inhibition on CRH secretion from the hypothalamus thereby inhibiting cortisol secretion [Reference Lim, Khoo, De Groot, Chrousos and Dungan60]. This process may result in increased density of opioid receptors in peripheral nerve terminals, contributing to antinociceptive effects of opiates [Reference Garcia, Cardoso and Dos-Santos59]. Furthermore, opioid administration may suppress the immune system thereby increasing vulnerability to infections, although endogenous opioids may not have such effects [Reference Plein and Rittner61]. Recent studies show that the role of opioid receptors in immune function is very complex and entails various different mechanisms. Experimental and clinical studies have clearly shown that activation of peripheral opioid receptors with exogenous opioid agonists and endogenous opioid peptides are able to produce significant analgesic and anti-inflammatory effects, without central opioid mediated side effects (e.g., respiratory depression, sedation, tolerance, dependence [Reference Iwaszkiewicz, Schneider and Hua62]. β-EP, an anti-nociceptive neuropeptide [Reference Bäckryd, Ghafouri, Larsson and Gerdle63], may be released in the brain in response to nociceptive stimuli indicating a possible mechanism for the organisms to cope with pain as observed in MDD patients [Reference Sprouse-Blum, Smith, Sugai and Parsa64].
Different opioids may have immunosuppressive effects and/or immunostimulatory effects [Reference Liang, Liu, Chen, Ji and Li22]. Finley et al. [Reference Finley, Happel, Kaminsky and Rogers65] suggested a broad role of opioids in the modulation of the immune system function and concluded that the kappa opioid receptor induces an antiinflammatory response through down-regulation of cytokines, chemokines and chemokine receptor expression, while activation of MOR may favor a more proinflammatory response. Al-Hashimi et al. [Reference Al-Hashimi, Scott, Thompson and Lambert66] concluded that opioids produce immune modulation in both humans and experimental animals. Furthermore, they found that MOR receptors are probably the main target for classical opioid immune modulation [Reference Al-Hashimi, Scott, Thompson and Lambert66]. Activated dendritic cells can be regulated by endogenous opioid through MOR expression. Moreover, MOR activation by EP activates IL-10 productions and suppresses IL-12 and IL-23 secretion [Reference Li, Chu, Shan, Gong, Yin and Jiang67], suggesting that MOR plays a role in the CIRS and attenuates some IRS components. In animal models, exogenous opioids (heroin and morphine) have a strong stimulatory effect on the release of anti-inflammatory cytokines including IL-10 [Reference Pacifici, di Carlo, Bacosi, Pichini and Zuccaro68]. Another possible pathway explaining the association between IL-10 and MOR revolves around B cell functions. Thus, B-lymphocytes express μ, δ, and κ opioid receptors [Reference Bidlack, Khimich, Parkhill, Sumagin, Sun and Tipton69], while μ, but not δ or κ, opioid receptors may regulate immune functions [Reference Nelson, Schneider and Lysle23]. During the later phase of the adaptive immune response, increased production of T cell cytokines helps to activate B cells [Reference Abbas, Pober, Abbas and Pober70]. In MDD, Maes et al. [Reference Maes, Stevens, DeClerck, Bridts, Peeters and Schotte71] noticed a significant increase in B-cells number and percentage as compared with controls. Therefore, the correlation between the MOR and IL-10 could in part be explained by the increased number of B-cells that express MOR and are activated during an adaptive immune response.
5. Limitations of the study
One limitation of this study is that we included only males and excluded females in order to eliminate any effects of changes in female hormones, an irregular menstrual cycle or menopausal status on the results.
6. Conclusions
Serum β-EP, MOR, IL-6 and IL-10 concentrations are significantly higher in MDD than in controls, while EM-2 did not show a significant difference. Endogenous opioids (β-EP and EM-2) play a crucial role in relaxation and regulation of mood of depressed patients through the reduction of stress while attenuating immune responsivity. We found a significant correlation between IL-10 and MOR indicating that there is a possible link between immune system and endogenous opioid receptors in MDD that needs more mechanistic research for absolute proof.
Funding/support
Not funded.
Conflict of interest disclosures
None reported.







Comments
No Comments have been published for this article.